It has recently been suggested that alterations of the layers of the retina could be a biomarker of specific mental disorders since they originate in the same embryonic layer as the brain and both are interconnected through the optic nerve. The purpose of this article is to offer a systematic review of the literature and a thematic synthesis on the current state of the alterations of the retina layers identified by optical coherence tomography in patients with schizophrenia, bipolar disorder and major depression. For this purpose, we performed a bibliographic search, a systematic review of the studies and a thematic synthesis of the reported findings.
Patients with schizophrenia have more abnormal findings followed by patients with bipolar disorder, with very few findings in depression. The nerve fibre layer is the retinal layer with more abnormal findings both in schizophrenia and in bipolar disorder, while no study in major depression found alterations in it. Of the clinical parameters, the duration of the illness correlates significantly and inversely with the thickness of the different layers in all disorders.
When interpreting these data, it is necessary to take into account the limitations and differences of the studies, especially the mean length of the disorders. Given that this was very different among the 3 disorders (more than doubled in the case of schizophrenia respect to major depression), the differences in the results found could be due more to the effect of the length of illness than to the disorder itself.
In summary, optical coherence tomography findings are promising, since they could provide biomarkers of neurodegeneration and/or neuroprogression of both schizophrenia and bipolar disorder.
Recientemente se ha planteado que las alteraciones de las capas de la retina podrían ser un biomarcador de determinados trastornos mentales, al derivar esta de la misma capa embrionaria que el cerebro y estar conectada con este a través del nervio óptico. El objeto del presente artículo es ofrecer una revisión sistemática de la literatura y una síntesis temática sobre el estado actual de las alteraciones de las capas de la retina identificadas mediante tomografía de coherencia óptica en los pacientes con esquizofrenia, trastorno bipolar y depresión mayor. Para ello se realizó una búsqueda sistemática de la literatura, la lectura crítica de los artículos seleccionados y la síntesis temática de los resultados.
Los pacientes con esquizofrenia son los que presentan más alteraciones, seguidos de los pacientes con trastorno bipolar, siendo muy escasos los hallazgos en la depresión. La capa de fibras nerviosas de la retina es la capa retiniana con más alteraciones en la esquizofrenia y en el trastorno bipolar, mientras que ningún estudio en depresión mayor encontró alteraciones en ella. De los parámetros clínicos, la duración de la enfermedad correlaciona significativa e inversamente con el grosor de las distintas capas en todos los trastornos.
A la hora de interpretar estos datos es necesario tener en cuenta las limitaciones y diferencias de los estudios, especialmente el tiempo medio de evolución de los trastornos. Dado que este era muy diferente entre los 3 trastornos (más del doble en el caso de la esquizofrenia respecto a la depresión mayor), las diferencias en los resultados encontrados podrían deberse más al efecto del tiempo de evolución que al trastorno en sí.
En conclusión, los hallazgos de la tomografía de coherencia óptica son esperanzadores, ya que podrían proporcionar biomarcadores de la neurodegeneración y/o neuroprogresión tanto de la esquizofrenia como del trastorno bipolar.
Heading towards the end of the second decade of the 21st century, there are still no consistent data on the pathophysiology of severe mental disorders and their diagnosis continues leaning towards identifying the characteristic signs and symptoms through the clinical interview. However, the clinical presentation, especially in initial phases, can vary widely; dominant manifestations range from psychotic and/or affective signs and symptoms to a reduction in the level of functioning, passing through cognitive changes – such variability making it considerably more difficult to diagnose the illness precisely. The past 15 years have consequently seen significant efforts to identify objective, quantifiable markers that help to understand the pathophysiology of these disorders and to improve our diagnostic precision by incorporating biological markers.1 Among the markers under study is brain imaging. However, despite its importance, neuroimaging has yet to demonstrate great usefulness in diagnosing these illnesses, besides being a relatively expensive method that is uncomfortable for patients.
Interest has recently been focused on the retina, given that it originates from the same embryonic layer as the brain and is connected to it through the optic nerve, like a window that makes it possible to observe the brain directly through its structure and/or function. Several authors2–4 have suggested that changes in retinal structure might parallel or reflect changes in brain tissue, so retinal changes would be an easily-accessed marker of functional and/or structural brain integrity.
Optical coherence tomography (OCT) is a rapid, non-invasive imaging technique conducted without body contact and lacking known side effects. It provides precise, detailed in vivo visualisation of retinal architecture, especially in the macula and fovea region. The axial slices that OCT provides make it possible to obtain an “optical biopsy” of the surface studied, showing the 10 layers conforming the retina in great detail. Considered the optical analogue of ultrasound imaging, the OCT technique features short pulses of infrared light aimed at the eye and a reference mirror. The difference between the pattern of spectral frequency returned by the eye (the profile of the original spectral frequency minus what is absorbed by the retinal structures) and the unaltered frequency returned by the reference mirror is used to reconstruct the retinal layer images in two or three dimensions. It represents the greatest technological advance in ophthalmology in recent years, becoming the diagnostic method par excellence for analysing and controlling the diseases that affect the retina and the optic nerve.
Currently, OCT is being used in researching neurodegenerative diseases such as multiple sclerosis, Parkinson's and Alzheimer's dementia. There is neurodegenerative parallelism between these illnesses and severe mental disorders, especially schizophrenia (SCH). Bearing this in mind, this technique has recently been incorporated into research on autism spectrum disorder, SCH, el bipolar disorder (BD) and major depression (MD), in order to establish whether its findings might be biomarkers of the neurodegeneration and/or neuroprogression characteristic of these disorders. The interest is also even greater because it is a non-invasive, rapidly applied technique that is more economical than the conventional neuroimaging techniques.
Several reviews have recently been published about retinal alterations identified using OCT in mental disorders.4–7 However, these are limited to SCH, even though Adams and Nasrallah6 also included a small summary of the findings in other mental disorders. That is the reason that our objective was to carry out a systematic review and qualitative thematic synthesis of alterations in the retina identified using OCT in individuals with SCH, BD and depression.
MethodsThis article presents a systematic review and a thematic synthesis on the retina alterations identified using OCT in individuals with severe mental disorders, specifically SCH, BD and MD. First of all, a systematic literature search was carried out; next, the studies selected were read critically. Finally, a thematic synthesis of the retinal alterations described was created, and the areas for improvement based on the weaknesses of the studies analysed were identified.
Systematic literature searchA systematic search of the PubMed database, with no time limitation at all, was performed using the following search terms: (Optical Coherence Tomography [All Fields]) AND ((Schizophrenia [All Fields]) OR (Bipolar Disorder [All Fields]) OR (Major Depression [All Fields])). A total of 45 articles were obtained, of which 19 were selected (10 on SCH, 5 on depression, 3 on BD and 1 on psychosis). The same search was also repeated, but this time with the mental disorder search terms operating as MeSH Terms instead of All Fields, yielding a total of 22 articles. Of these, 19 had already been obtained in the previous search, while there were 3 new articles relevant for this review (1 for each disorder).
The relevance criteria established for selecting an article for analysis of results were that the article had to describe OCT results in patients with the chosen mental disorder and had to be written in Spanish or English. There were no restrictions as to sample size or study design. Based on these criteria, 8 more articles were eliminated, as follows: 1 on psychosis,8 because it included 6 patients with 2 mental disorders (3 with SCH and 3 with BD) and did not provide the results separately for the two disorders; 5 on SCH, because they were reviews4–7 or they included patients with schizoaffective disorder as well2; and 2 on depression,9,10 because they consisted of a letter to the editor about the limitations of the article by Schönfeldt-Lecuona et al.11 and the article authors’ reply to the letter comments.
A search was also conducted in PsycINFO, as well as a manual search of the references of the articles identified, but no additional articles were found.
Critical reading of the articlesThe articles on SCH were critically read by LGA, those on BD by LFT and those on depression by AVI. The first-named author (MPGP) also read all the articles independently. After that, a consensus was reached among the 4 authors on the weaknesses and strengths of the studies, and on the results obtained.
Thematic synthesisTo carry out the thematic synthesis, 2 main topics of interest were identified: alterations in the retina and choroid in each of the mental disorders, and the correlation between the retinal and choroidal alterations and the clinical variables for each disorder (time of evolution, psychometric and clinical severity, hospitalisations, treatment refractoriness, drug treatment). Each of the reviewers extracted the findings individually, organising them into these 2 topics, along with the limitations of the disorder studies. The first-named author unified and synthesised the findings on the 3 disorders and noted the pertinent assessments or functions.
ResultsThis systematic review and thematic synthesis on retinal alterations in individuals with severe mental disorder is based on a total of 14 articles: 6 on SCH, 4 on BD and 4 on depression.
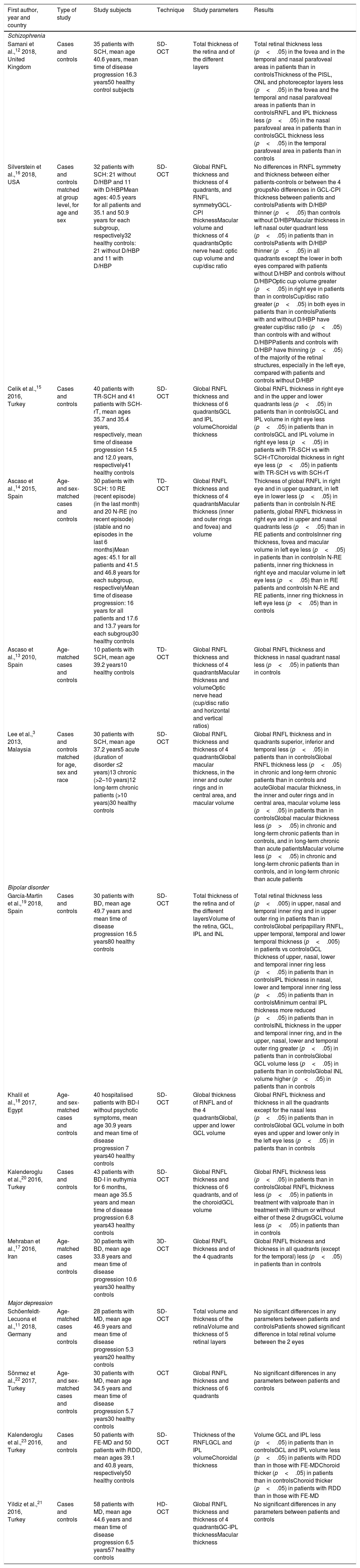
Alterations in the retina in severe mental disorders (Table 1)SchizophreniaThe few studies conducted using OCT in patients with SCH have shown mainly a global thinning of retinal thickness. This occurs especially at the expense of the photoreceptor layer, in the fovea and the nasal and temporal parafoveal areas,12 but also of the global thickness of the retinal nerve fibre layer (RNFL)3,13–15 and in the superior and inferior quadrants,3,14 and loss of volume in the ganglion cell layer (GCL)15 and in the inner plexiform layer (IPL)15 compared with healthy controls. In addition, patients that had comorbidities with systemic diseases such as diabetes or high blood pressure (HBP) showed thinner GCL and IPL areas than the controls that did not have diabetes or HBP, and a thinner macula than the patients and controls without diabetes or HBP.6
Differences between patients and healthy control subjects in the optical coherence tomography parameters.
| First author, year and country | Type of study | Study subjects | Technique | Study parameters | Results |
|---|---|---|---|---|---|
| Schizophrenia | |||||
| Samani et al.,12 2018, United Kingdom | Cases and controls | 35 patients with SCH, mean age 40.6 years, mean time of disease progression 16.3 years50 healthy control subjects | SD-OCT | Total thickness of the retina and of the different layers | Total retinal thickness less (p<.05) in the fovea and in the temporal and nasal parafoveal areas in patients than in controlsThickness of the PISL, ONL and photoreceptor layers less (p<.05) in the fovea and the temporal and nasal parafoveal areas in patients than in controlsRNFL and IPL thickness less (p<.05) in the nasal parafoveal area in patients than in controlsGCL thickness less (p<.05) in the temporal parafoveal area in patients than in controls |
| Silverstein et al.,16 2018, USA | Cases and controls matched at group level, for age and sex | 32 patients with SCH: 21 without D/HBP and 11 with D/HBPMean ages: 40.5 years for all patients and 35.1 and 50.9 years for each subgroup, respectively32 healthy controls: 21 without D/HBP and 11 with D/HBP | SD-OCT | Global RNFL thickness and thickness of 4 quadrants, and RNFL symmetryGCL-CPI thicknessMacular volume and thickness of 4 quadrantsOptic nerve head: optic cup volume and cup/disc ratio | No differences in RNFL symmetry and thickness between either patients-controls or between the 4 groupsNo differences in GCL-CPI thickness between patients and controlsPatients with D/HBP thinner (p<.05) than controls without D/HBPMacular thickness in left nasal outer quadrant less (p<.05) in patients than in controlsPatients with D/HBP thinner (p<.05) in all quadrants except the lower in both eyes compared with patients without D/HBP and controls without D/HBPOptic cup volume greater (p<.05) in right eye in patients than in controlsCup/disc ratio greater (p<.05) in both eyes in patients than in controlsPatients with and without D/HBP have greater cup/disc ratio (p<.05) than controls with and without D/HBPPatients and controls with D/HBP have thinning (p<.05) of the majority of the retinal structures, especially in the left eye, compared with patients and controls without D/HBP |
| Celik et al.,15 2016, Turkey | Cases and controls | 40 patients with TR-SCH and 41 patients with SCH-rT, mean ages 35.7 and 35.4 years, respectively, mean time of disease progression 14.5 and 12.0 years, respectively41 healthy controls | SD-OCT | Global RNFL thickness and thickness of 6 quadrantsGCL and IPL volumeChoroidal thickness | Global RNFL thickness in right eye and in the upper and lower quadrants less (p<.05) in patients than in controlsGCL and IPL volume in right eye less (p<.05) in patients than in controlsGCL and IPL volume in right eye less (p<.05) in patients with TR-SCH vs with SCH-rTChoroidal thickness in right eye less (p<.05) in patients with TR-SCH vs with SCH-rT |
| Ascaso et al.,14 2015, Spain | Age- and sex-matched cases and controls | 30 patients with SCH: 10 RE (recent episode) (in the last month) and 20 N-RE (no recent episode) (stable and no episodes in the last 6 months)Mean ages: 45.1 for all patients and 41.5 and 46.8 years for each subgroup, respectivelyMean time of disease progression: 16 years for all patients and 17.6 and 13.7 years for each subgroup30 healthy controls | TD-OCT | Global RNFL thickness and thickness of 4 quadrantsMacular thickness (inner and outer rings and fovea) and volume | Thickness of global RNFL in right eye and in upper quadrant, in left eye in lower less (p<.05) in patients than in controlsIn N-RE patients, global RNFL thickness in right eye and in upper and nasal quadrants less (p<.05) than in RE patients and controlsInner ring thickness, fovea and macular volume in left eye less (p<.05) in patients than in controlsIn N-RE patients, inner ring thickness in right eye and macular volume in left eye less (p<.05) than in RE patients and controlsIn N-RE and RE patients, inner ring thickness in left eye less (p<.05) than in controls |
| Ascaso et al.,13 2010, Spain | Age-matched cases and controls | 10 patients with SCH, mean age 39.2 years10 healthy controls | TD-OCT | Global RNFL thickness and thickness of 4 quadrantsMacular thickness and volumeOptic nerve head (cup/disc ratio and horizontal and vertical ratios) | Global RNFL thickness and thickness in nasal quadrant nasal less (p<.05) in patients than in controls |
| Lee et al.,3 2013, Malaysia | Cases and controls matched for age, sex and race | 30 patients with SCH, mean age 37.2 years5 acute (duration of disorder ≤2 years)13 chronic (>2–10 years)12 long-term chronic patients (>10 years)30 healthy controls | SD-OCT | Global RNFL thickness and thickness of 4 quadrantsGlobal macular thickness, in the inner and outer rings and in central area, and macular volume | Global RNFL thickness and in quadrants superior, inferior and temporal less (p<.05) in patients than in controlsGlobal RNFL thickness less (p<.05) in chronic and long-term chronic patients than in controls and acuteGlobal macular thickness, in the inner and outer rings and in central area, macular volume less (p<.05) in patients than in controlsGlobal macular thickness less (p>.05) in chronic and long-term chronic patients than in controls, and in long-term chronic than acute patientsMacular volume less (p<.05) in chronic and long-term chronic patients than in controls, and in long-term chronic than acute patients |
| Bipolar disorder | |||||
| García-Martín et al.,19 2018, Spain | Cases and controls | 30 patients with BD, mean age 49.7 years and mean time of disease progression 16.5 years80 healthy controls | SD-OCT | Total thickness of the retina and of the different layersVolume of the retina, GCL, IPL and INL | Total retinal thickness less (p<.005) in upper, nasal and temporal inner ring and in upper outer ring in patients than in controlsGlobal peripapillary RNFL, upper temporal, temporal and lower temporal thickness (p<.005) in patients vs controlsGCL thickness of upper, nasal, lower and temporal inner ring less (p<.05) in patients than in controlsIPL thickness in nasal, lower and temporal inner ring less (p<.05) in patients than in controlsMinimum central IPL thickness more reduced (p<.05) in patients than in controlsINL thickness in the upper and temporal inner ring, and in the upper, nasal, lower and temporal outer ring greater (p<.05) in patients than in controlsGlobal GCL volume less (p<.05) in patients than in controlsGlobal INL volume higher (p<.05) in patients than in controls |
| Khalil et al.,18 2017, Egypt | Age- and sex-matched cases and controls | 40 hospitalised patients with BD-I without psychotic symptoms, mean age 30.9 years and mean time of disease progression 7 years40 healthy controls | SD-OCT | Global thickness of RNFL and of the 4 quadrantsGlobal, upper and lower GCL volume | Global RNFL thickness and thickness in all the quadrants except for the nasal less (p<.05) in patients than in controlsGlobal GCL volume in both eyes and upper and lower only in the left eye less (p<.05) in patients than in controls |
| Kalenderoglu et al.,20 2016, Turkey | Cases and controls | 43 patients with BD-I in euthymia for 6 months, mean age 35.5 years and mean time of disease progression 6.8 years43 healthy controls | SD-OCT | Global RNFL thickness and thickness of 6 quadrants, and of the choroidGCL volume | Global RNFL thickness less (p<.05) in patients than in controlsGlobal RNFL thickness less (p<.05) in patients in treatment with valproate than in treatment with lithium or without either of these 2 drugsGCL volume less (p<.05) in patients than in controls |
| Mehraban et al.,17 2016, Iran | Age-matched cases and controls | 30 patients with BD, mean age 33.8 years and mean time of disease progression 10.6 years30 healthy controls | 3D-OCT | Global RNFL thickness and of the 4 quadrants | Global RNFL thickness and thickness in all quadrants (except for the temporal) less (p<.05) in patients than in controls |
| Major depression | |||||
| Schöenfeldt-Lecuona et al.,11 2018, Germany | Age-matched cases and controls | 28 patients with MD, mean age 46.9 years and mean time of disease progression 5.3 years20 healthy controls | SD-OCT | Total volume and thickness of the retinaVolume and thickness of 5 retinal layers | No significant differences in any parameters between patients and controlsPatients showed significant difference in total retinal volume between the 2 eyes |
| Sönmez et al.,22 2017, Turkey | Age- and sex-matched cases and controls | 30 patients with MD, mean age 34.5 years and mean time of disease progression 5.7 years30 healthy controls | OCT | Global RNFL thickness and thickness of 6 quadrants | No significant differences in any parameters between patients and controls |
| Kalenderoglu et al.,23 2016, Turkey | Cases and controls | 50 patients with FE-MD and 50 patients with RDD, mean ages 39.1 and 40.8 years, respectively50 healthy controls | SD-OCT | Thickness of the RNFLGCL and IPL volumeChoroidal thickness | Volume GCL and IPL less (p<.05) in patients than in controlsGCL and IPL volume less (p<.05) in patients with RDD than in those with FE-MDChoroid thicker (p<.05) in patients than in controlsChoroid thicker (p<.05) in patients with RDD than in those with FE-MD |
| Yildiz et al.,21 2016, Turkey | Cases and controls | 58 patients with MD, mean age 44.6 years and mean time of disease progression 6.5 years57 healthy controls | HD-OCT | Global RNFL thickness and thickness of 4 quadrantsGC-IPL thicknessMacular thickness | No significant differences in any parameters between patients and controls |
BD: bipolar disorder; D: diabetes; FE-MD: first episode of major depression; GCL: ganglion cell layer; GC-IPL: ganglion cell layer and inner plexiform layer; HBP: high blood pressure; HD-OCT: high-domain optical coherence tomography; INL: inner nuclear layer; IPL: inner plexiform layer; MD: major depression; OCT: optical coherence tomography; ONL: outer nuclear layer; PISL: photoreceptor inner segment layer; RDD: recurrent depressive disorder; RNFL: retinal nerve fibre layer; SCH: schizophrenia; SCH-rT: schizophrenia with response to treatment; SD-OCT: spectral-domain optical coherence tomography; TD-OCT: time-domain optical coherence tomography; TR-SCH: treatment-resistant schizophrenia.
Likewise, differences between patients and controls have been described at the level of the macula3,14,16 and optic disc.16 Comparing patient macula with those of healthy controls, patients presented less macular thickness3,16 and volume,3,14,16 as well as thinning in the fovea and the outer3 and inner rings.3,14 As for optic disc alterations, patients presented greater cup volume and a greater cup/disc ratio than healthy controls.16
Bipolar disorderThe 4 studies published on BD showed a considerable reduction in RNFL thickness in the patients with BD compared with the healthy controls, the location of this alteration differing. Mehraban et al.17 described a notable reduction in the inferior, superior and nasal quadrants; Khalil et al.,18 in the inferior, superior, temporal and global quadrants; García-Martín et al.,19 in the upper temporal, temporal, lower temporal and global quadrants; and Kalenderoglu et al.,20 in only the global. In addition, Kalenderoglu et al.,20 Khalil et al.18 and García-Martín et al.19 showed that GCL volume was less in the patients with BD than this volume in the control subjects. The last-mentioned authors also found reduced inner ring thickness in the 4 quadrants of the GCL compared with the healthy controls. As for IPL, García-Martín et al.19 described a reduction in the inner ring of the inferior, nasal and temporal quadrants, along with a reduction in the minimum central thickness in comparison with the controls. Lastly, these same authors found a global increase in the volume of the inner nuclear layer, specifically in inner ring thickness in the superior and inferior quadrants, and in the outer ring in the 4 quadrants.
Major depressionIn the case of MD, 3 of the 4 studies published did not show significant differences between the patients and the healthy controls in any of the OCT parameters studied.11,21,22 Only Kalenderoglu et al.23 found less GCL and IPL volume in patients with MD than in healthy controls, and in patients with multiple episodes than in those with a single episode.
Schöenfeldt-Lecuona et al.11 found the total volume of the left eye retina was significantly greater than that of the right eye (8.72 vs 8.69mm3, p=.03) in patients with MD.
Choroidal alterations in severe mental disorders (Table 1)The studies reviewed described alterations in choroidal thickness solely in the patients with MD. Specifically, Kalenderoglu et al.23 found that the patients with MD showed a greater choroidal thickness than the healthy controls did; the same occurred for the patients with multiple episodes compared with the patients that had had a single episode.
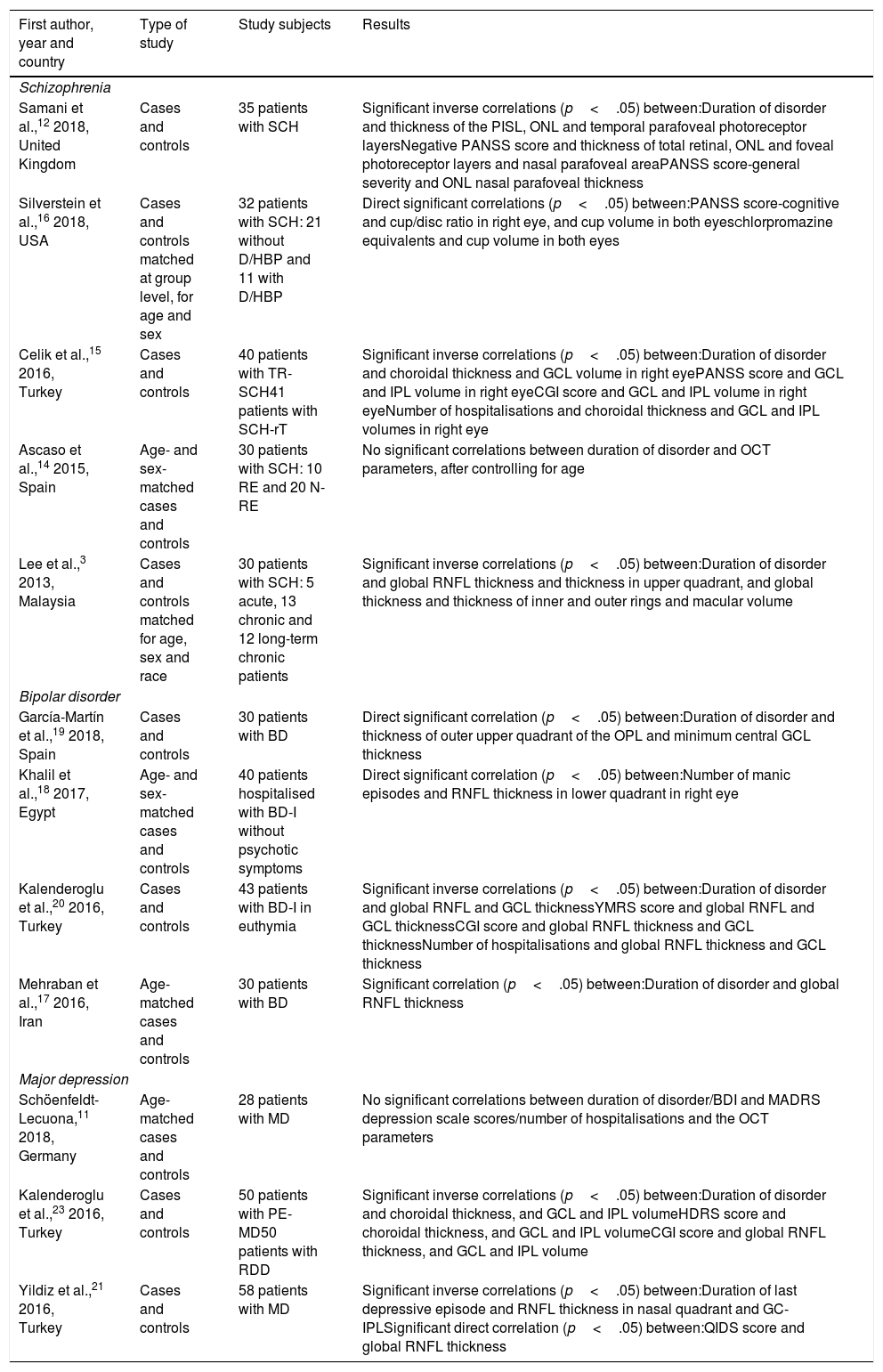
Correlation between ocular alterations and clinical dimensions (Table 2)SchizophreniaWith respect to the impact of time of evolution on ocular alterations, Lee et al.3 demonstrated that global RNFL was thinner in patients with more than 2 years of disorder evolution than in patients with less than 2 years of evolution and in controls. In addition, in patients without a recent episode (defined as >6 months since the last episode), they found significant reductions in global RNFL thickness and in the thickness of the superior, inferior and nasal quadrants compared with the controls; the thickness of the inner ring of the macula and macular volume were reduced as well.14 Likewise, they observed a thinning in the inner ring of the macula3,14 and a loss of macular volume3,14 in advanced stages of the disease compared with the control subjects.16
Correlations between the optical coherence tomography parameters and the clinical characteristics of the illness.
| First author, year and country | Type of study | Study subjects | Results |
|---|---|---|---|
| Schizophrenia | |||
| Samani et al.,12 2018, United Kingdom | Cases and controls | 35 patients with SCH | Significant inverse correlations (p<.05) between:Duration of disorder and thickness of the PISL, ONL and temporal parafoveal photoreceptor layersNegative PANSS score and thickness of total retinal, ONL and foveal photoreceptor layers and nasal parafoveal areaPANSS score-general severity and ONL nasal parafoveal thickness |
| Silverstein et al.,16 2018, USA | Cases and controls matched at group level, for age and sex | 32 patients with SCH: 21 without D/HBP and 11 with D/HBP | Direct significant correlations (p<.05) between:PANSS score-cognitive and cup/disc ratio in right eye, and cup volume in both eyesChlorpromazine equivalents and cup volume in both eyes |
| Celik et al.,15 2016, Turkey | Cases and controls | 40 patients with TR-SCH41 patients with SCH-rT | Significant inverse correlations (p<.05) between:Duration of disorder and choroidal thickness and GCL volume in right eyePANSS score and GCL and IPL volume in right eyeCGI score and GCL and IPL volume in right eyeNumber of hospitalisations and choroidal thickness and GCL and IPL volumes in right eye |
| Ascaso et al.,14 2015, Spain | Age- and sex-matched cases and controls | 30 patients with SCH: 10 RE and 20 N-RE | No significant correlations between duration of disorder and OCT parameters, after controlling for age |
| Lee et al.,3 2013, Malaysia | Cases and controls matched for age, sex and race | 30 patients with SCH: 5 acute, 13 chronic and 12 long-term chronic patients | Significant inverse correlations (p<.05) between:Duration of disorder and global RNFL thickness and thickness in upper quadrant, and global thickness and thickness of inner and outer rings and macular volume |
| Bipolar disorder | |||
| García-Martín et al.,19 2018, Spain | Cases and controls | 30 patients with BD | Direct significant correlation (p<.05) between:Duration of disorder and thickness of outer upper quadrant of the OPL and minimum central GCL thickness |
| Khalil et al.,18 2017, Egypt | Age- and sex-matched cases and controls | 40 patients hospitalised with BD-I without psychotic symptoms | Direct significant correlation (p<.05) between:Number of manic episodes and RNFL thickness in lower quadrant in right eye |
| Kalenderoglu et al.,20 2016, Turkey | Cases and controls | 43 patients with BD-I in euthymia | Significant inverse correlations (p<.05) between:Duration of disorder and global RNFL and GCL thicknessYMRS score and global RNFL and GCL thicknessCGI score and global RNFL thickness and GCL thicknessNumber of hospitalisations and global RNFL thickness and GCL thickness |
| Mehraban et al.,17 2016, Iran | Age-matched cases and controls | 30 patients with BD | Significant correlation (p<.05) between:Duration of disorder and global RNFL thickness |
| Major depression | |||
| Schöenfeldt-Lecuona,11 2018, Germany | Age-matched cases and controls | 28 patients with MD | No significant correlations between duration of disorder/BDI and MADRS depression scale scores/number of hospitalisations and the OCT parameters |
| Kalenderoglu et al.,23 2016, Turkey | Cases and controls | 50 patients with PE-MD50 patients with RDD | Significant inverse correlations (p<.05) between:Duration of disorder and choroidal thickness, and GCL and IPL volumeHDRS score and choroidal thickness, and GCL and IPL volumeCGI score and global RNFL thickness, and GCL and IPL volume |
| Yildiz et al.,21 2016, Turkey | Cases and controls | 58 patients with MD | Significant inverse correlations (p<.05) between:Duration of last depressive episode and RNFL thickness in nasal quadrant and GC-IPLSignificant direct correlation (p<.05) between:QIDS score and global RNFL thickness |
BD: bipolar disorder; BDI: Beck Depression Inventory; CGI: Clinical Global Impression Scale; D: diabetes; FE-MD: first episode of major depression; GC-IPL: ganglion cell layer and inner plexiform layer; GCL: ganglion cell layer; HBP: high blood pressure; HD-OCT: high-domain optical coherence tomography; HDRS: Hamilton Depression Rating Scale; IPL: inner plexiform layer; MADRS: Montgomery-Asberg Depression Rating Scale; MD: major depression; N-RE: no recent episode (stable and no episodes in the last 6 months); OCT: optical coherence tomography; ONL: outer nuclear layer; OPL: outer plexiform layer; PANSS: Positive and Negative Syndrome Scale; PISL: photoreceptor inner segment layer; QIDS: Quick Inventory of Depressive Symptomatology; RDD: recurrent depressive disorder; RE: recent episode (in the last month); RNFL: retinal nerve fibre layer; SCH: schizophrenia; SCH-rT: schizophrenia with response to treatment; SD-OCT: spectral-domain optical coherence tomography; TD-OCT: time-domain optical coherence tomography; TR-SCH: treatment-resistant schizophrenia; YMRS: Young Mania Rating Scale.
The relationship between the different psychopathological dimensions of SCH and changes in retinal architecture have also been investigated. Samani et al.12 found a significant moderate inverse correlation between the severity of negative SCH symptoms and the thickness of the photoreceptor (r=−.54) and outer nuclear layers (r=−.47) in the fovea. Cognitive symptoms were shown to be related with increased cup volume in both eyes (left, r=.48; right, r=.45) and increased cup/optic disc ration in the right eye (r=.41).16 These authors also found a significant direct correlation between antipsychotic drug dose (chlorpromazine equivalents) and cup volume in both eyes (left, r=.41; right, r=.38).
Lastly, patients with treatment-refractory SCH showed a greater reduction in GCL and IPL volume than patients with an adequate response to treatment.15
Bipolar disorderThe time of BD evolution correlated significantly and inversely with global RNFL thickness (r=−.250,20 non-proportional value of r17) and GCL thickness (r=−.466).20 Disorder evolution time directly correlated with the minimum central thickness of the GCL (r=.70)19 and the upper outer quadrant thickness of the outer plexiform layer (r=.49).19 In addition, there was significant direct correlation between the number of manic episodes and RNFL thickness in the inferior quadrant of the right eye (r=.335).18
As for clinical severity, Khalenderoglu et al.20 demonstrated significant inverse correlations between the scores on the Young Mania Rating Scale and the Clinical Global Impression Scale (CGI) and the number of hospitalisations with global RNFL thickness (r=−.265, −.280 and −.232, respectively) and with GCL thickness (r=−.407, −.456 and −.431, respectively).
Major depressionA significant inverse correlation of time of disorder evolution has been described with GCL and IPL layer volume (r=−.247 and −.252, respectively) and with choroidal thickness (r=−.329).23 Inverse correlations between the duration of the last depressive episode and the thickness of the RNFL in the nasal quadrant (r=−.31) and of the ganglion cell and inner plexiform layers (GC-IPL) (r=−.32) have also been described.21
Likewise, there were significant correlations between the clinical severity of the depression and the OCT parameters. Scores on the Hamilton Depression Rating Scale correlated inversely and significantly with GCL and IPL volume (r=−.200 and −.221, respectively) and with choroidal thickness (r=−.180).23 Scores on the Quick Inventory of Depressive Symptomatology-Self Report directly correlated with total RNFL thickness (r=.28). Although CGI scores correlated inversely and significantly with GCL and IPL volume (r=−.248 and −.268, respectively), they correlated with global RNFL thickness (r=−.162) instead of with that of the choroid.23
Lastly, taking antipsychotic drugs was significantly associated with less GCL and IPL volume.23
DiscussionAs has been indicated in the result section, there are few studies using OCT in mental disorders such as SCH, BD and MD. This is in contrast with what happens in dementia or in neurological diseases such as Parkinson's. In addition, the results obtained are sometimes divergent, and even contradictory.
Among all the retinal parameters evaluated, RNFL thickness is undoubtedly the one that has provided the most significant findings in the 3 mental disorders reviewed. With a single exception,16 all the studies conducted on patients with SCH or BD show significant thinning compared with the healthy controls, while none of the 4 studies on MD find significant alterations. These differences among the 3 disorders might indicate that MD, in contrast to SCH and BD, is not associated with a magnocellular pathway alteration, as Sönmez et al.22 noted. However, based on the hypothesis of neurodegeneration and the capability of OCT to identify it, the differences might be related to the time of evolution of the disorder more than to the disorder itself: the time of evolution for the patients with MD (from 5.5 to 6.5 years in the 4 studies) was much shorter than the time of evolution for the patients with BD (in 3 studies, it was between 7 and 10 years and was only greater than 10 years – specifically 16.5 years – in 1 study) and, above all, shorter than that of the patients with SCH (between 12 and 16 years). In the patients with SCH, the findings of Lee et al.3 and Ascaso et al.14 were congruent with this, which would reinforce the hypothesis that the retinal alterations were the expression of the progressive neurodegeneration of the disorder. These researchers showed that RNFL thinning was greater in long-term chronic (>10 years of evolution) and chronic (>2–10 years) patients than in acute patients (≤2 years) and healthy controls.3 They also showed that such thinning was more in patients without a recent episode (no episodes in the last 6 months) than in patients with a recent episode (the last month) and healthy control subjects.14
Other retinal layers that have provided significant results are the GCL and the IPL. Significant differences are obtained in comparison to results for the controls in the 3 disorders studied, even though the consistency of the findings is low. GCL thickness/volume is significantly less than those of the controls in 2 of the 6 studies on SCH,12,15 in 3 of the 4 studies on BD18–20 and in 1 of the 4 studies on MD.23 In addition, the IPL also presented less volume in SCH,15 in BD19 and in MD.23 The fact that the GCL-IPL alterations are more pronounced than those of the RNFL in BD20 and that they are the only retinal alterations described for MD23 would support the hypothesis of González-López et al.24 that the GCL-IPL would be more sensitive than the RNFL in the detection of alterations in retinal structure, bearing in mind the findings obtained in patients with multiple sclerosis. In addition, the shorter time of disease evolution in the case of MD and BD compared with that of SCH would also support the hypothesis of neuroprogression: patients with shorter time of evolution would only show alterations in the GCL-IPL, which would progress until reaching the RNFL. Along this line, Kalenderoglu et al.23 indicated that there was less volume in both layers in the patients with multiple episodes of depression than in the patients with a single episode. However, even though Silverstein et al.16 found no significant differences between patients and controls, they did find them between the subgroup of patients with diabetes or hypertension compared with the subgroup of controls that did not have these conditions. These authors, in contrast to what occurred in most of the studies conducted, included a subgroup of patients and controls with diabetes or hypertension without identifiable retinal involvement in order to determine whether the alterations described in the studies were truly due to the mental disorder or if they might be due to systemic diseases with a known ocular impact that are frequently comorbid with SCH. Their results demonstrated that the retinal alterations were due more to the presence of diabetes or hypertension than to SCH (although it is necessary to point out that the group of patients with diabetes and hypertension were significantly older than the patients and the controls without diabetes or hypertension). As for the impact of drug treatment on these 2 layers (GCL and IPL), less volume of both was found in the patients with treatment-resistant SCH than in those responding to treatment,15 and in the patients with MD under antipsychotic drug treatment than in those not taking such drugs.23 These results show that being refractory to drugs is related to greater neurodegeneration in both disorders.
The impact of psychopharmaceutical drugs on the retinal alterations found is a controversial subject that requires additional, in-depth studies. It has been proposed that the effect might be explained by the dopaminergic block of antipsychotics16 or by their serotoninergic effect and their impact on intraocular pressure.25 However, it should be pointed out that valproate (which lacks these mechanisms of action) has also been associated with a thinner RNFL, while lithium has not.20
Alterations in choroidal thickness have seldom been described and the data have been controversial. While no differences were found between the patients and controls in the study on SCH,15 in MD Kalederoglu et al.23 described a thickening of the choroidal layer in the patients compared with the controls, a fact that they attributed to the inflammatory state characterising the active disease. The results of Celik et al.15 were to a certain degree contrary to this hypothesis: in spite of the fact that treatment-resistant SCH has been associated with low-grade chronic peripheral inflammation,26 these authors found thinning of the choroid in the patients with treatment-resistant SCH compared with patients responding to treatment.
The relationship between the ocular alterations and the clinical parameters varies widely and has not been regularly replicated in these studies, except for the time of disease evolution. In all the disorders, and in most of the studies on each disorder, a direct relationship has been described between the time of evolution and the retinal alterations described, which supports the hypothesis of neuroprogression in these mental disorders. In the case of SCH, correlations have also been described, as expected, between the scores on the Positive and Negative Syndrome Scale-negative and general,12 the Positive and Negative Syndrome Scale-cognitive,16 the Positive and Negative Syndrome Scale and the CGI-G15 with different retinal alterations. In addition, the chlorpromazine equivalents16 and the number of hospitalisations correlated as expected with the optic cup volume in both eyes, and choroidal thickness and IPL and GCL volume in the right eye, respectively. In the case of BD, only 1 study out of the 320 found direct correlations between psychopathological severity (scores on the Young Mania Rating Scale and on the CGI) and retinal alterations (thickness of the RNFL and the GCL). However, considering other indexes of disorder severity, the number of hospitalisations20 has been associated with RNFL and GCL thickness, and the number of manic episodes,18 with RNFL thickness. Nonetheless, it should be pointed out that, for the number of manic episodes, the correlation was not in the expected sense (that is, the greater the number of manic episodes, the thicker the RNFL), and the authors18 did not offer a plausible explanation for this finding. Lastly, the results described are contradictory in the case of MD: while greater self-evaluated clinical severity (as indicated by scores on the Hamilton Depression Rating Scale and the CGI) has been associated with retinal structure thinning,23 greater self-evaluated clinical severity (Quick Inventory of Depressive Symptomatology scores) has also been associated with RNFL thickening.21
In evaluating the findings described here, the important methodological limitations that the studies reviewed present have to be considered. These limitations involve, in the first place, the study sample: study size and patient selection, the process of matching with control subjects, and the inclusion and exclusion criteria are questionable and vary from one study to another. In the majority of the studies, sample size ranges from 30 to 50 subjects per group, but the groups are sometimes subdivided and analysed based on some specific criterion (for example, with/without diabetes/hypertension, acute vs chronic vs long-term chronic patients, etc.); this reduces the sample size and statistical power even more. A very important parameter is the time of disease evolution, as has been seen upon comparing the results of the 3 disorders reviewed: it can be essential when interpreting the results. Disorder stage and evolution (acute vs chronic and single episodes vs multiple episodes) present similar importance. In addition, given the complex nature of BD, patient heterogeneity is crucial to control. Matching between patients and controls is not always present in the studies, nor is it always at an individual level. In the most recent studies, the criteria for subject inclusion and exclusion are stricter and more numerous than in the initial studies; and the fact that patients with somatic comorbidities without identifiable clinical ophthalmological effects were included16 makes some of the results for SCH to date questionable. As for technique, when the studies were performed once again generates differences: the most recent studies use spectral domain equipment, so the capability of detecting anomalies is better than that of the previous studies that used temporal domain OCT. The fact that the discrepancies in the findings might be partially caused by the non-homologation of devices used should also be considered. Finally, the statistical analysis is problematic. Most of the studies, especially the oldest, did not control for possibly confounding variables such as age, time of evolution of the disease and other factors that can interfere with the results. Although the limitations that we have indicated are numerous, they are ones that are frequently common in pioneering studies using a novel technique or intervention, and are overcome bit by bit as knowledge accumulates.
Among the limitations of this study, the fact that the sole source of information was PubMed should be mentioned. Nonetheless, this was because the PsycINFO search did not add any new studies. Although the lack of statistical analyses could be considered another limitation, the studies for each disorder are very limited and are on very diverse optical zones; these facts rule out conducting a metaanalysis of the results.
In short, the results of the OCT studies on patients with severe mental disorders back the existence of a gradient of neurodegeneration in these disorders, with the retinal alterations being more numerous and more consistent in SCH and in BD. The fact that such findings have not been found for MD might be due to either the shorter time of disease progression in the patients studied (which would lend weight to the hypothesis of neuroprogression), or to a lack of degeneration or an incapacity of OCT to identify it in this disorder. The fact that one of the studies has identified alterations in the GCL and IPL (layers that have shown to have a greater sensitivity in diseases such as multiple sclerosis) would also support neuroprogression in these 3 mental disorders. Lastly, except for time of disease progression, the retinal findings do not seem to mark differential clinical or psychopathological aspects in any of these disorders.
Bearing in mind everything that has been indicated above, the OCT findings are promising, because they could provide biomarkers of neurodegeneration and/or neuroprogression in both SCH and BD.
Conflict of interestsThe authors have no conflicts of interest to declare.
Please cite this article as: García-Portilla MP, García-Álvarez L, de la Fuente-Tomás L, Velasco-Iglesias Á, Sáiz PA, González-Blanco L, et al. Los cambios estructurales de la retina, ¿nuevos biomarcadores de los trastornos mentales? Una revisión sistemática y síntesis temática de la literatura. Rev Psiquiatr Salud Ment (Barc.). 2019;12:116–129.