Weight gain is an important and common side effect of second-generation antipsychotics (SGAs). Furthermore, these drugs can induce other side effects associated with higher cardiovascular morbidity and mortality, such as insulin resistance, diabetes or metabolic syndrome. Preliminary studies show that inter-individual genetic differences produce varying degrees of vulnerability to the different SGA-induced side effects. The Second-generation antipsychotic Long-term treatment Metabolic side effects (SLiM) study aims to identify clinical, environmental and genetic factors that explain inter-individual differences in weight gain and metabolic changes in drug-naïve patients after six months of treatment with SGAs.
Materials and methodsThe SLIM study is a multicenter, observational, six-month pharmacogenetic study where a cohort of 307 drug-naïve pediatric and adult patients (age range 8.8–90.1 years) and a cohort of 150 age- and sex- matched healthy controls (7.8–73.2 years) were recruited.
ResultsThis paper describes the rationale, objectives and design of the study and provides a description of the sample at baseline.
ConclusionsResults from the SLiM study will provide a better understanding of the clinical, environmental, and genetic factors involved in weight gain and metabolic disturbances associated with SGA treatment.
El aumento de peso es un efecto secundario frecuente e importante de los antipsicóticos de segunda generación (ASG). Además, estos fármacos pueden inducir otros efectos secundarios que están asociados a un aumento de la morbimortalidad cardiovascular, tales como la resistencia a la insulina, la diabetes o el síndrome metabólico. Estudios preliminares indican que las diferencias genéticas interindividuales producen distintos grados de vulnerabilidad a los efectos secundarios inducidos por los ASG. El estudio SLiM (por sus siglas en inglés, Second-generation antipsychotic Long-term treatment Metabolic side effects) tiene como objetivo identificar en pacientes no tratados previamente con ASG (pacientes naive), aquellos factores clínicos, genéticos y ambientales que expliquen las diferencias interindividuales en relación con el aumento de peso y los cambios metabólicos generados tras 6 meses de tratamiento con estos fármacos.
Material y métodosEl estudio SLiM es un estudio farmacogenético multicéntrico, observacional, prospectivo, de 6 meses de duración, en el que se ha reclutado una cohorte de 307 pacientes pediátricos y adultos (rango de edad entre 8,8 a 90,1 años) naive a ASG y una cohorte de 150 controles sanos (rango de edad entre 7,8 y 73,2 años) emparejados por edad y sexo.
ResultadosEn este artículo se presentan la justificación, los objetivos y el diseño del estudio y se ofrece una descripción de la muestra al inicio del estudio.
ConclusionesLos resultados del estudio SLiM permitirán una mejor comprensión de los factores clínicos, ambientales y genéticos implicados en el aumento de peso y los trastornos metabólicos asociados al tratamiento con ASG.
The prescription of second-generation antipsychotics (SGAs) has dramatically increased in recent years in pediatric and adult population, both for psychotic and non-psychotic disorders.1,2 Despite a better extra-pyramidal side effect profile than classic antipsychotics, overall, SGAs are associated with a higher prevalence of metabolic and endocrine disturbances, such as weight gain, dyslipidaemia or glycemic abnormalities.3,4 These disturbances lead to a higher risk of cardiovascular disease and to an increased morbidity and mortality in these patients, as compared with the general population.5,6 Furthermore, metabolic adverse events have been associated with non-adherence to treatment and poor quality of life.7–9
Currently, optimization of antipsychotic treatment is limited by the high variability of response to treatment and tolerability among individuals. This variability is related to clinical heterogeneity, genetic, environmental and social factors10 and has hindered the use of pharmacogenomic (PGx) testing or genotype-based prescription in clinical practice.11,12 However, PGx-related techniques could help the identification of patients at higher risk of developing SGA-induced side effects through the identification of PGx biomarkers.13,14
The purpose of the second-generation antipsychotic Long-term treatment metabolic side effect study (SLiM study), a multicenter, longitudinal, 6-month follow-up PGx study, was to identify clinical, environmental and genetic predictive factors of weight gain and metabolic changes in a Spanish sample of drug-naïve pediatric and adult patients treated with SGAs, and a cohort of age- and sex- matched healthy controls. The specific goals of the study were: (1) to assess the prevalence of anthropometric and metabolic changes after six months of treatment with SGAs, (2) to assess the association between weight gain/metabolic changes and clinical improvement/non-adherence to treatment/quality of life, (3) to analyze polymorphisms in candidate genes related to appetite control, central nervous system homeostatic regulation, and obesity, (4) to assess the association between the analyzed genotypes, weight gain and metabolic changes, and (5) to identify risk genotypes that explain inter-individual differences in SGA-induced weight gain and metabolic disturbances.
This paper describes the rationale, objectives and design of the SLiM study and provides a description of the sample at baseline.
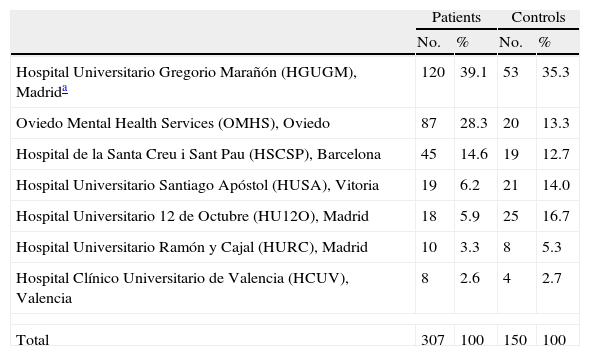
Subjects and methodsRecruitment procedureFrom January 2007 to December 2010, seven sites from the Spanish Psychiatric Research Network (CIBERSAM) (see www.cibersam.es) recruited a sample of 307 pediatric and adult patients consecutively attended in their clinical facilities and prescribed an SGA for the first time or with a prior lifetime exposure to antipsychotics of no more than 10 days. A cohort of 150 age- and sex- matched healthy control subjects was also recruited. Table 1 displays the number of patients and controls included in the study per participating site.
Included patients and controls per participating site.
| Patients | Controls | |||
| No. | % | No. | % | |
| Hospital Universitario Gregorio Marañón (HGUGM), Madrida | 120 | 39.1 | 53 | 35.3 |
| Oviedo Mental Health Services (OMHS), Oviedo | 87 | 28.3 | 20 | 13.3 |
| Hospital de la Santa Creu i Sant Pau (HSCSP), Barcelona | 45 | 14.6 | 19 | 12.7 |
| Hospital Universitario Santiago Apóstol (HUSA), Vitoria | 19 | 6.2 | 21 | 14.0 |
| Hospital Universitario 12 de Octubre (HU12O), Madrid | 18 | 5.9 | 25 | 16.7 |
| Hospital Universitario Ramón y Cajal (HURC), Madrid | 10 | 3.3 | 8 | 5.3 |
| Hospital Clínico Universitario de Valencia (HCUV), Valencia | 8 | 2.6 | 4 | 2.7 |
| Total | 307 | 100 | 150 | 100 |
Patients were recruited from emergency rooms, inpatient units, and outpatient clinics. The inclusion criteria for patients were: (i) presence of a psychiatric disorder according to DSM-IV criteria,15 (ii) capacity to give written informed consent (from participants and from their parents or legal guardians as appropriate), and (iii) being drug-naïve patients (first prescription of an SGA, or, if previously treated, a total lifetime exposure to antipsychotics of no more than 10 days). The initial design of the SLiM study only considered those patients who were firstly prescribed risperidone, olanzapine and quetiapine. Later on, this inclusion criterion was broadened and prescription with other SGAs was also allowed. Concomitant treatment with stimulants, mood stabilizers, antidepressants, anticholinergic agents, and benzodiazepines/hypnotics was also allowed. Presence of a severe medical condition was the unique exclusion criterion for patients.
Healthy controls were recruited among patients’ friends, colleagues and neighbors. Inclusion criteria for controls were (i) absence of any psychiatric diagnosis according to DSM-IV criteria15 and (ii) capacity to give written informed consent (from participants and from their legal guardians/representatives when needed). Exclusion criteria for controls were (i) presence of a severe medical condition, and (ii) current or past treatment with any antipsychotic drug. The controls were compensated for their time with a 25€ gift card at each visit.
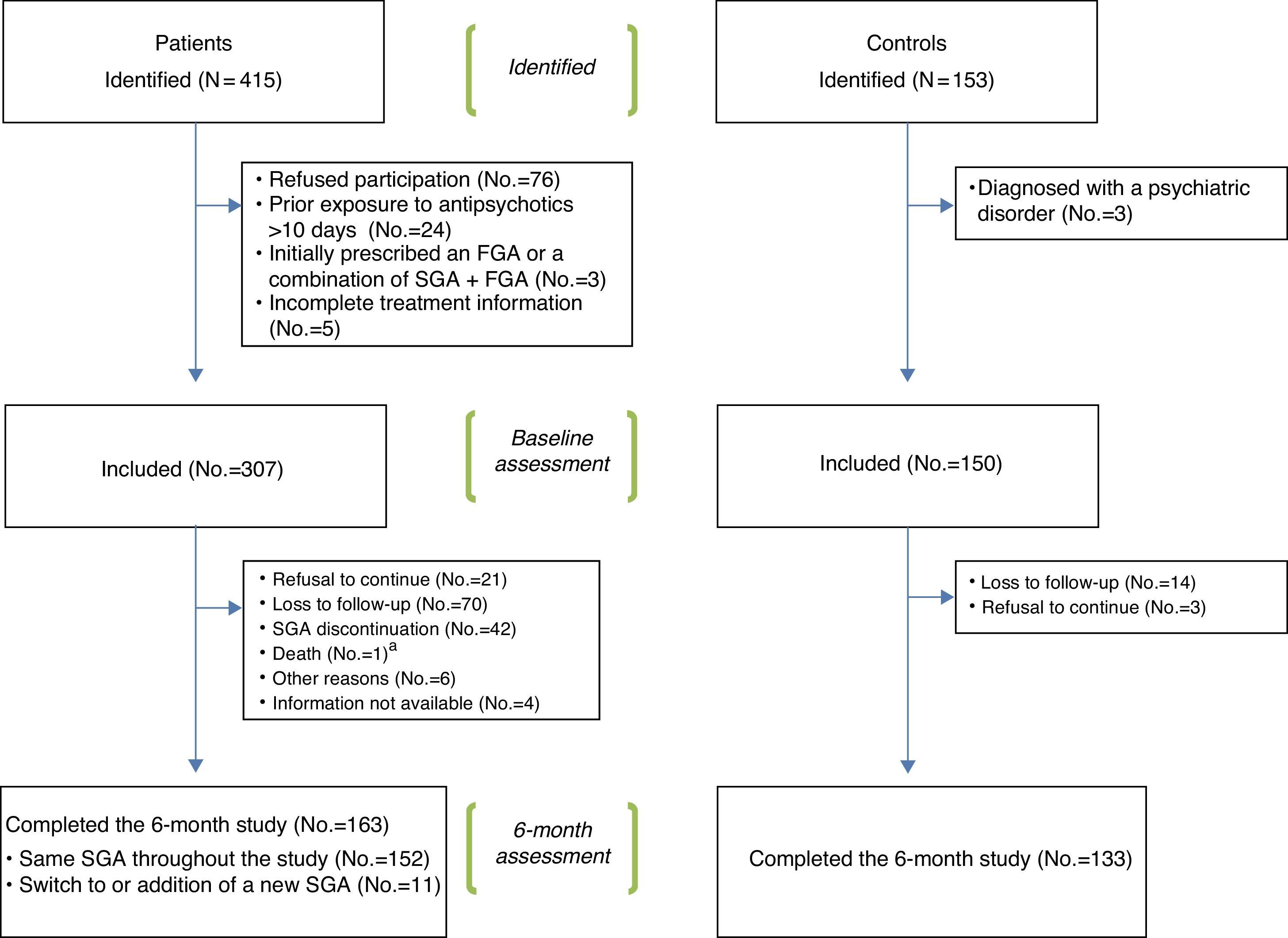
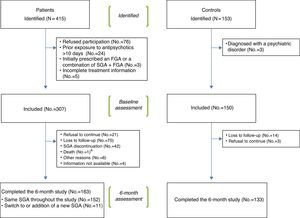
Fig. 1 shows a flowchart of the recruitment procedure. Out of the 415 patients and 153 controls initially identified, 307 patients and 150 controls met the inclusion criteria and completed the baseline assessment. Out of these, 163 patients and 133 controls completed the 6 month follow-up period. Reasons for drop-out were: refusal to continue, loss to follow-up, SGA discontinuation (only for patients), death and other reasons (reason was specified). Shift to or addition of a new SGA during the follow-up was not considered a reason for drop-out.
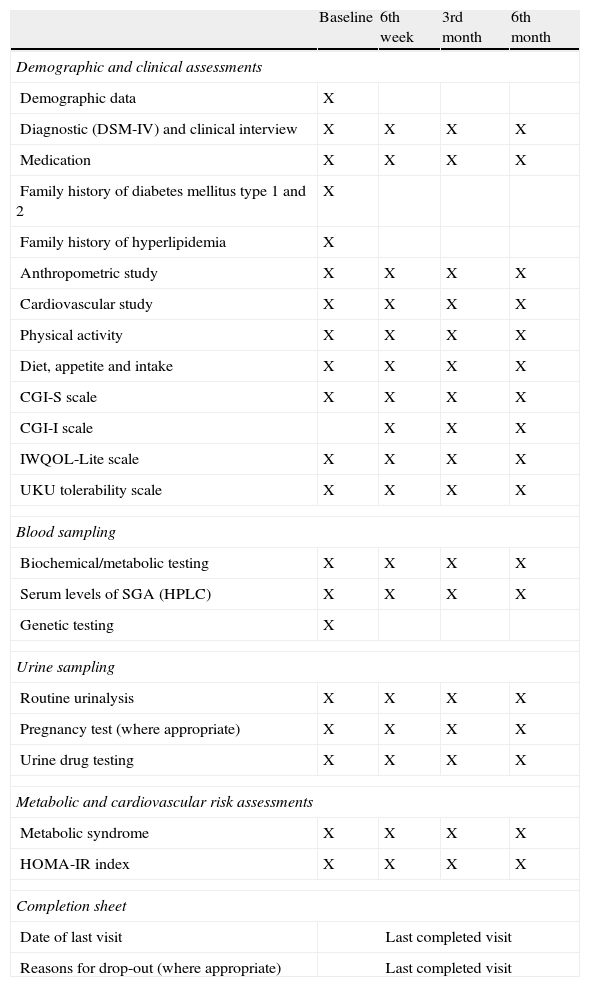
DesignThe SLiM study is a prospective, observational, six-month study, in which four assessment visits (at baseline, 6 weeks, 3 months and 6 months) were performed for patients and controls. At baseline, a complete evaluation (which included recording of demographic, diagnostic and clinical data, clinical scales, recording of psychopharmacological treatment and side effects, an anthropometric and cardiovascular study, and collection of fasting blood and urine samples) was performed in the setting where subjects had been recruited. Later on, subjects were contacted by telephone and the same assessments were repeated at 6 weeks, 3 months and 6 months in a psychiatric outpatient setting. A review of the diagnosis and of the ongoing pharmacologic treatment, and an assessment of change in global psychopathology were also conducted. Taking each individual's anthropometric, cardiovascular and blood test data, and based on established criteria, presence of insulin resistance16 and presence of metabolic syndrome17–19 were determined at each visit. For each participant, after the last completed visit, a completion sheet form was filled out. Date of last visit and reasons for drop-out (where appropriate) were registered. Table 2 summarizes the assessments and their timing throughout the study.
Assessments and timing.
| Baseline | 6th week | 3rd month | 6th month | |
| Demographic and clinical assessments | ||||
| Demographic data | X | |||
| Diagnostic (DSM-IV) and clinical interview | X | X | X | X |
| Medication | X | X | X | X |
| Family history of diabetes mellitus type 1 and 2 | X | |||
| Family history of hyperlipidemia | X | |||
| Anthropometric study | X | X | X | X |
| Cardiovascular study | X | X | X | X |
| Physical activity | X | X | X | X |
| Diet, appetite and intake | X | X | X | X |
| CGI-S scale | X | X | X | X |
| CGI-I scale | X | X | X | |
| IWQOL-Lite scale | X | X | X | X |
| UKU tolerability scale | X | X | X | X |
| Blood sampling | ||||
| Biochemical/metabolic testing | X | X | X | X |
| Serum levels of SGA (HPLC) | X | X | X | X |
| Genetic testing | X | |||
| Urine sampling | ||||
| Routine urinalysis | X | X | X | X |
| Pregnancy test (where appropriate) | X | X | X | X |
| Urine drug testing | X | X | X | X |
| Metabolic and cardiovascular risk assessments | ||||
| Metabolic syndrome | X | X | X | X |
| HOMA-IR index | X | X | X | X |
| Completion sheet | ||||
| Date of last visit | Last completed visit | |||
| Reasons for drop-out (where appropriate) | Last completed visit | |||
Abbreviations: CGI-I: Clinical global impression-improvement; DSM-IV: Diagnostic and statistical manual of mental disorders, 4th edition; HOMA-IR: Homeostasis model assessment-estimated insulin resistance; HPLC: High-performance liquid chromatography; IWQOL-Lite: Impact of weight on quality of life; SGA: Second-generation antipsychotic; UKU: Udvalg for Kiniske Undersogelser.
An “ad hoc” protocol for recording of demographic data was designed. Age, sex, ethnicity, civil status, educational and occupational level of the participant (or his/her parent or legal guardian if under 18 years of age) were recorded.
Diagnosis was made by experienced psychiatrists according to DSM-IV criteria,15 by means of a clinical interview and a review of medical records of the participant. Also, information on personal medical history (including type 1 and 2 diabetes mellitus), family history of type 1 and 2 diabetes mellitus and/or hyperlipidemia, personal and family history of psychiatric disorder, number and duration of hospitalizations (for patients), pregnancy, and substance misuse was recorded. Data on somatic and psychopharmacological treatment was also recorded, including dosage, dosage changes, and start and discontinuation dates of any previous and/or current treatment. Antipsychotic doses were derived to chlorpromazine equivalents and current and cumulative doses at each visit were calculated.20,21
The anthropometric study was performed by trained nurses. Weight (in kilograms) and height (in meters) were measured at each visit with the same scale: SECA GMBH & Co., Model 797, in Hospital General Universitario Gregorio Marañón (HGUGM) and Oviedo Mental Health Service (OMHS); Año Sayol, Model Atlantida, in Hospital de la Santa Creu i Sant Pau (HSCSP) and Hospital Universitario Ramón y Cajal (HURC); Soenhle 7831 Digital Scale in Hospital Universitario Santiago Apóstol (HUSA); Body Composition Analyzer BF-350, Tanita Corporation, in Hospital Universitario 12 de Octubre (HU12O); OMROM BF-500 in Hospital Clínico Universitario Valencia (HCUV). Using this data, body mass index (BMI) was calculated as follows: weight (kilograms)/height (square meters). Since BMI varies with age and sex, the BMI value was adjusted using a conversion to a z-score. For children and adolescents, BMI was adjusted for age and sex using Spanish normative charts.22 For adults, raw scores were transformed to z-scores based on the BMI of the sex-matched control group at each visit. Abdominal circumference was measured using a flexible tape measure placed above the uppermost border of the iliac crests. Total body fat mass was measured at each visit with the same bioelectrical impedance scale: OMRON BF-500 in HGUGM, OMHS, HUSA, HURC and HCUV; OMRON BF-511 in HSCSP; Tanita BF-350 in HU12O). At each visit, participants were asked whether they were on diet and whether they had experienced changes in appetite and/or in food intake. Information on levels of physical activity (i.e., hours per week of physical activity during the previous week) was also recorded.
A Cardioline Delta 1 Plus Digital ECG, Versione Base (Renco, Italy) in HGUGM, Cardioline AR600ADV ECG (Cavareno, Italy) in OMHS, Philips M1772A ECG (Andover, MA, USA) in HSCSP, Philips Page Writer Trim I ECG (Eindhoven, The Netherlands) in HUSA, Philips Page Writer TC30 ECG (Andover, MA, USA) in HURC, Cardiovit AT-2 ECG, Schiller AG (Baar, Switzerland) in HU12O and Nihon Kohden Cardio FAX V Ecaps 12 (Rosbach, Germany) in HCUV were used for the ECG assessment. Recording of sitting blood pressure (BP) and heart rate was also performed. For children, raw BP values were derived to percentiles according to the International Task Force for BP.23
The Clinical Global Impression-Severity (CGI-S) scale24 and the Clinical Global Impression-Improvement (CGI-I) scale24 were completed by experienced psychiatrists. These scales assess severity and improvement, respectively, of global symptomatology on a scale of 1–7. They are particularly helpful in longitudinal studies as they provide an ordinal repeated measure of global clinical status.
The Impact of Weight on Quality of Life (IWQOL-Lite) scale25 and the Udvalg for Kiniske Undersogelser (UKU) scale26 were also administered throughout the study by trained psychiatrists. The IWQOL-Lite scale is a 31-item self-report scale for assessment of obesity-specific quality of life which consists of a total score and five subscores, ech one derived from five specific subscales (i.e., physical function, self-esteem, sexual life, public distress, and work). The UKU scale is a comprehensive rating scale including a total of 54 items (divided in four categories) scored 0–3, designed to assess general side effects of psychotropic drugs.
Biochemical determinationsVenous blood samples were collected by nursing staff in 7 polypropylene EDTA-containing and serum tubes in the morning (between 8:00 and 10:00) after fasting overnight and stored initially at 4°C. One of each extracted blood samples was sent to the Biochemistry Department at each participating site, where biochemical determinations were performed. Determinations included lipid profile, glucose, glycated hemoglobin A1 (HbA1c), and insulin levels. In all the participating sites, blood glucose, total cholesterol, high density lipoprotein (HDL) cholesterol, low density lipoprotein (LDL) cholesterol, and triglycerides (TG) were determined by enzymatic procedures with an Automatic Chemical Analyzer. HbA1c was analyzed by high-performance liquid chromatography (HPLC). Insulin was measured by immunoassay with chemiluminescence detection. The Biochemistry Department at each participating site recorded the biochemical data using pre-established criteria for normality (minimum and maximum) for each parameter. The reference values at each site were recorded and individual values were corrected and homogenized in the common database.
For both patients and controls, one of the blood tubes was centrifuged (2000×g×10min, at room temperature) after 1h of storage. The resultant serum samples were carefully collected in serum tubes and stored at ≤−20°C until they were sent to the Biochemistry laboratory at the coordinating site (HGUGM), where adiponectin and leptin levels were determined (in duplicate) by Enzyme-Linked Immune Sorbent (ELISA) assay (Human Leptin ELISA [ref: RD191001100], Human Adiponectin ELISA [ref: RD195023100]; all Biovendor Laboratory Medicine Inc., Brno; Czech Republic). All these determinations were performed by a trained and experienced biochemist.
Serum SGA determinationsFor each patient and at each visit, one of the stored serum samples was also sent to the Biochemistry laboratory at the coordinating site (HGUGM), where HPLC was performed by a trained and experienced biochemist using a Waters 2695 series HPLC system (Alliance HPLC system, Waters Corp. Milford, MA, USA). The analytes were separated on an Agilent Eclipse XDB C-18 column (150×4.6mm i.d., 5μm) with a 4.6×12.5mm Eclipse XDB C-18 guard column, following the protocol described by Zhang et al.27 This semi-quantitative technique provided an indirect measure of patient's adherence to SGAs at each visit (by means of a ‘positive’ or ‘negative’ HPLC result).
Genotype analysesFor each participant, one of each collected blood samples was stored at ≤−20°C until it was sent to the College of Biology at University of Barcelona, where an experienced and trained geneticist performed DNA processing (i.e., coding, storage and extraction, using a commercial extraction kit) and DNA genotyping. Candidate genes of the SLiM PGx study and their selected SNPs and MAFs are summarized in Supplementary Table 1 (Annex). Candidate genes related to mechanism of action of SGAs, appetite control, homeostatic regulation and weight gain or related phenotypes (e.g., obesity) were selected based on previous association and genome wide association studies (GWAS).28–35 Single Nucleotide Polymorphisms (SNPs) of these candidate genes were selected based on previous literature and the SYSNPS program (www.sysnps.org). SNPs were chosen from the SYSNPS program only if they were TagSNPs of the candidate gene and presented a Minor Allele Frequency (MAF)>0.2. This MAF criterion was used to increase the statistical power.
Genotyping was blind to group membership (patient vs control) and SGA treatment. The 5-HTTLPR polymorphism (SLC6A4 gene) was genotyped following a standard protocol.36 The rs6265 (BDNF gene) and rs1049353 (CNR1 gene) were genotyped using Taqman 5′-exonuclease assay. All other SNPs were genotyped by competitive quantitative PCR using allele specific probes with FRET signal detection. A randomly selected subsample of individuals was regenotyped in order to confirm the pattern of reproducibility.
Urine samplingAt each visit and for each participant, urine samples were collected and a routine urinalysis, pregnancy test (where appropriate) and urine drug test were performed. Amphetamines, cannabis, cocaine, opiates, benzodiazepines and barbiturates were determined by immunoassay in HGUGM, HSCSP, HU12O, HUSA, and HURC; by endpoint spectrophotometry with monochromatic light in OMHS; and by thin-layer chromatography in HCUV.
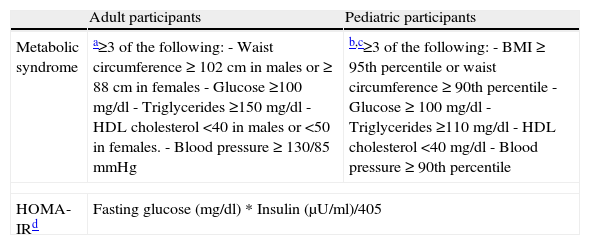
Metabolic and cardiovascular risk assessmentTaking each individual's anthropometric and cardiovascular data, presence of metabolic syndrome was established for each participant at each visit. For adult participants, metabolic syndrome was defined following the American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement.19 For pediatric participants, metabolic syndrome was defined following adapted criteria derived from Cook et al., 2003,17 and Correll and Carlson, 2006.18 These criteria include a clustering of the most relevant risk factors for cardiovascular disease: abdominal obesity raised fasting plasma glucose and/or diabetes, abnormal lipid profile and high blood pressure (see Table 3).
Criteria for presence of metabolic syndrome and insulin resistance in pediatric and adult populations.
| Adult participants | Pediatric participants | |
| Metabolic syndrome | a≥3 of the following:- Waist circumference ≥ 102cm in males or ≥ 88cm in females- Glucose ≥100mg/dl- Triglycerides ≥150mg/dl- HDL cholesterol <40 in males or <50 in females.- Blood pressure≥130/85mmHg | b,c≥3 of the following:- BMI≥95th percentile or waist circumference≥90th percentile- Glucose≥100mg/dl- Triglycerides ≥110mg/dl- HDL cholesterol <40mg/dl- Blood pressure≥90th percentile |
| HOMA-IRd | Fasting glucose (mg/dl)*Insulin (μU/ml)/405 | |
Abbreviations: BMI: body mass index. HDL: high density lipoprotein, HOMA-IR: homeostasis model assessment-insulin resistance.
Both for pediatric and adult participants, insulin resistance was estimated using the homeostasis model assessment- insulin resistance (HOMA-IR) index,16 which is easily calculated from a single measurement of fasting insulin and glucose (see Table 3).
Data processing and analysisIn order to integrate all the available information, and to facilitate data management and exploitation, a common database was created. Data entry, database design and management were conducted at the coordinating site.
For the description of the sample at baseline, continuous variables were expressed as means, standard deviations (SD) and ranges, and categorical variables were expressed as frequencies and percentages. Group differences were tested for baseline demographic and clinical data. The comparison groups were patients (whole sample) vs controls (whole sample), pediatric vs adult patients, and pediatric vs adult controls. Chi-square or Fisher exact tests were used for discrete categorical variables. Since all the quantitative variables included in the analysis (age, days of previous SGA exposure, current and cumulative doses of SGA at baseline) showed non-normal distributions, non-parametric tests (Mann–Whitney U) were used. Statistical analyses were carried out with the SPSS 18.037 and differences of p<0.05 were considered significant. All tests were two-tailed.
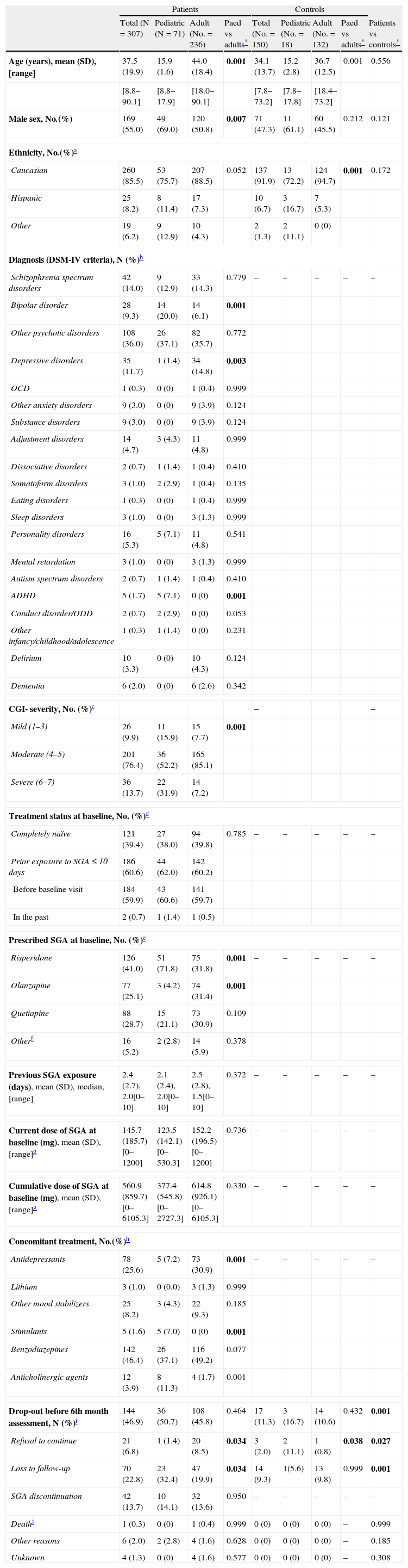
ResultsSample description at baselineThe demographic and clinical characteristics at baseline of the 307 (71 pediatric and 236 adult) patients and 150 (18 pediatric and 132 adult) healthy controls who were included in the SLiM study are shown in Table 4. Data on SGA and concomitant treatments, both for the whole sample and for each age group, are also shown in Table 4.
Demographic and clinical characteristics at baseline of pediatric and adult patients and controls.
| Patients | Controls | ||||||||
| Total (N=307) | Pediatric (N=71) | Adult (No.=236) | Paed vs adults* | Total (No.=150) | Pediatric (No.=18) | Adult (No.=132) | Paed vs adults* | Patients vs controls* | |
| Age (years), mean (SD), [range] | 37.5 (19.9) | 15.9 (1.6) | 44.0 (18.4) | 0.001 | 34.1 (13.7) | 15.2 (2.8) | 36.7 (12.5) | 0.001 | 0.556 |
| [8.8–90.1] | [8.8–17.9] | [18.0–90.1] | [7.8–73.2] | [7.8–17.8] | [18.4–73.2] | ||||
| Male sex, No.(%) | 169 (55.0) | 49 (69.0) | 120 (50.8) | 0.007 | 71 (47.3) | 11 (61.1) | 60 (45.5) | 0.212 | 0.121 |
| Ethnicity, No.(%)a | |||||||||
| Caucasian | 260 (85.5) | 53 (75.7) | 207 (88.5) | 0.052 | 137 (91.9) | 13 (72.2) | 124 (94.7) | 0.001 | 0.172 |
| Hispanic | 25 (8.2) | 8 (11.4) | 17 (7.3) | 10 (6.7) | 3 (16.7) | 7 (5.3) | |||
| Other | 19 (6.2) | 9 (12.9) | 10 (4.3) | 2 (1.3) | 2 (11.1) | 0 (0) | |||
| Diagnosis (DSM-IV criteria), N (%)b | |||||||||
| Schizophrenia spectrum disorders | 42 (14.0) | 9 (12.9) | 33 (14.3) | 0.779 | – | – | – | – | – |
| Bipolar disorder | 28 (9.3) | 14 (20.0) | 14 (6.1) | 0.001 | |||||
| Other psychotic disorders | 108 (36.0) | 26 (37.1) | 82 (35.7) | 0.772 | |||||
| Depressive disorders | 35 (11.7) | 1 (1.4) | 34 (14.8) | 0.003 | |||||
| OCD | 1 (0.3) | 0 (0) | 1 (0.4) | 0.999 | |||||
| Other anxiety disorders | 9 (3.0) | 0 (0) | 9 (3.9) | 0.124 | |||||
| Substance disorders | 9 (3.0) | 0 (0) | 9 (3.9) | 0.124 | |||||
| Adjustment disorders | 14 (4.7) | 3 (4.3) | 11 (4.8) | 0.999 | |||||
| Dissociative disorders | 2 (0.7) | 1 (1.4) | 1 (0.4) | 0.410 | |||||
| Somatoform disorders | 3 (1.0) | 2 (2.9) | 1 (0.4) | 0.135 | |||||
| Eating disorders | 1 (0.3) | 0 (0) | 1 (0.4) | 0.999 | |||||
| Sleep disorders | 3 (1.0) | 0 (0) | 3 (1.3) | 0.999 | |||||
| Personality disorders | 16 (5.3) | 5 (7.1) | 11 (4.8) | 0.541 | |||||
| Mental retardation | 3 (1.0) | 0 (0) | 3 (1.3) | 0.999 | |||||
| Autism spectrum disorders | 2 (0.7) | 1 (1.4) | 1 (0.4) | 0.410 | |||||
| ADHD | 5 (1.7) | 5 (7.1) | 0 (0) | 0.001 | |||||
| Conduct disorder/ODD | 2 (0.7) | 2 (2.9) | 0 (0) | 0.053 | |||||
| Other infancy/childhood/adolescence | 1 (0.3) | 1 (1.4) | 0 (0) | 0.231 | |||||
| Delirium | 10 (3.3) | 0 (0) | 10 (4.3) | 0.124 | |||||
| Dementia | 6 (2.0) | 0 (0) | 6 (2.6) | 0.342 | |||||
| CGI- severity, No. (%)c | – | – | |||||||
| Mild (1–3) | 26 (9.9) | 11 (15.9) | 15 (7.7) | 0.001 | |||||
| Moderate (4–5) | 201 (76.4) | 36 (52.2) | 165 (85.1) | ||||||
| Severe (6–7) | 36 (13.7) | 22 (31.9) | 14 (7.2) | ||||||
| Treatment status at baseline, No. (%)d | |||||||||
| Completely naïve | 121 (39.4) | 27 (38.0) | 94 (39.8) | 0.785 | – | – | – | – | – |
| Prior exposure to SGA≤10 days | 186 (60.6) | 44 (62.0) | 142 (60.2) | ||||||
| Before baseline visit | 184 (59.9) | 43 (60.6) | 141 (59.7) | ||||||
| In the past | 2 (0.7) | 1 (1.4) | 1 (0.5) | ||||||
| Prescribed SGA at baseline, No. (%)e | |||||||||
| Risperidone | 126 (41.0) | 51 (71.8) | 75 (31.8) | 0.001 | – | – | – | – | – |
| Olanzapine | 77 (25.1) | 3 (4.2) | 74 (31.4) | 0.001 | |||||
| Quetiapine | 88 (28.7) | 15 (21.1) | 73 (30.9) | 0.109 | |||||
| Otherf | 16 (5.2) | 2 (2.8) | 14 (5.9) | 0.378 | |||||
| Previous SGA exposure (days), mean (SD), median, [range] | 2.4 (2.7), 2.0[0–10] | 2.1 (2.4), 2.0[0–10] | 2.5 (2.8), 1.5[0–10] | 0.372 | – | – | – | – | – |
| Current dose of SGA at baseline (mg), mean (SD), [range]g | 145.7 (185.7)[0–1200] | 123.5 (142.1)[0–530.3] | 152.2 (196.5)[0–1200] | 0.736 | – | – | – | – | – |
| Cumulative dose of SGA at baseline (mg), mean (SD), [range]g | 560.9 (859.7)[0–6105.3] | 377.4 (545.8)[0–2727.3] | 614.8 (926.1)[0–6105.3] | 0.330 | – | – | – | – | – |
| Concomitant treatment, No.(%)h | |||||||||
| Antidepressants | 78 (25.6) | 5 (7.2) | 73 (30.9) | 0.001 | – | – | – | – | – |
| Lithium | 3 (1.0) | 0 (0.0) | 3 (1.3) | 0.999 | |||||
| Other mood stabilizers | 25 (8.2) | 3 (4.3) | 22 (9.3) | 0.185 | |||||
| Stimulants | 5 (1.6) | 5 (7.0) | 0 (0) | 0.001 | |||||
| Benzodiazepines | 142 (46.4) | 26 (37.1) | 116 (49.2) | 0.077 | |||||
| Anticholinergic agents | 12 (3.9) | 8 (11.3) | 4 (1.7) | 0.001 | |||||
| Drop-out before 6th month assessment, N (%)i | 144 (46.9) | 36 (50.7) | 108 (45.8) | 0.464 | 17 (11.3) | 3 (16.7) | 14 (10.6) | 0.432 | 0.001 |
| Refusal to continue | 21 (6.8) | 1 (1.4) | 20 (8.5) | 0.034 | 3 (2.0) | 2 (11.1) | 1 (0.8) | 0.038 | 0.027 |
| Loss to follow-up | 70 (22.8) | 23 (32.4) | 47 (19.9) | 0.034 | 14 (9.3) | 1(5.6) | 13 (9.8) | 0.999 | 0.001 |
| SGA discontinuation | 42 (13.7) | 10 (14.1) | 32 (13.6) | 0.950 | – | – | – | – | – |
| Deathj | 1 (0.3) | 0 (0) | 1 (0.4) | 0.999 | 0 (0) | 0 (0) | 0 (0) | – | 0.999 |
| Other reasons | 6 (2.0) | 2 (2.8) | 4 (1.6) | 0.628 | 0 (0) | 0 (0) | 0 (0) | – | 0.185 |
| Unknown | 4 (1.3) | 0 (0) | 4 (1.6) | 0.577 | 0 (0) | 0 (0) | 0 (0) | – | 0.308 |
In all cells, % refers to percentages (within columns) of participants for whom information was available. Abbreviations: CGI: clinical global impression; Paed: pediatric; SGA: second-generation antipsychotic.
Statistically significant p-values in bold. For qualitative variables, Chi-square tests (χ2) (Fisher exact test when needed). For quantitative variables, Mann–Whitney U tests.
Information available for 300 patients. Diagnosis refers to the psychiatric condition for which the SGA was prescribed. Forty-five patients had a co-morbid Axis I or Axis II diagnosis (data available upon request). ADHD: Attention deficit and hyperactivity disorder. Depressive disorders: major depressive disorder, dysthymia, and depressive disorder not otherwise specified. OCD: Obsessive-compulsive disorder. ODD: Oppositional defiant disorder. Other anxiety disorders: generalized anxiety disorder, anxiety disorder not otherwise specified, and posttraumatic stress disorder. Other infancy/childhood/adolescence: other disorders of infancy, childhood, or adolescence. Other psychotic disorders: brief reactive psychosis, psychotic disorder not otherwise specified, major depressive disorder with psychotic symptoms, substance-induced psychosis, and delusional disorder. Schizophrenia spectrum disorders: schizophrenia, schizophreniform and schizoaffective disorder.
Information available for 307 patients. Completely naïve refers to those patients who were prescribed an SGA for the first time just at the time the baseline visit was performed. Prior exposure to SGA≤10 days refers to those patients who had a prior lifetime exposure to antipsychotics of no more than 10 days, either because they had been prescribed an SGA right before the baseline visit or in the past.
Other SGAs: paliperidone (n=6 adult patients), aripiprazol (n=2 pediatric and 4 adult patients), amisulpride (n=2 adult patients), asenapine (n=1 adult patient), combination of olanzapine+ sulpride (n=1 adult patient).
A significantly higher proportion of patients (47%) than healthy controls (11%) dropped out of the study through the follow-up. The main reasons for drop-out are shown in Fig. 1 and Table 4.
DiscussionThe SLiM study is the first 6-month prospective, observational, multicenter, pharmacogenetic (PGx) study including a large cohort of SGA drug-naïve patients across a large age span (8–90 years) and with a variety of diagnoses, and a cohort of healthy controls matched by age, sex and ethnicity. In our sample, the group of pediatric patients had a significantly higher proportion of males, a higher proportion of subjects diagnosed with bipolar disorder and a higher proportion of severe patients (defined as having a CGI-S score≥6) than the group of adult patients. Irrespective of age, almost 40% of patients were completely naïve to SGAs at baseline. Risperidone was the most commonly prescribed SGA across the entire age span. Pediatric patients had higher prescription rates of risperidone, anticholinergic agents and stimulants at baseline than their adult counterparts. Conversely, olanzapine and antidepressants were more commonly prescribed in adults. Although not statistically significant, mean daily doses of prescribed SGAs were slightly lower in the pediatric than in the adult group.
SGAs other than risperidone, olanzapine and quetiapine were scarcely prescribed in our sample. This may be partly due to the study design. At the beginning of this study, only those patients who were firstly prescribed one out of these three SGA drugs were included; further on, the inclusion criteria were broadened and prescription of any SGA was allowed. This may have biased the results regarding prescription rates in both age groups. Nonetheless, our results were congruent with previous observational studies in similar settings, in which those were the most prescribed SGAs, both in paediatric38,39 and adult samples.40 Except for a patient that had been prescribed risperidone for aggression in the context of a diagnosed conduct disorder and low intelligence quotient, the rest of pediatric patients enrolled in the SLiM study were taking SGA drugs for off-label uses. That said, the fact that olanzapine was poorly prescribed in the pediatric group, points toward the increasing awareness of the metabolic profile of this drug in child and adolescent psychiatrists.41 Regarding concomitant treatments, even though antidepressants are increasingly co-prescribed with antipsychotics in children and adolescents,42,43 co-prescription rates appear to be higher in adult population.40,44
The SLiM study has a number of limitations that are inherent in its naturalistic design. Prescribed SGA treatment was not controlled and doses were chosen by the treating psychiatrists, based on clinical criteria. Also, the sample was quite heterogeneous in setting provenance, diagnoses, use of concomitant medications or presence of alcohol and substance misuse. This will be taken into account when carrying out further analyses on the clinical, environmental, and genetic factors that might be associated with weight gain and metabolic disturbances. The main difficulties that we encountered were the recruitment of drug-naïve patients and the control group matching strategy. That said, a relatively large cohort of drug-naïve patients and healthy controls was recruited and followed-up during 6 months. Through the follow-up, one of the main difficulties that we encountered was the large drop-out rate (around 50% of patients and 11% healthy controls dropped out of the study). The main reason for drop-out both in adult and pediatric patients was the loss to follow-up (19.9% and 32.4%, respectively), while refusal to continue was more frequent in adults than in young people (8.5% vs 1.4%, respectively). Discontinuation of the prescribed SGA was a reason for drop-out in around 14% of patients (both in adult and pediatric subgroups). Nonetheless, discontinuation rates may have been higher, since discontinuation might have happened for some of the patients who were lost to follow-up or refused to continue participation. Furthermore, the controls were compensated with a gift card for their participation in the SLiM study. This may have led to a ‘volunteer bias’ (i.e., the bias resulting from the fact that a particular sample can contain only those participants who are actually willing to participate in the study or experiment45). Nonetheless, the authors tried to control for this by means of a careful control group matching strategy. Another relevant limitation is that serum SGA determinations at each visit only provide an indirect measure of treatment adherence. Nonetheless, these determinations were carried out at one site, where a trained and experienced biochemist performed the analyses following a standardized protocol.
The main strengths of this study include the prospective design of the study, with a 6-month follow-up, the homogeneity of the patient and control sample, the large age span of the recruited sample and the relatively large sample size considering the above mentioned characteristics of the sample. This strategy will increase the power of the PGx study with few confounding factors and carry-over effects.
Results from the SLiM study will provide a better understanding of the clinical, environmental, and genetic factors involved in weight gain and metabolic disturbances associated with SGA treatment. Additionally, we will be able to explore the effect of age in such changes and the impact of weight gain in clinical outcome and quality of life of drug-naïve patients treated with SGAs. Finally, the study will enable the identification of potential PGx biomarkers as predictors for SGA tolerability. In the near future, this could potentially assist the individualization of antipsychotic treatment and facilitate the development of new therapeutic tools with better safety and tolerability profiles.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Declaration of Helsinki.
Confidentiality of DataThe authors declare that there is no confidential data appearing in this article. Privacy and confidentiality of data were warranted throughout the study.
Right to privacy and informed consentThe authors obtained the informed consent of the participants and/or their parents or legal guardians as appropriate mentioned in the article. The author for correspondence is in possession of this document.
Funding sourcesThis study was funded by Fondo de Investigaciones Sanitarias (FIS), Instituto de Salud Carlos III, Spanish Ministry of Economy and Competitiveness (PI 06/1165, PI 07/0815, PI 07/0163, and PI 07/0452), Beca Intramural CIBERSAM 2008 and Fundación Mutua Madrileña 2009.
Conflict of interestLP-C and CMD-C have received a grant ‘Río Hortega’ from Instituto de Salud Carlos III, Spanish Ministry of Economy and Competitiveness and from Fundación Alicia Koplowitz.
JB has been a consultant to or has received honoraria or grants from Adamed, AstraZeneca, Bristol-Myers Squibb, CIBERSAM, D&A Pharma, Elan, European Commission- FP6 and FP7-, Forest, Instituto de Salud Carlos III, Janssen Cilag, Lilly, Lundbeck, Ministerio de Sanidad, Plan Nacional sobre Drogas, Otsuka, Pfizer, Roche, Servier and Shire.
IC has received honoraria or grants from Lilly, Janssen and Otsuka.
RRJ has been a consultant to or has received honoraria or grants from CIBERSAM, Instituto de Salud Carlos III, Lundbeck, Otsuka, Pfizer.
JS has been a consultant or has received research grants from Spanish Ministry of Economy and Competitiveness, Conselleria de Sanidad, ISCIII, TV3, Lilly, Janssen, Otsuka, Roche, Pfizer, and Astra Zeneca.
AG-L has received research grants and served as consultant advisor or speaker for AstraZeneca, Eli Lilly, Boëhringer Ingelheim, Lundbeck, Pfizer and Servier.
DF has been a consultant and/or advisor to or has received honoraria from Astra-Zeneca, Bristol-Myers-Squibb, Janssen, Lundbeck, Otsuka and Pfizer.
CA has been a consultant to or has received honoraria or grants from Abbot, AMGEN, AstraZeneca, Bristol-Myers Squibb, Caja Navarra, CIBERSAM, Fundación Alicia Koplowitz, Instituto de Salud Carlos III, Janssen Cilag, Lundbeck, Merck, Spanish Ministry of Science and Innovation, Spanish Ministry of Health, Spanish Ministry of Economy and Competitiveness, Fundación Mutua Madrileña, Otsuka, Pfizer, Roche, Servier, Shire, Takeda and Schering Plough.
PAS, EG, MF, CT-C, MA-B, MM and BA have no conflict of interest to declare.
Supported by the Spanish Ministry of Economy and Competitiveness. Instituto de Salud Carlos III, CIBERSAM. Madrid Regional Government (S2010/BMD-2422 AGES), European Union Structural Funds, Fundación Alicia Koplowitz, Fundación Mutua Madrileña and The Comissionat per a Universitats i Recerca del DIUE de la Generalitat de Catalunya (2009 SGR 827).
Please cite this article as: Pina-Camacho L, Díaz-Caneja CM, Saiz PA, Bobes J, Corripio I, Grasa E, et al. Estudio farmacogenético del tratamiento a largo plazo con antipsicóticos de segunda generación y sus efectos adversos metabólicos (Estudio SLiM): justificación, objetivos, diseño y descripción de la muestra. Rev Psiquiatr Salud Ment (Barc). 2014;7:166–178.