Clozapine plasma levels are useful to monitor drug compliance, and also to assess and to prevent some side effects. Recently, routine monitoring to all clozapine-treated patients has been proposed to prevent relapses. However, high intra-individual variability in plasma levels has been reported too, although these studies have some limitations.
MethodsWe analysed differences between two clozapine plasma levels separated by at least one year in a subgroup of 28 outpatients (82% male, mean age 47.9 years-old) with diagnosis of non-affective psychosis in clinical remission whose clozapine doses and smoking habits remained unchanged.
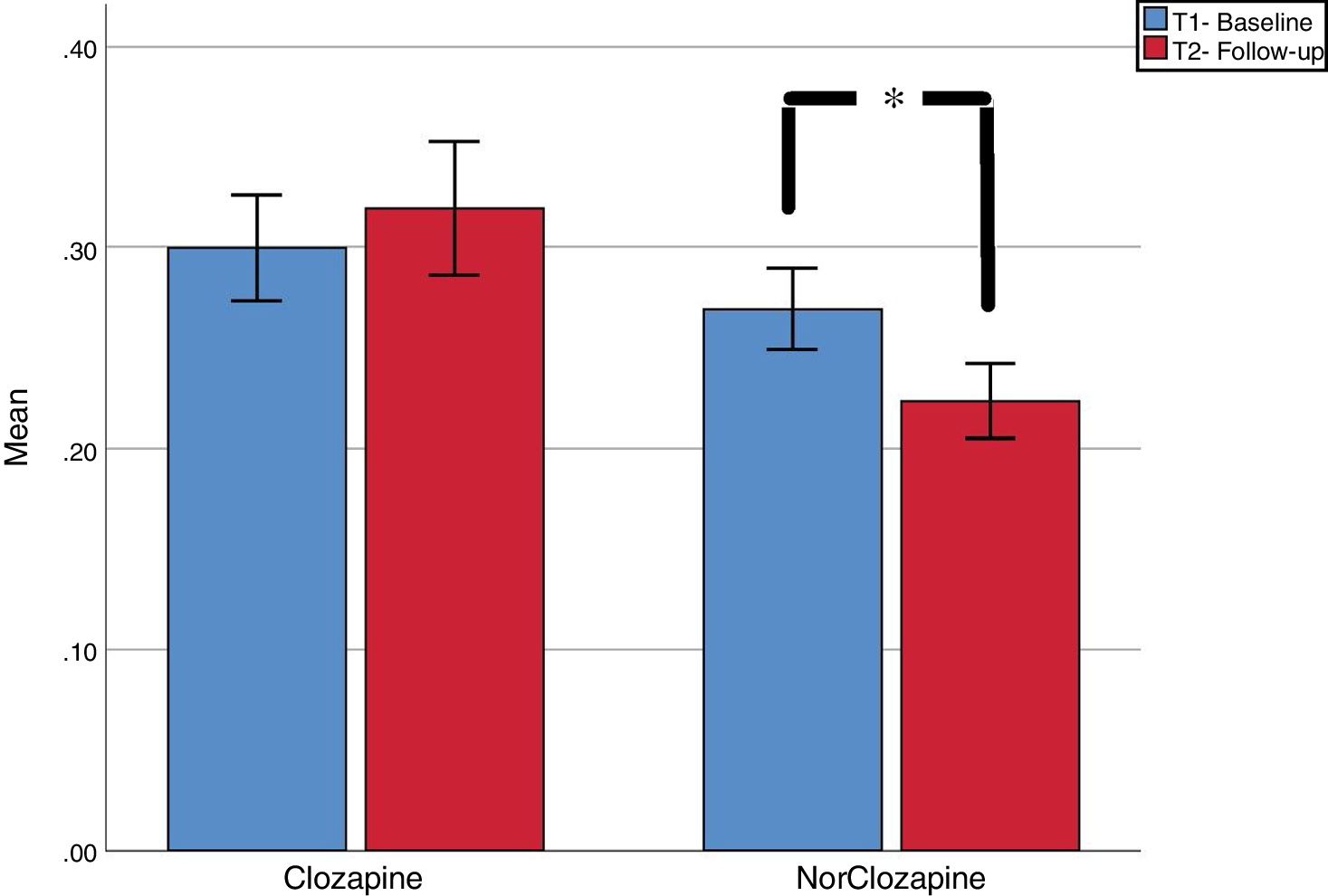
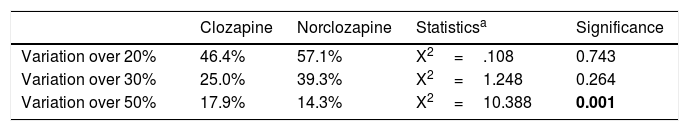
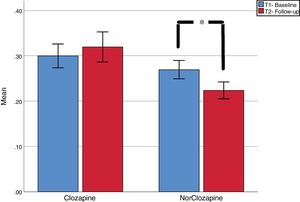
ResultsWe found a non-significant increase in clozapine plasma levels [.30mg/L (SD=.14) vs .32 (SD=.17); t=−.858, p=.40] and a significant decrease in norclozapine plasma levels [.27 (SD=.11) vs .22 (SD=.10); t=3.27; p=.003]. Absolute coefficient of variation (CV) between first and second assessment were calculated. Forty-six and fifty-seven percent of cases had CV 20% in clozapine and norclozapine, respectively. CV of 50% was seen in 20.7% and 13.8% of clozapine and norclozapine test respectively. We discussed potential causes of such high CV.
ConclusionsOur study suggest high intra-individual variation even in a subgroup of very stable patients, which suggest that routine monitoring of these levels may be indicated in order to detect significant plasma variations. We think that clinicians should act with caution in case of a sudden decrease in plasma level. In the absence of obvious symptom severity variation, sources of intra-individual fluctuations might be considered first, before assuming poor compliance.
Los niveles plasmáticos de clozapina son útiles para monitorizar el cumplimento farmacológico pero también para evaluar y prevenir los efectos secundarios. Recientemente, se ha propuesto realizar controles plasmáticos a todos los pacientes para prevenir recaídas. A pesar de ello, también se ha documentado una alta variabilidad intra-individual aunque los estudios tienen algunas limitaciones.
MétodosAquí analizamos las diferencias entre dos determinaciones de niveles plasmáticos con al menos un año de diferencias en un subgrupo de 28 pacientes (82% hombres, media de edad=47.9 años) con diagnósticos de psicosis no afectiva y en remisión clínica con dosis de clozapina y niveles de tabaquismo estable.
ResultadosEncontramos un incremento no significativo de los niveles de clozapina [.30mg/L (SD=.14) vs .32 (SD=.17); t=−.858, p=.40] y un descenso significativo de la norclozapina [.27 (SD=.11) vs .22 (SD=.10); t=3.27; p=.003]. Calculamos el coeficiente de variación absoluto (CV) entre la primera y segunda determinación. CV del 20% en los niveles de clozapina y norclozapina se encontró en el 46% y 57% de los casos respectivamente, mientras que el CV 50% se obtuvo en el 20.7% y 13.8%. Discutimos las causas potenciales de CV tan altos.
ConclusionesNuestro estudio encontró una alta variación intra-individual incluso en un subgrupo de pacientes especialmente estables, lo que sugiere que la monitorización rutinaria sería adecuada para detectar cambios significativos de los niveles. Creemos que los clínicos deberían ser cautos a la hora de asumir pobre cumplimiento terapéutico a la hora de explicar cambios de los niveles plasmáticos no acompañados de cambios clínicos.
Clozapine is the only approved drug for the treatment resistant schizophrenia.1 Its metabolism is influenced by age, gender, smoking habit and pharmacological interaction with other drugs. Its efficacy has been established above 0.35mg/mL2 and monitoring plasma levels has been exclusively recommended for assessing compliance.1,3 In practice they are also requested to prevent serious dose-dependant side effects, such as constipation, sedation or even epileptic seizures, or for dose adjustment after changes in smoking habits.2,4 Recently, routine monitoring to all clozapine-treated patients has been proposed5 as reductions of 20% in clozapine plasma levels could predict relapse6 and rehospitalisation.7 Notably, several studies found that intra-individual variability in clozapine plasma levels may range between 10 and 70%.8–11 However, these studies did not consider major variables that might have affected these levels, such as changes in smoking habit or treatment adherence.
Herewith, we aimed to understand whether plasma levels change in the group of stable patients. We analyse intra-individual differences between clozapine plasma levels separated by at least one year in patients with diagnosis of non-affective psychosis in long-term clinical remission, whose clozapine doses and smoking habits remained unchanged using a large cohort of clozapine-treated patients. We hypothesised that, in this specific clinical population, intra-individual clozapine plasma levels would not differ significantly between both assessments.
Subjects and methodsStudy designThis is a single-centre retrospective study of a cohort of clozapine-treated schizophrenia patients at the Cambridgeshire and Peterborough NHS Foundation Trust, a community mental health trust in the UK. The study used the electronic clinical records of 233 cases from September 2012 to December 2017, which are embedded in an ethically approved database for research and clinical purposes (13/EE/0121).
Clinical assessments and scales included in the electronic recordsAll cases were reviewed by the same psychiatrist (EFE) at least once annually, and included a full psychiatric history, mental state examination, current psychiatric and non-psychiatric medication list, smoking habit (number of cigarettes or equivalent per day), legal and illegal drugs self-reported habits, a comprehensive evaluation of clozapine-related side effects and overall physical health, among others. Routine clinical evaluations use a battery of questionnaires12 including the Clinical Global Impression for Schizophrenia,13 which measures severity of positive, negative, depressive, cognitive symptoms domains as well as an overall score, all ranging from 1 (no symptoms) to 7 (extremely severe).
Inclusion and exclusion criteria for the study sampleWe included patients who met the following criteria: (1) on clozapine since at least 2010, (2) at least two plasma level evaluations separated for at least one year; if more than two plasma levels were available, the oldest and the most recent were included; (3) no more than +/− 25mgs change in prescribed clozapine dose between both evaluations; (4) absence of acute psychotic symptoms during the follow-up according to the CGI-Schizophrenia for positive symptoms (score <3); and (5) no change in smoking habits, based on number of self-recorded cigarettes per day.
Clozapine plasma levelsAs per internal protocol, in our clinic clozapine and norclozapine plasma levels have been routinely assessed at least every three years, unless otherwise clinically required. Plasma levels (mg/L) were measured at the Toxicology Department, King's College Hospital, London, UK.
Statistical analysesAll statistical analyses were conducted using SPSS v25.0 for Mac, with a two-tail 0.05 significance level. Two main variables were evaluated: clozapine and norclozapine plasma levels. As described in the results section, we explored group level changes using paired T-test, but also changes at individual level.
For the latter, we calculated the coefficient of variation (CV) of clozapine and norclozapine between baseline (t1) and last assessment (t2). We described the prevalence of cases with absolute variations above 20% (CV20), 30% (CV30) and 50% (CV50). We compared the degree of change of the three outcome variables between t1 and t2, using paired-test or chi-square when required, as described below. Finally, we also explored whether t2 levels could be predicted with t1 levels by using multiple regression analysis, using t2_clozapine as independent variable and gender, age (in years), time gap between t1-t2 (in days), smoking habit, t1_clozapine and dose as predictors. Same models were developed for norclozapine.
ResultsNotably, only 28 out of the 233 cases (12%) met our inclusion and exclusion criteria. All had a primary diagnosis of non-affective psychosis. Twenty-three were males (82.1%), with a mean age of 47.9 (SD=12.2). Mean treatment dose was 305.4mg/day (SD=143) and 8 (39%)% were smokers. The average time between t1 and t2 was 1092 days (SD=363; range 476–1854).
Group analysisAs seen in Fig. 1, Compared to baseline, we found a non-significant increase in clozapine plasma levels [.30 (SD=.14) vs .32 (SD=.17); t=-.858, p=.40] and a significant decrease in norclozapine plasma levels [.27 (SD=.11) vs .22 (SD=.10); t=3.27; p=.003].
Individual analysesTable 1 shows the percentage of subjects with CV20, CV30 and CV50 in the two measures of clozapine and norclozapine levels. Fifty-two percent of patients experienced at least 20% variation in clozapine and norclozapine plasma levels between both tests. There were significantly more patients with CV50 in clozapine than with norclozapine, whereas no significant differences were seen in those with smaller variation (CV20 and CV30). Time gap between tests did not correlate with net change in clozapine (r=−.23; p=.27) or norclozapine (r=.08; p=.71).
Comparison of percentage of subjects with clozapine and norclozapine coefficient of variations above 20%, 30% and 50%.
| Clozapine | Norclozapine | Statisticsa | Significance | |
|---|---|---|---|---|
| Variation over 20% | 46.4% | 57.1% | X2=.108 | 0.743 |
| Variation over 30% | 25.0% | 39.3% | X2=1.248 | 0.264 |
| Variation over 50% | 17.9% | 14.3% | X2=10.388 | 0.001 |
Regression model for predicting T2_clozapine level were significant (R2=.64; F=7.7; p<.001) showing only t1_clozapine level as significant predictor (p<.001), but not for gender, age, smoking habit or t1-t2_time gap (all p>.25). For t2_norclozpaine level, the model was significant (R2=.60; F=6.7; p=.001). Only T1_norclozapine was a significant predictor (p<.001), but not for gender, age, smoking habit or t1-t2_time gap (all p>.32).
All analyses were re-done excluding the six cases with 25mg medication change (which was considered as clinically insignificant) and reported exactly the same results. Analyses can be found in the supplementary material.
DiscussionContrary to our hypothesis, we found significant decrease in norclozapine plasma levels and high intra individual variability. Half of patients presented at least 20% variation between tests separated by one year or longer. These findings cannot be attributed to changes in doses, smoking habits or medication adherence. The decrease in norclozapine level is particularly significant as this metabolite has a 22.5h half-life, approximately 8h longer than clozapine’s14; and therefore its plasma concentration may be less affected less than clozapine’s by differences in blood extraction times or anecdotal missing doses.. Clozapine’s mean value was below therapeutic threshold recommendation, but this is common in patients highly stable, as our sample.
Our results of high intra-individual variation replicate prior work,7,9–11 with the difference that our sample was a clinically stable and with no change in smoking habit. The high intra-individual variation found in clozapine levels may be due to a wide variety of reasons, as several substances are CYP1A2 and CYP2C19 inductors, the liver metabolic route for clozapine. The list includes psychiatric (e.g. antidepressant, anticonvulsants) and non-psychiatric medications (e.g. antibiotics), but also commonly used non-medical substances, such as caffeine, smoking or even grapefruit.5 Additionally, clozapine plasma levels may also be affected by passive smoking15 during minor infections10 by total pharmacological load or even the long term action of clozapine and inhibition over CYP2D6. More remarkably, in the clinical practice, the prescribing specialist is likely to be unaware of most of these factors as they can take place away for the clinic and out of the control of the clinician. Said this, none of the patients included in our sample was exposed to clinically-relevant clozapine inhibitors such as fluvoxamine, ciprofloxacine (and related antibiotics) and oral contraceptives, or major clozapine inducers, such as phenytoin, phenobarbital, carbamazepine or rifampicin.
The lack of evidence and unclear cost-effectiveness are the main arguments that preclude routine monitoring of clozapine levels in clinical practice. However, our study and others11 show that plasma level variations over time may be remarkable and subject to variables very difficult to control. Noteworthy, our analysis only included the subgroup of patients with greater stability (∼12% of the total caseload), hence the implication is that ∼88% of cases have experienced significant changes in dose, smoking habit or mental state, not to mention concomitant medication It is reasonable to assume that if significant variation is found in the more stable patients, even greater variation will be found in the rest of patients. In line with recent recommendations,5 our data suggest that routine monitoring of these levels, at least annually and probably even more frequently, may be indicated in order to detect significant plasma variations. Although additional awareness and psycho-education on factors that may affect plasma levels should also be recommended, the list of potential interactions and factors is so vast, that regular monitoring is the more feasible option.
Our results should be understood in light of the following limitations. First, we could not ascertain medication adherence. However, our sample was in long-term psychotic remission, hence, it could be argued that they were concordant with medication as prescribed. Second, the use of other medications was not an exclusion criterion as we aimed to mimic routine clinical practice. In any case, potent cytochrome inductors are well known, but there is also a wide range of moderate and weak inductors/inhibitors, which are very difficult to control in practice. Third, it could be argue that the inclusion of patients whose clozapine dose varied minimally (+/− 25mgs) could still have affected our results. However, only 6 patients had the dose change within that range and a subanalysis excluding this group provided similar findings (See supplementary material).
Finally, in our study we excluded cases with changes in mental state, so we could not rule out a threshold for predicting relapse as proposed by others.5,7 Nevertheless, our findings suggest intra-individual plasma level fluctuations may not always be related to treatment adherence but likely caused by the endless factors that affect clozapine metabolism in clinical practice. Therefore, these levels should be measured routinely as sudden variations may trigger either relapses or tolerability and safety problems.
Role of fundingDr. Fernandez-Egea was supported by 2009 Young investigator award and intramural funding from CPFT/NIHR-CRN supported the database. Prof. Bernardo is funded by the Spanish Ministry of Science and Education, the Spanish Ministry of Economy and Competiveness, Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), from the Government of Catalonia, Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement (2014SGR441), from the Foundation European Group for Research In Schizophrenia (EGRIS), and from the 7th Framework Program of the European Union.
Conflicts of interestMs. Turrion has no conflicto of interest to disclose. Prof. Perez has received honoraria for academic presentations from Eli Lilly, Janssen-Cilag, AstraZeneca, Lundbeck, Otsuka and Bristol-Myers Squibb. He has also taken part in advisory panels for Eli Lilly, Lundbeck, Otsuka and Roche. Prof. Bernardo has received fees for consultant and/or research grants from ABBiotics, Adamed, Ferrer, Forum Pharmaceuticals, Gedeon, Janssen-Cilag, Lundbeck, Otsuka, Pfizer, Roche, Servier. Dr. Fernandez-Egea has received consultant fees from Recordati and Angelini.
To patients and to Tomas Fernandez-Bruna for assistance in preparing Fig. 1.
Please cite this article as: Turrion MC, Perez J, Bernardo M, Fernandez-Egea E. Variación intraindividual en los niveles plasmáticos de clozapina y norclozapina en la práctica clínica. Rev Psiquiatr Salud Ment (Barc.). 2020;13:31–35.