Therapeutic Drug Monitoring (TDM) of antipsychotics in schizophrenia is a powerful tool that allows tailoring the treatment in an individualized approach. Our goals are to develop and validate a Dried Blood Spot (DBS) method for monitoring some commonly used antipsychotics (aripiprazole, clozapine, and paliperidone) and to evaluate its usefulness as a compliance biomarker, as well as in drug-dose adjustment to personalize the antipsychotic treatment to improve its efficacy and safety.
Methods31 first-psychotic episode (FEP) and schizophrenia patients were included; 5 refer to naïve FEP who started antipsychotic treatment; 26, to patients with more than one episode and under antipsychotic treatment: aripiprazole (7 cases), clozapine (17), paliperidone (11). For DBS sample collection, 25μl of capillary blood were placed in the spot of a FTA™DMPK-C-card. After completely dryness, antipsychotics were extracted and analyzed by a validated UHPLC-MS/MS-method. DBS antipsychotic results were compared with those obtained in venous blood/plasma.
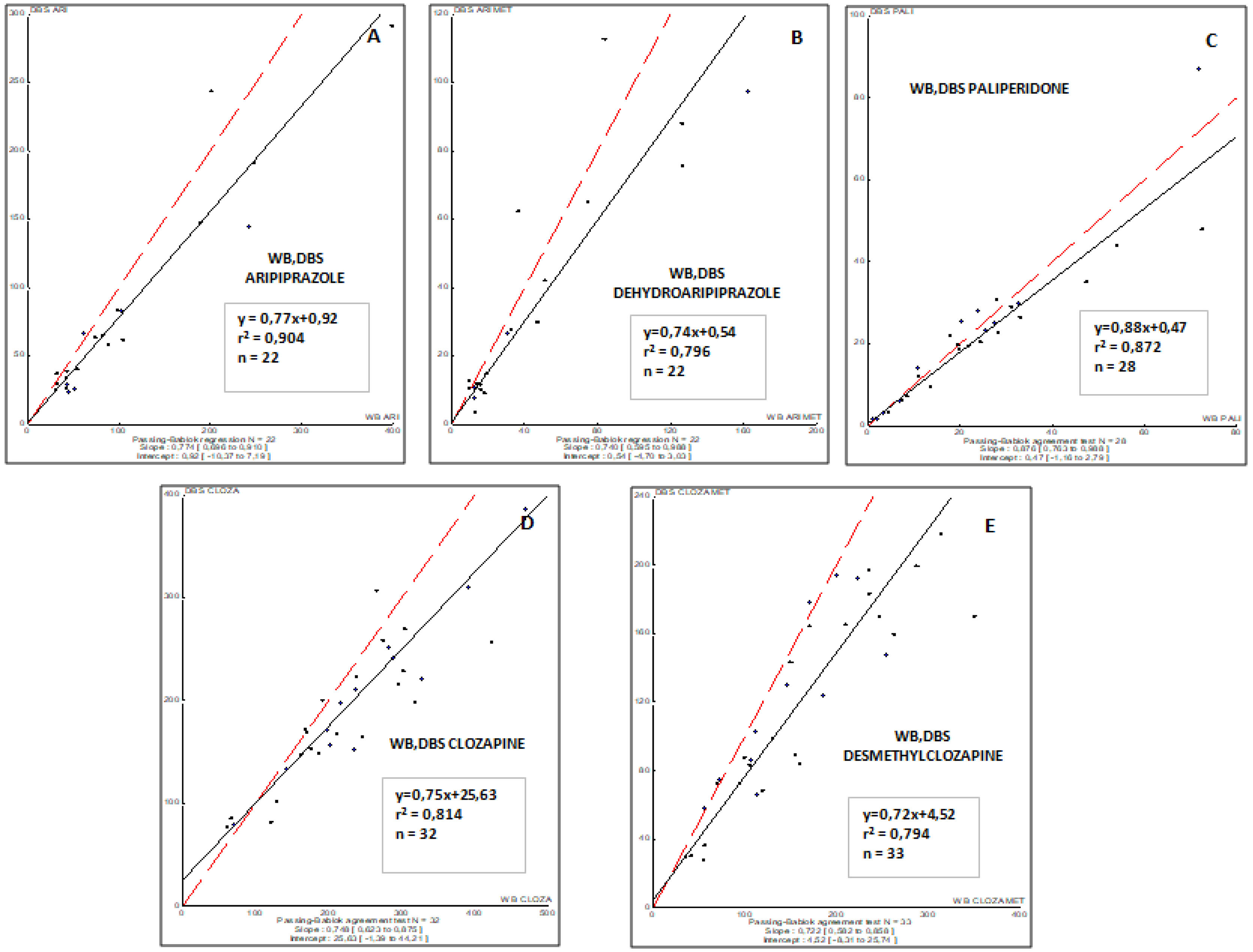
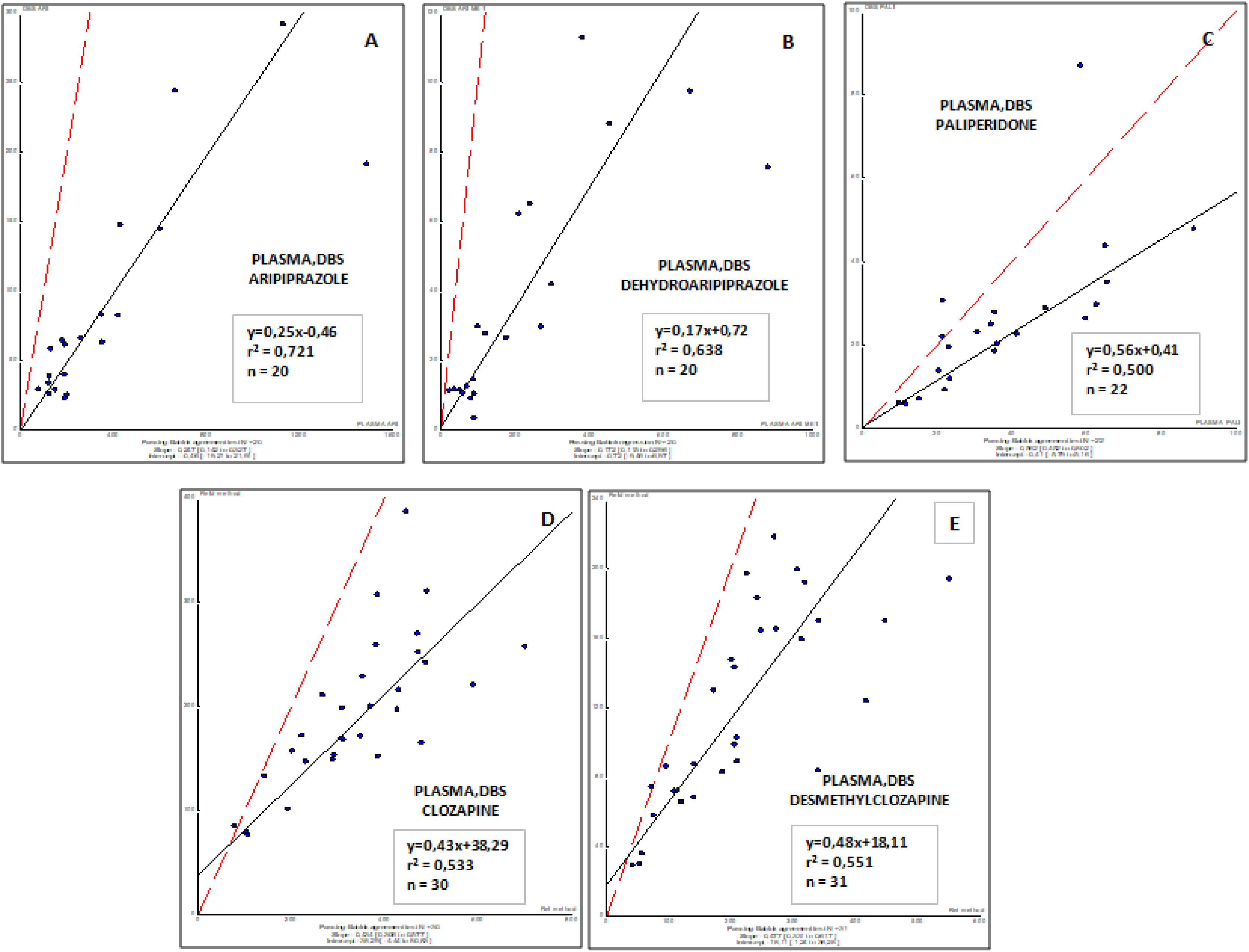
ResultsAripiprazole, paliperidone and clozapine showed from good to excellent correlations between concentrations in venous blood and DBS capillary blood (r2, from 0.500 to 0.721). The correlation between conventional plasma and DBS concentrations for paliperidone, aripiprazole, clozapine, and their metabolites were moderate, suggesting that optimal drug target concentrations should be established for DBS.
ConclusionsIn this study, for aripiprazole, dehydroaripiprazole, paliperidone, clozapine and desmethylclozapine, DBS has provided good analytical performance for TDM. Thus, DBS sampling can offer a great alternative over conventional sampling for plasma measurement. The assay provides good analytical performances for TDM and clinical research applicability, suggesting that DBS is a promising clinical application in TDM in psychiatry.
La monitorización terapéutica de medicamentos (Therapeutic Drug Monitoring [TDM]) de los antipsicóticos en la esquizofrenia es una potente herramienta que permite adaptar el tratamiento de forma individualizada y personalizada. Los objetivos del presente estudio son desarrollar y validar un método de análisis en sangre seca (Dried Blood Spot [DBS]) para la monitorización de algunos antipsicóticos de uso común (aripiprazol, clozapina y paliperidona) y evaluar su utilidad como biomarcador de cumplimiento y adherencia terapéutica, así como en el ajuste de la dosis del fármaco para personalizar el tratamiento antipsicótico para mejorar su eficacia y su seguridad.
MetodologíaSe incluyeron 31 pacientes con un primer episodio psicótico (PEP) o un diagnóstico de esquizofrenia; 5 eran PEP que iniciaron tratamiento antipsicótico, y 26 eran pacientes con más de un episodio y que seguían tratamiento con antipsicóticos: aripiprazol (7 casos), clozapina (17), paliperidona (11). Para la recogida de muestras de DBS se colocaron 25μl de sangre capilar en el punto de una tarjeta FTATMDMPK-C. Tras secarse completamente, se extrajeron los antipsicóticos y se analizaron mediante un método UHPLC-MS/MS validado. Los resultados de los antipsicóticos según la DBS se compararon con los obtenidos en sangre venosa/plasma.
ResultadosEl aripiprazol, la paliperidona y la clozapina mostraron de buenas a excelentes correlaciones entre las concentraciones en sangre venosa y en sangre capilar del DBS (r2, de 0,500 a 0,721). La correlación entre las concentraciones plasmáticas convencionales y las del DBS para la paliperidona, el aripiprazol, la clozapina y sus metabolitos fue moderada, lo que sugiere que se deberían definir y establecer concentraciones óptimas del fármaco para el DBS.
ConclusionesEn este estudio, para el aripiprazol, el dehidroaripiprazol, la paliperidona, la clozapina y la desmetilclozapina, el DBS ha proporcionado un buen rendimiento analítico para la monitorización de estos medicamentos. Por lo tanto, los análisis realizados con DBS pueden ofrecer una gran alternativa sobre los análisis convencionales para la medición del plasma. Los resultados sugieren un buen rendimiento analítico para la TDM y su aplicabilidad en la investigación clínica, lo que sugiere que el DBS tiene una aplicación clínica prometedora en la monitorización terapéutica en psiquiatría.
Schizophrenia is a complex and severe psychiatric disorder characterized by the presence of positive and negative symptoms and often associated with cognitive impairment and affective, mainly depressive symptomatology. Antipsychotic medication is the primary intervention for stabilization of acute psychotic episodes and prevention of recurrences and relapses in patients with this disease.1,2
The antipsychotic treatment is a principal therapeutic approach to several and severe mental illnesses. People with psychiatric diseases, such as schizophrenia, bipolar disorder and related disorders, are usually treated with a long-term treatment with antipsychotics3 and the adherence to it is related to the course and outcome.4–6 Even more, compliance is also an important predictor of relapse in patients with psychosis.7
The lack of adherence to medication is very common, especially in patients with psychiatric problems. Drug-related adverse events are the major causes of non-adherence mainly when self-administered treatments are prescribed, depending also on the complexity, cost and duration of them.7,8
Considering the mentioned above, it is thought that there is a requirement to improve the clinical care of these patients, as well as adherence by giving more instructions to patients, increasing the communication between doctors and patients, including reminders in daily habits or pill packaging, among others.9 In addition, therapeutic drug monitoring (TDM) can help on assessing adherence, optimize dosage, minimize toxicity risk and on the knowledge of drug interactions.10,11
Mainly in psychiatric settings, adherence assessment is performed only with standardized scales, as Drug Attitudes Inventory (DAI)12 and Morisky-Green13 that could be added to improve clinical profiles, to complement by various biochemical measurements such as drug concentration in plasma, whole blood, saliva or Dried Blood Spot (DBS).7,14
Monitoring of plasma drug concentrations and clinical Biochemistry tests is a key component of modern personalized pharmacological therapy in psychiatry. Usually, TDM is based on the quantification of drug concentrations in blood (i.e., serum or plasma) and can efficiently guide pharmacotherapy to achieve drug target concentrations. TDM in psychiatry should be used in situations of unclear adherence, lack of clinical response, adverse drug reactions (ADRs), polypharmacy and treatment, especially in those vulnerable patients with lack of adherence, lack of insight or other vulnerable groups, as children, pregnant women, elderly patients, etc.11 In addition, TDM can help on optimize dosage, minimize toxicity risk and on the knowledge of drug interactions.15,16 Thus, TDM is a powerful tool that allows tailor-made treatment for the specific needs of individual patients.
Conventionally, monitoring antipsychotics concentrations has been analyzed in plasma obtained by classic blood withdrawal. This is highly recommended for all these therapeutic agents because there is a high interindividual pharmacokinetic variability and drug-effects are concentration-related.17 Therefore, the narrow therapeutic ranges have a high risk of associated severe adverse events and measuring concentrations of these drugs in serum or plasma could prevent these side effects and the consequence of some pharmacokinetic interactions, increasing safety, efficacy and detecting poor compliance.15
In schizophrenia and related psychotic disorders, whole blood withdrawal is often experienced as frightening and unpleasant. In addition, venous sampling can only be performed in equipped health-care facilities and as such poses another burden for the patient. Therefore, new methods for TDM, such as saliva or DBS, need to be studied.8,14
During the last years, our laboratory group and other groups have been investigating new non-invasive strategies for monitoring adherence in psychotic treatment. Oral fluid has been proved as a useful matrix for antipsychotic concentration detection and some groups have shown the potential of saliva to analyze these drugs for treatment adherence.10,16,18–20 Nowadays, the analysis of antipsychotic drugs by DBS method with further LC-MS/MS detection is gaining importance due to its advantages: DBS sampling does not need a specialist and could be performed at home by patients or relatives, is less invasive than classical whole blood withdrawal as just one drop of capillary finger prick is needed, it is also less expensive in sampling, transport and storage, and it presents better patient comfort.19
Taking all the background into account, our objectives are to develop and validate a DBS method for monitoring some commonly used antipsychotics in schizophrenia (aripiprazole, clozapine, and their metabolites, and paliperidone) and to evaluate its usefulness as a compliance biomarker, as well as in drug–dose adjustment to personalize antipsychotic treatment in order to improve their efficacy and safety in terms of toxicity.
Materials and methodsSubjectsThe sample size was composed by 31 cases; 5, refer to first-episode patients who started an antipsychotic treatment; 26 cases were patients with a first-episode of schizophrenia or with more than one episode under antipsychotic treatment: aripiprazole (7 cases), clozapine (17), and paliperidone (11). Inclusion criteria were: age between 18 and 45 years, meet diagnostic criteria DSM-521 for Schizophrenia and other related psychotic diseases and sign the informed consent. Exclusion criteria: subjects that have had a history of head trauma with loss of consciousness or an organic disease with mental repercussions. Blood collection was done just before the morning dose and patients had to be in steady state conditions, which mean taking the same antipsychotic drug dose during the last 7 days.
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice and the Hospital Clinic of Barcelona Ethics and Research Board. All participants provided written informed consent prior to their inclusion in the study.
Chemicals and reagentsStandards were purchased in Sigma–Aldrich (Madrid, Spain): aripiprazole (A-119), paliperidone (9-hydroxyrisperidone (H-079)), N-desmethylclozapine (D-048), clozapine (C-059), desmethylolanzapine (D-069), dehydroaripiprazole (D-053), aripiprazole-d8 (A-081), and clozapine-d4 (C-091). Methyl-tert butyl eter (MTBE), methanol and acetonitrile UHPLC purity were bought in Panreac (Castellar del Vallès, Barcelona, Spain); glacial acetic acid, from VWR (Llinars del Vallès, Barcelona, Spain); and ammonium acetate, ascorbic acid, potassium carbonate (K2CO3) and potassium hydrogen carbonate (KHCO3) were also purchased from Sigma–Aldrich.
For DBS collection: automatic lancet single-use Accu-check, Safe-T-Pro plus (Roche, Sant Cugat del Vallès, Barcelona, Spain), 25μl capillary end-to-end microsafe pipette (Microsafe, Safetec) (Tens Medical Services, Central House, Gate Lane, Boldmere, Sutton Coldfield, West Midlands) and FTA™ DMPK-C Cards Whatman (Sigma–Aldrich).
StandardsWorking solutions (WS) of 100, 10 and 1μg/ml were prepared from methanolic stock solutions of 1mg/ml of the antipsychotic drugs aripiprazole, clozapine and their metabolites, and paliperidone.
From the 100μg/ml stock solutions, internal standards (IS) for DBS were prepared as a mix of the same volumes of paliperidone-d4, aripiprazole-d8 and clozapine-d4 with the following concentrations: 30, 150 and 150ng/ml respectively. The lower limit of quantification (LLOQ) is 20ng/ml for aripiprazole, 10ng/ml for dehydroaripiprazole, 50ng/ml for clozapine, 10ng/ml for N-desmethylclozapine, and 1ng/ml for paliperidone. Calibrators and controls were processed following the same operational procedure established for samples.
Sample collectionBlood collection was done just before the morning dose and patients had to be in steady state conditions, which mean taking the same antipsychotic drug dose during the last 7 days. For aripiprazole, due to its half-life, to reach steady state were necessary at least 25 days. Frequency of whole blood (WB), DBS and saliva sample collection: 1, 3, 6, 9 and 12 months.
DBS: The chosen finger was disinfected with alcohol 96°C and let it dry. Capillary blood flow was increased by applying a massage in that finger. After that, the finger prick was punched with a single-use automatic lancet and the first drop was discarded. Then, the second drop was collected by a 25μl capillary end-to-end microsafe pipette until it is completely filled and the capillary was emptied in the marked circle of a FTA™ DMPK-C card, where previously 0.5μl of ascorbic acid 25% were added.
WB: Via the classical venous blood withdrawal early morning previous drug administration.
Sample extractionDBS: The 25μl placed in the FTA™ DMPK-C card were allowed to dry for at least 12h (overnight, O/N) and 10μl IS-mix were added. After completely dryness, the spot was cut off and placed into a 2ml Eppendorf, were 450μl methanol and 150μl MTBE were added for the extraction. After 8min shake at 1800rpm in an Eppendorf MixMate, the extract was transferred to a 1.5ml Eppendorf and evaporated at 38°C with a nitrogen flow until dryness. Samples were reconstituted with 50μl of mobile phase (10mM aqueous ammonium acetate at pH 3.7 and acetonitrile (9:1, v/v)). After 30 s vortex and 2min at 10,000×g centrifugation, samples were placed in an autosampler vial and 10μl were injected in the UHPLC-MS/MS. Adapted from Patteet and collaborators.19
WB: 200μl of WB are placed in a 2ml Eppendorf where 20μl acetonitrile, 20μl IS-mix, 50μl buffer carbonate 1M pH 9.5 (K2CO3+KHCO3) and 1ml MTBE were added. After 1min shake at 2000rpm in an Eppendorf MixMate and 5min at 9000×g centrifuge, the organic phase was transferred into a 1.5ml Eppendorf and evaporated until dryness at 40°C under a nitrogen flow. Samples were reconstituted with 50μl acetonitrile and after 30s vortex and a 4000×g centrifuge of 2min, samples were placed into an autosampler vial. To dilute samples and achieve a better signal, 200μl of 10mM aqueous ammonium acetate at pH 3.7 were added at chromatographic vials and 15μl were injected in UHPLC-MS/MS. Based on Pattet and collaborators22 with some minor modifications.
Equipment and analytical methodSamples were analyzed in an Acquity™ Ultra Performance LC Waters coupled with a Triple Quadrupole mass spectrometer detector (Waters Cromatografía, S.A., Cerdanyola del Vallès, Barcelona, Spain). MassLynx 4.1 software version 1.40.2532 was used to acquire and analyze data (Waters).
Separation was achieved using an Acquity UPLC HSS T3 1.8μm 2.1×50mm analytical column (Waters) at 40°C. The MS conditions were optimized as: ESI positive polarity; nitrogen as nebulizer gas; capillary voltage: 1kV; cone voltage: 30V; source temperature: 130°C; desolvation temperature: 350°C; desolvation gas flow: 900l/h; collision gas flow: 0.3ml/min. Two ion transitions for each analyte were monitored.
ResultsMethods validationValidation methods for DBS and WB were performed according to EMA Guideline on bioanalytical method validations.23
DBS validationSpecificityThe response obtained for the analytes was less than 20% of the response of the Limit of quantification (LQ) and for the IS, the response was less than 5%. Therefore, we could affirm that the analytical method is specific for the determination of aripiprazole, dehidroaripiprazole, clozapine, n-desmethylclozapine, and paliperidone.
Sensibility (LQ): The limit of quantification was accepted since the accuracy and precision were less than ±20%.
Precision: The within-day and between-day precision, expressed as % of coefficient of variation (CV%) were summarized in Table 1.
Results for DBS within-day and between-day precision, expressed by the % of coefficient of variation (CV%) for aripiprazole, dehidroaripiprazole, paliperidone, clozapine and N-desmethylclozapine.
| Precision (CV%) | Within day | Between day |
|---|---|---|
| Aripiprazole | 3.39–15.97 | 9.85–12.94 |
| Dehidroaripiprazole | 3.18–7.63 | 9.77–11.56 |
| Paliperidone | 9.09–18.64 | 7.69–12.12 |
| Clozapine | 2.20–10.42 | 1.71–7.15 |
| N-desmetilclozapine | 4.99–14.35 | 7.10–10.04 |
Specificity: The response obtained for the analytes was less than 15% of the response of the Limit of quantification (LQ) and for the IS, the response was less than 5%. Therefore, we could affirm that the analytical method is specific for the determination of aripiprazole, dehidroaripiprazole, clozapine, n-desmethylclozapine, and paliperidone.
Sensibility (LQ): The Limit of quantification was accepted since the accuracy and precision were less than ±10%.
Precision: The within-day and between-day precision, expressed as % of coefficient of variation (CV%) were summarized in Table 2.
Results for WB within-day and between-day precision, expressed by the % of coefficient of variation (CV%) for aripiprazole, dehidroaripiprazole, paliperidone, clozapine and N-desmethylclozapine.
| Precision (CV%) | Within day | Between day |
|---|---|---|
| Aripiprazole | 6.55–13.75 | 1.76–9.93 |
| Dehidroaripiprazole | 5.62–11.28 | 5.29–11.69 |
| Paliperidone | 6.44–7.43 | 2.67–6.58 |
| Clozapine | 7.88–11.03 | 2.57–9.37 |
| N-desmetilclozapine | 4.74–8.34 | 4.08–12.26 |
The correlations obtained between DBS capillary and WB venous sample concentrations for all antipsychotic drugs involved in this study are summarized in Fig. 1. To remark, a good correlation was observed for all drugs and their respective metabolites (r2, from 0.794 to 0.904).
Correlations obtained between plasma (usual matrix for TDM) and DBS (capillary Blood) sample concentrations for all antipsychotic drugs involved in this study are shown in Fig. 2. These results demonstrate that a moderate to weak correlation was observed for all drugs and their respective active metabolites (r2, from 0.500 to 0.721), and suggest the requirement to consider specific target concentrations for each drug and metabolite in blood. Further studies are needed to properly evaluate this factor.
DiscussionIn the present clinical validation study in thirty-one first-episode and schizophrenia patients, it has been compared the venous plasma concentrations of antipsychotic drugs (gold standard) with dried blood spots concentrations obtained in our clinical device. Our results demonstrate that monitoring aripiprazole, dehydroaripiprazole, paliperidone, clozapine, and desmethylclozapine by DBS is a useful clinical tool to evaluate adherence to the treatment and may reflect the corresponding venous plasma concentration in first-episode and schizophrenia patients.
The first validation of DBS for quantification of different antipsychotics has been performed by Patteet and collaborators.20 This team developed a fast and easy to perform DBS method for quantification of ten APs (clozapine, amisulpride, aripiprazole, bromperidol, haloperidol, paliperidone, pipamperone, quetiapine, risperidone and zuclopentixol) and 6 metabolites that showed pharmacological activity. In agreement with our results, concentrations found in blood and DBS were quite similar for all of them.20 Tron and collaborators also found DBS as a valid tool suitable for TDM for risperidone and aripiprazole.24 In our study, paliperidone, clozapine, and aripriprazole and their metabolites were analyzed. Similar to Patteet study,20 aripiprazole, paliperidone and clozapine showed from good to excellent correlations between concentrations in blood and DBS. As TDM guidelines highly recommend monitoring of almost all antipsychotics, these results suggest that DBS has a good and promising clinical application in psychiatry in TDM and, moreover, for assessment of adherence or dose adjustment (for some drugs) in case of remarkable adverse effects using standard doses.16 On the other hand, the correlation between DBS and plasma concentrations for paliperidone, clozapine, and aripriprazole and their metabolites is from moderate to weak. In agreement with this finding, previous results reported that DBS and plasma concentrations of aripiprazole, pipamperone, and their major metabolites have good agreement based on Deming regression analysis, but do not fulfill Bland-Altman acceptance limits. Therefore, not all criteria for a completely successful clinical validation have been met.25
In summary, the good correlation between DBS and WB drug concentrations demonstrates that the new friendly strategy, using capillary blood spotted in specific cards, for sample withdrawal, offers similar results compared with those obtained from samples in venous whole blood (conventional sample). Thus, DBS can be considered as a feasible method for monitoring the exposure (C0) of the evaluated antipsychotic drugs and guiding drug dose adjustment.
Currently, TDM is carried out in plasma samples from treated patients. Thus, the correlation between DBS and plasma levels is of interest for physicians to know how to adjust the dose in treated patients. From this pilot study, a moderate to weak correlation was observed between DBS and plasma concentrations suggesting that optimal target concentrations should be established for DBS.
Our methodology provides a standardized method for sample withdrawal, using a specific volume of capillary blood, and considering all factors that may influence the diffusion of whole blood in the marked circle (Spot) of the card. Thus, the complete spot is used for sample pretreatment, drugs and metabolites extraction and later LC/MS/MS analysis. The results obtained in the process of validation of our methodology showed good precision and adequate lower limit of quantification and linear range, and make this LC/MS/MS method, suitable for determination of aripiprazol, dehydroaripiprazol, paliperidone, clozapine, and desmethylclozapine in DBS samples and could be implemented in clinical routine.
Despite the fact that DBS has achieved more attention over the last decade, and that it presents potential application in routine clinical care, this sampling method stills plays a marginal role in daily's clinical care.26 DBS sampling can present advantages and disadvantages. From on hand, DBS can be obtained by a minimally invasive procedure; it is required a small amount of blood; sampling could be performed at home; it can be cost-saving as compared to conventional blood sampling.16 In contrast, some disadvantages in relation with DBS should have taken into consideration: not all antipsychotic drugs could be analyzed by DBS, and there is a requirement of a fine tune therapeutic range on DBS.
The present study has some limitations. Firstly, the small sample size that could be compromising the statistical power. It is also unknown whether the subjects included are representative for the whole first-episode and schizophrenia population using antipsychotic drugs, and it is not known to what degree the patients were adherent to the treatment. However, the naturalistic design of the study could also be considered strength.
ConclusionsIn conclusion, TDM of antipsychotic drugs is a powerful tool that allows tailoring the treatment to the patients’ needs in an individualized and personalized approach. It can help in monitoring adherence, in dose adjustment, in minimizing the risk of adverse events and toxicity and in cost-effectiveness in the treatment of schizophrenia and related disorders. In the present study, for a selected number of antipsychotic drugs, such as aripiprazole, dehydroaripiprazole, paliperidone, clozapine, and desmethylclozapine, DBS has provided good analytical performance for TDM. Thus, DBS sampling can offer a great alternative over conventional sampling for plasma measurement. The assay provides good analytical performances for TDM and clinical research applications.
As future directions, although DBS antipsychotics assays are becoming available, clinical assays in greater and homogeneous sample within schizophrenia spectrum are still scarce. Thus, future research should focus on those antipsychotics currently used in clinical practice and blood parameters carried out in daily and regular clinical practice in psychiatry. Going in this direction, it may be possible to improve patient's adherence and getting them involved in the management of their illness (shared decisions between the clinician and the patient), getting closer to the patient's real world and, consequently, promoting its feasibility.
FundingThis work was totally supported by the Catalan Pons Balmes Grant, Fundació Clínic Recerca Biomèdica (Ajut a la Recerca Pons Balmes: FCRB_PB_2016).
Conflict of interestAll authors have declared that there are no conflicts of interest in relation to the subject of this study. None of the authors received any payment from the pharmaceutical industry to write this article.
We are grateful to all participants. We want to agree to the Fundació Clínic Recerca Biomèdica (Ajut a la Recerca Pons Balmes: FCRB_PB_2016). We would also like to thank the Carlos III Healthcare Institute, the Spanish Ministry of Science, Innovation and Universities, the European Regional Development Fund (ERDF/FEDER) (PI08/0208, PI11/00325, PI14/00612); CIBERSAM; CERCA Program; Catalan Government, the Secretariat of Universities and Research of the Department of Enterprise and Knowledge (2017SGR1355) and Institut de Neurociències, Universitat de Barcelona.