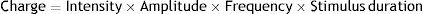The process of normalization electroconvulsive therapy (ECT) requires, among other actions, disseminating the latest information on this technique. One of the most complex aspects is the electrical stimulus, whose knowledge should be spread and put into practice.
In this paper, we review the available information about frequency and number of ECT sessions, and efficacy of each electrode placement. We also present two approaches to determine the ECT charge: stimulus titration versus age-based method; and the limitations of the summary metrics of charge, being necessary to expand our knowledge of the parameters that configure the stimulus: duration, current amplitude frequency and pulse width.
El proceso de normalización y dignificación de la terapia electroconvulsiva (TEC) requiere, entre otras acciones, la difusión de la información más actualizada sobre esta técnica. Uno de los aspectos que ha alcanzado mayor sofisticación es el relacionado con el estímulo eléctrico, un conocimiento que es preciso consensuar, extender y llevar a nuestra práctica.
Se revisa en este trabajo la información disponible sobre frecuencia y número de sesiones de TEC, y la utilidad de las distintas localizaciones de electrodos; se describen y valoran los métodos de cálculo de la carga a emplear, titulación o cálculo directo con base en la edad, y se destaca la insuficiencia de las medidas globales de magnitud del estímulo, siendo preciso ampliar nuestro conocimiento sobre los parámetros que configuran la carga: tiempo, intensidad, frecuencia y amplitud de pulso.
Electroconvulsive therapy (ECT) is a particularly effective treatment in the case of clearly defined indications,1 still surrounded by a certain degree of stigma in our setting, and negatively affected by inefficient resource management, unequal access for patients, significant variability in rates and standards of application, and insufficient training of professionals.2,3 Several initiatives linked to the Spanish Society of Biological Psychiatry (SEPB in its Spanish acronym) have posed the need to dignify and standardize this technique in our setting.4 The greater amount of research on ECT, the dissemination of findings, and the drafting of guidelines and consensuses (such as the recently published Guide to Best Clinical Practice on Electroconvulsive Therapy)5 are at the crux of these actions. Along these lines of further researching and disseminating information, the following review addresses aspects related to the technique of application, which are included in the guides as general guidelines6 but are rarely considered in clinical practice. The most up-to-date information is reviewed on the frequency and number of sessions, position of the electrodes and characteristics of the electrical stimulus, setting out the different opinions on this, and summarizing with subtle views, following the author's criteria. In general, results obtained with samples were collected from depressed patients, specifying the cases in which other pathologies were treated. In addition, we shall refer in principle to the results of treatments in the acute phase, indicating the data on continuation/maintenance if applicable.
MethodologyThe search results for articles published between 1965 and 2016 on the subject in Medline, Psychinfo, Embase and Cochrane originals have been thoroughly reviewed, as well as the main guides on the subject, including articles in English, French and Spanish, from original research texts to published cases, recommendations, consensuses or narrative reviews. Particularly considered were experimental studies, especially meta-analyses, series of studies on topics by defined groups, and regularly updated guides. The basic search terms were: “Electroconvulsive Therapy Instrumentation, Methods, Standards, Therapeutic use, Electrode placement, Unilateral, Stimulus intensities, Convulsion duration, Pulse, Ultra-brief, Brief, Suprathreshold, Threshold, Titration, Electrical stimulus, Duration, Current amplitude, Frequency, Pulse width”. The search was completed with a manual and retrospective review of the studies selected, using a final total of 160 different references of particular interest.
ResultsAs with many other empirical medical treatments, the mechanism of action of ECT is not completely known, despite the enormous advances over the last few decades,7 and the particular insistence with which knowledge of this technique is demanded.8 What is clearly known is that ECT produces this improvement by provoking a convulsion by means of an electrical stimulus.9 Historically, crises were achieved through chemical means, an option that Fink continues to defend in order to highlight the role of the crisis as opposed to the way it is produced.10,11 The objective is a clinically effective crisis, which is associated with a minimum duration, spread across both hemispheres, and an electroencephalographic pattern of a certain quality.6 This requires an appropriate stimulus: sufficiently powerful to maximize efficacy but not so high that it causes side effects.12 This risk-benefit balance will be, as in the case of all medical action, the crux of this study. It is necessary to consider the patient's own variables, such as their age, concomitant medication, medical situation, pre-medication, anaesthetic technique, hydration, and O2 and CO2 levels. However, in this study we shall focus on the variables of the stimulation which the crisis is provoked with: the number of sessions, the break between them, the position of the electrodes, and the electrical stimulus used; not only considering the total charge but also the electrical parameters that compose it, the handling of which can decisively influence the clinical outcome.13 Although we shall analyze each aspect separately, the decisions about the technique that will be used with a particular patient at a specific point in time must be taken before the sessions, as a strictly individualized unit, depending on the characteristics of both the particular patient (age, medication, medical and anaesthetic risk, cognitive risk and the urgency of response, etc.). However, it must also be borne in mind that the day-to-day reality of healthcare includes different contexts for application which are not always ideal,2 as well as various different levels of training of the professionals involved, or the stability and knowledge of the anaesthesia team, which can sometimes lead to one action or another.
Number of sessionsIt is not possible to anticipate the number of sessions that will be required during acute treatment of ECT. In general, the treatment is reconsidered after each session, and it is decided to withdraw the treatment when no further improvement has been obtained. The decision to continue results from a balance between individual risk, the seriousness of the patient's condition, and the expectations of improvement. In general, the first sessions are an indicator of the expected response14: a 30% decrease in symptoms after the first 6 sessions predicts complete remission.15 If there is no improvement in the first 4–6 sessions it will be necessary to reconsider the technique used, reviewing the suitability of the crises being obtained and, where appropriate, the position of the electrodes, going from unilateral (UL) to bilateral. If after that there is no improvement, it will be appropriate to rethink the indication.12 Although most patients improve before 9 sessions,16 some depressed patients present complete late responses, so it is appropriate to reach 12 sessions if there is a partial initial response.17,18 In general, a greater number of sessions is needed to obtain maximum improvement in psychotic symptoms (around 12–14 sessions), and fewer in depressive ones (6–12 sessions).
Frequency of applicationThe interval between sessions in acute treatment has been one of the topics in the process of change over recent years. The most frequent practice has been to apply 3 weekly sessions as the basic norm, and this is still the usual frequency in the United States and most other countries. In Spain, the practice has remained stable over the last decade in this respect: in 2006, 75.9% of the centres applied ECT 3 times per week, and 20.4% twice.19 At the current time, this distribution stands at 79.6% versus 19.5%.2 In the UK the basic frequency of 2 weekly sessions has been applied for a considerable time: in the 199520 guide of the Royal College this was already alluded to in a general way, however in the 200412 edition it became the standard, which in 201322 had still been maintained. The basis of this change was the meta-analysis by the UK's ECT Review Group in 2003,22 which did not find any differences in effectiveness between 3 and 2 weekly sessions but did, however, find more cognitive effects with the greater frequency. Over the last decade the frequency of twice weekly sessions has been extended to Europe and other countries, such as Ireland, India or Australia.23–25 This is a practice proposed for the acute phase, bilateral positions, depressive symptoms and non-urgent response.22,26 In UL positions this lower frequency is not clearly indicated.27 Lower frequencies in the acute phase, such as one weekly session, generally reduce the effectiveness,28 and if what we are looking for is to further attenuate cognitive impairment, it will be preferable to apply low charges, handling stimulus parameters, or use UL29 positions. In emergency situations (catatonia, neuroleptic malignant syndrome [NMS], agitated mania, starvation etc.) cognitive involvement will be secondary, and the frequency can be increased to reach daily sessions. Recently, a daily regimen has been proposed when ultra-brief stimuli are used with UL positions, seeking to advance the response while retaining the advantage of this type of stimulation30 in cognitive impairment. The 2001 APA guide31 maintained the option of more than one stimulation in the same session—the so-called multiple, monitored ECT—in life-threatening situations, action that is no longer taken.
Placement of stimulation electrodesThe optimal position of electrodes remains a debated issue, in close connection with advances in our knowledge of brain structures. Although32–34 these were explored, and numerous positions are still being proposed that aim to minimize damage or increase effectiveness in situations of resistance,35–37 there are 3 which are still regularly used:
Bilateral frontotemporal (BFT)This is the classic position with the electrodes at the midpoint of the canthomeatal line on both sides. Repeatedly associated with a greater speed of action38,39 and efficacy,11,22,40 it also produces more cognitive involvement.41
UnilateralWith different variants, the most frequently dealt with in the literature and used in clinical treatment is the classic D’Elia: one stimulation electrode in the non-dominant hemisphere, next to the vertex, on the parietal lobe, and the other one at the midpoint of the canto-metal line on the same side.31 Developed to minimize cognitive impairment, this is usually associated with lower efficacy and slower response, although depending on the technique used and the target population, the balance may be more efficient.42 Although UL is proposed as stimulation in the non-dominant hemisphere, the difficulty in determining dominance,12 and the low prevalence in the population of clear right hemispheric dominance, especially in language, means that, in practice, the right unilateral is systematically used (ULD/RUL). Only in cases of transient dysphasia in the postictal period would the left unilateral (ULI/LUL) be used to prevent the involvement of verbal memory.29 UL stimulation presents certain practical difficulties in achieving correct impedances, due to the presence of hair; greater difficulty in obtaining a good contact surface in the parietal electrode; or the risk of shunt due to proximity, which will be handled respectively using conductive gel, pressure on the electrodes with a non-conductive object, with the position of the head held steady by an auxiliary nurse, and correct position and cleaning to minimize the stimulus passing through the surface. With this position, cases of awakening have been described before the end of the effect of the succinylcholine.43,44
Bilateral frontal (BF)Although different variants45–49 have been explored, bilateral frontotemporal—the most widespread position—places the stimulation electrodes symmetrically at 5cm over the orbital axis.50 The objective will be to obtain an effectiveness equivalent to BFT, with a cognitive impairment as low as UL, by avoiding the involvement of the areas most related to cognitive impairment and, for some authors, through the direct stimulation of the structures involved in depression: the orbitomedial frontal cortex.50–52 The spatial distribution and intensity of the electric field induced by the stimulation depend to a large extent by the position of the electrodes, which will result in involvement—for better or for worse—of different brain structures to varying extents. The field produced with a small distance between electrodes will be more focal when directly covering less surface area, and less intense when a certain degree of shunt occurs on surface layers.13 Thus, in the UL positions, the highest field strength is between the electrodes, while in the bilateral positions it is more focused under each electrode.53 The result will be, in general, that UL positions will tend to cause less postictal disorientation and retrograde/anterograde memory involvement,54 by not acting on areas related to cognitive impairment—and perhaps stimulating those that are considered areas of interest—although they will do this with less energy and will tend to achieve a lower level of effectiveness. On the contrary, BFT stimuli achieve greater psychopathological improvement at the cost of greater cognitive impairment due to more intense but less selective stimulation.41 As we said, the BF positions are proposed as the solution: as effective as BFT and with as few unwanted effects as UL55–57—the ideal balance between efficacy and cognitive impairment.54,58,59 And not only in depression, also in severe mania, with fewer cognitive effects60 and a faster response61 in the elderly,62 and even in catatonia.63 It also includes the same or lower cardiovascular risk than other positions65–76 However, the literature has always been contradictory,66 and thorough studies in recent years have not found significant differences between BF and classical BFT,22,38 so recent editions of the guidelines do not consider BF as a regular alternative.21 The search for a point of equilibrium that would enable the cognitive advantage in the UL position to be maintained, with an effectiveness comparable to BFT, led Sackeim's group in Columbia to consider individually adjusting the charge (“dose” of the stimulus) used, depending on the patient's convulsion threshold.67 In an essential study,68 this group showed that with low charges (1.5–2.5 times the threshold charge [U×1.5–2.5]), the response was not comparable, but greater UL stimuli, at 6 times the threshold (U×6), were as effective as BFT at 1.5 times the threshold (U×1.5), maintaining the cognitive advantage. Subsequent work has not always confirmed these results, and a decade later, the also fundamental work of Kellner et al.,38 found no differences in efficacy or cognition between BFT with U×1.5, BF and U×1.5 and RUL with U×6. The possibility that the ideal level in the balance may be smaller charges such as RUL at U×469 is still an open question, and the question of optimal suprathreshold charge in UL remains an issue pending research for the Royal College guide.21 The controversy over positions will therefore not have a unique answer. It is not possible to affirm categorically that a position is always better, it being necessary to individualize the decision, applying this balance of efficacy-damage to each particular case, depending on the patient and the specific circumstances.70
The electrical stimulusThe importance attributed to electrical stimulus in ECT has varied throughout the history of the technique, and this remains very different, depending on the author. The classic approach10,71 considers that ECT produces its beneficial effect through a convulsion, provoked in different ways, currently by means of an electrical stimulus. The greater or lesser quality of this crisis will mean greater or less effectiveness, and that quality will depend, among other variables, on the characteristics of the electrical stimulus used. The alternative view72–74 would argue that the electrical stimulus itself has a direct therapeutic effect (as it has on undesired effects) on the brain structures involved in the disorders treated. The different techniques of direct stimulation, electrical or magnetic without a crisis, and which are currently on the increase,75–77 would support this perspective.78
Evidently both alternatives are compatible and, in any case, the magnitude and quality of the electrical stimulus used are enormously important, either as a tool to provoke a therapeutic crisis, or as a direct actor of the effect.
General measurements of the size of the stimulus. The chargeThe general measures of the “dose” of stimulus are used as a simplification to calculate or describe the stimulus to be used. Currently, the usual measure is the charge delivered (measured in megacoulombs [mC]), but we recall that previously this was the energy (in joules) which was the standard “dose” measure.31–79 Once stimulus with sinusoidal waves had been done away with as they were physiologically inefficient and produced more cognitive effects,80 the main devices now currently available (MECTA Spectrum and Thymatron IV) supply rectangular pulse trains with alternating polarity. The total charge given to the patient in a stimulation (we could say the electrons directed at the stimulated areas) will depend on the intensity of the current (that is, the charge that flows per unit of time, in milliamps [mA]) and the duration of stimulation, a product in turn of the amplitude of the pulses administered (in milliseconds [ms]), their frequency, and the total duration of the stimulus (in seconds [s]):
The charge necessary to cause a crisis in a given patient at a specific time depends on a large number of factors with different weighting, varying from age and sex to the patient's usual pharmacological treatment and the anaesthetics used, their hydration level, or the ventilation technique during anaesthesia, among many others. The current strategies to decide on this charge are essentially two: the anticipated charge calculation based on the variables with greater weight, and the progressive titration seeking the threshold. Other methods described are mixed strategies where one starts from a certain basis,81 or the benchmark charge.82 Fixed high doses,80 no longer used, are limited by the APA to exceptional situations where subconvulsive stimuli are contraindicated due to medical fragility.31
The methods of direct calculation of the charge start from the greater weighting attributed to some of the variables alluded to: essentially, age, but also sex and the position of the electrodes. We have come to investigate complex statistical models that include numerous variables,83,84 however in practice the approach of Abrams and others along the same lines still persists.85 This is based on the age variable to calculate a charge that is anticipated as clinically effective for that patient. This perspective has in practice materialized as the method of Petrides and Fink in 199686 who, in bilateral positions (BFT or BF), propose giving a charge in mC equal to the patient's age, multiplied by 2.5, and in UL positions 5 times the patient's age. In devices with a charge indicator (MECTA), this will be the charge in mC that is sought, then later deciding on the most suitable intensity, frequency, pulse amplitude and duration. This method of calculation underlies the design of one of the aforementioned stimulator models, the Thymatron, which divides the maximum charge that the device can deliver into percentages: each section will be the charge that a patient of that age needs in order to respond with UL stimuli (approximately their age×5); in BF or BFT half will be needed (their age×2.5). The psychiatrist has to predetermine the amplitude of the pulses given (0.25, 0.5 and 1ms), and for each percentage of charge of the dial the stimulator is programmed for a frequency of pulses and a total duration of the stimulus; the programming carries the maximum duration for a frequency between 10 and 70Hz. If desired, the current versions can be individualized, setting the frequency, amplitude and duration of the stimulus manually, always with a fixed intensity of 900mA. This method of calculating the charge will be quick because it directly proposes therapeutic charges; and simple, since it uses a single variable to calculate. We emphasize that this strategy directly calculates the anticipated charge as effective in obtaining a clinical response in a patient, not only for obtaining a convulsion. In contrast to the direct charge calculation method, the titration or empirical method is proposed. This seeks to identify the charge that a specific patient needs under certain conditions. Given that the variables that influence it are very numerous and change over time, the pragmatic strategy will be to test charges until finding the appropriate one. In practice, this is a question of starting with deliberately low stimuli until a crisis greater than 15 s21 is reached. The charge that was necessary for this will be called the convulsion threshold for that patient under those conditions. This charge, sufficient to achieve a small crisis, will usually not suffice to achieve therapeutic effect, so that it will be necessary to multiply it in the following stimulations by a constant, higher in UL, lower in BFT/FB. This will result in a “clinically effective” charge for that patient at that point in time.
We find ourselves again up against the importance of the stimulus: it is not enough to exceed the threshold and obtain a crisis, and there are no minimum or sufficient crises durations for all patients. A sufficient stimulus to overcome the threshold and achieve a minimal response in EEG may not lead to a clinically effective response, especially in UL positions.87,88 The threshold concept continues to be discussed. Its definition is arbitrary and based on modest evidence31,80,89,90 which is also contradictory,91 however it seems to be the best predictor of charge and response available to us. The range of variation between individuals is important, between 150 and 400mC according to the studies.11,91 A large number of variables influence the threshold92: it will be higher in males, at advanced age, bilateral electrode placement, lower intensities, brief duration of the stimulus, higher body mass index, estrogenic deprivation, hypoxaemia and hypercapnia; the opposite conditions lower the threshold.67,91,93–95 The threshold increases by an average of 60% with successive sessions (more in BL than UL),87 but this is unrelated to clinical improvement, as was suggested.96 We would like to highlight the effectiveness of hyperventilation to reduce high thresholds, since with minimal effort during anaesthesia we will obtain hyperoxygenation and hypocapnia, which will enable us to use smaller charges.97 Drugs are also an important factor due to their weight and because they can affect this: the anaesthetics Propofol and Pentotal raise the threshold, unlike Etomidate and Ketamine.98–102 Anticonvulsants, benzodiazepines and lidocaine, raise the threshold, while methylxanthines, some antidepressants and antipsychotics, and stimulants decrease it.103,104 The sum of numerous external factors and the high individual variability will result in difficulty in predicting the threshold of a specific subject at any given time.31 Although it is already known that long crises do not necessarily mean effective crises, we emphasize that increasing the charge does not mean increasing the duration of the crisis: the longest are obtained with charges above the threshold, and high charges shorten the duration of convulsions, in addition to generating more cognitive impairment.83,87 Not only the clinical response is related to an excess dose with respect to the threshold; it seems that also cognitive effects are not related to the absolute dose but to an excess of doses over the threshold.105,106
Overall, it appears that calculating the charge using titration would enable the stimulus to be individualized for a specific patient at a specific time and circumstances. In contrast, at least theoretically, age-based formula calculations would, in principle, constitute an overdose in many young women107 and under-dose patients with UL54 positions, as age is only one of the many variables that influence the patient's threshold. As a consequence, the main official guidelines on ECT practice21,31 recommend the calculation of individualized charges using titration as a standard practice, although it is acceptable to calculate directly by age in situations of emergency or clinical severity, where it is not advisable to extend the response time, and the risk-benefit balance of high charges is acceptable.21
The guides do not completely agree on the figure for multiplying the threshold to obtain a clinically effective charge. The 2001 APA guide31 states that the charge will be in UL position, between 2.5 and 6 times the threshold, and bilaterally, between 1.5 and 2.5 times the threshold. The UK's latest edition of the Royal College 2013 guide21 indicates bilateral charges of at least 1.5 times the threshold, while for UL it recognizes that this is not clearly established, proposing between 4 and 6 times the threshold in situations of cognitive risk. This is, indeed, a topic open to new research, as the cognitive impact benefit of UL ECT could be lost at over 4 times the threshold,69,108 however not all patients will respond to these moderate charges. Titration dosing presents objective drawbacks in daily practice, as it may delay the response by occasionally losing a first session in the calculation of the charge. In addition, it is possible that premature re-stimulations in the refractory period do not obtain a response.109 Contradictory data has been put forward on cardiac risks of subconvulsive stimulations110,111 and the technique has been related to the appearance of late convulsions.112 A possible increase in cognitive impairment appears to be reasonably ruled out.91,113–115 Nevertheless, in addition to the drawbacks and risks associated with titration, the often firm defenders of the systematic use of direct calculation question its theoretical approach—based on a model of individual neuron depolarization versus a model based on kindling—78,116 and, above all, the return on the effort when comparing the charges finally applied by both methods.
Thus, in 2009, Petrides et al.,81 applying their half-age method described above to a sample of 402 patients, found a clinically effective charge only 18% greater than the threshold obtained by titration in the same sample. When multiplied by 1.5 (as they use BFT positions), the clinically effective charge by titration is 31% greater than with their method. Based on this, they firmly conclude that their method is openly superior to titration. Rasmussen117 questions these conclusions by proposing alternative titration strategies to those of the Consortium for Research in ECT (CORE) group, such as minor increases in the charge during titration, which minimize the final dose. It could also be added that higher charges are not necessarily worse if they are what the patient needs in order to obtain a response. On the other hand, also in 2009,118 Van Waarde et al. ran a meta-analysis of the threshold found in UL (68.2mC) and bilateral (111.6mC) positions. They indicated that multiplying this by 6 (UL) produces a charge similar to the “fixed dose” methods, considered over-stimulators, while multiplying by 1.5–2.5 in bilateral positions produces the same charges as if a calculation had been made using the half age. Their conclusion is that the methods of direct calculation of the charge are still legitimate. In 2013, the same authors analyzed the weight of each variable in the threshold, finding that age and bilateral position explained 49% of the variation, enough to justify the direct calculation.119 Finally, this group has recently presented data from a prospective study that compares both methods, resulting in equal efficacy and cognitive effects but a greater total number of sessions required for the titration method.120 Recently, Fink11 has made definitive statements on the subject. In a review of the publications by the CORE group between 1997 and 2011, he stated that there is no justification for measuring the threshold, since his data does not show a variation in this throughout the sessions. This absence of change would invalidate the threshold as a predictor of response. It should be noted that the threshold value derives from its ability to individualize the stimuli, regardless of their variation throughout the sessions.
The charge adjustment parametersIncreasingly more data is becoming available that indicates that overarching stimulus dose measurements are, as we said, an oversimplification, and that we will obtain different results by handling the stimulus variables that influence this charge.121 The role of the charge parameters is to be an intuitive hypothesis, as we understand that different pulse amplitudes produce different lengths of time that the neuron is stimulated in each pulse. The frequency varies the neuronal recovery time between stimuli, and the total duration will mean a different number of stimuli.122 But, in addition, the data shows that the same overall “dose” of electrical stimulus (in mC or joules) given in more or less time, or with different pulse amplitudes, frequencies or intensities, can produce very different effects on the convulsion sought; on the mechanism that produces it78; and on the final clinical effect123,124 in the same way that the same dose of drug translates into different effects in different formulations. The available devices make handling these parameters easier or less easy, depending. As we pointed out, Thymatron is designed to handle general charges, but if desired, it allows individualized adjustment options for frequency, amplitude and duration, but not intensity. The individualization of the parameters in MECTA is, however, inevitable due to its design, with external controls laid down for this purpose. We shall briefly review the generally scarce information on these subjects, which it is undoubtedly necessary to deepen research into. Often these parameters are not even usually specified when describing the methodology of a ECT trial, and when considered, their analysis is often interfered with by other variables such as the position of the electrodes or the frequency and number of sessions.125 Despite its probable practical effect, the duration of application of the stimulus is one of the least studied variables. As we have pointed out, stimuli of the same charge given over a longer period of time translate into lower convulsion thresholds.126–128 It is also suggested that this could be related to better indicators of the quality of the crisis in EEG, and its longer duration.129
Therefore, by applying the expected charges over longer times during titration, it is possible to find lower thresholds; this will mean lower charges finally given, by multiplying by the constant corresponding to the position. Although the clinical significance of this has not been demonstrated,13 the usefulness of an action that obtains a crisis in high threshold situations, beyond the titration process, is evident. This lengthens the stimulation time while maintaining the same charge. Variations in the polarity of the neurons are related to the electrical field produced by the stimulus. As we pointed out, the field varies depending on the size and position of the electrodes, and is very different depending on individual anatomical variables130; but it will also be directly proportional to the intensity parameter of the stimulus. Despite this, intensity is one of the least studied parameters of the stimulus, and sometimes it is considered without bearing in mind the other variables.131,132 The commercial stimulators enable little room for manoeuvre in the case of MECTA (between 500 and 800mA), and none at all in Tymatron (900mA).
However, the differences in the stimulated area—and therefore the neurons recruited—as a function of intensity, are very important, especially for UL positions, and more focal stimuli could be considered with lower intensities, which could result in less cognitive impairment. On the other hand, we have data indicating that using high intensities makes it possible to specify lower threshold charges.133,134 The clinical consequences of these alternatives have still to be assessed.135 An ideal frequency has not been well established, but most of the few available studies that assess this variable suggest that low frequencies (20–40Hz) are more efficient in inducing convulsions, as these are obtained with lower charges, fewer subconvulsive stimuli, and even less cognitive impairment.136–138 The clinical significance of this data has also not been well evaluated, and the stimulation time can be considered as a variable which confuses the results.139 Once the sinusoidal waves of pulse amplitude had been exceeded by 10ms, since the 1980s we have used square pulses of 0.5–2ms nm, classified as brief, with equal efficacy and fewer cognitive effects.73,121,140,141 Interest in experiencing pulse amplitudes of less than 0.5ms, usually classified as ultra-brief,142 has fluctuated since then, this being one of the few parameters which is actively researched. The effectiveness of ultra-brief pulses has been described in UL143 and bilateral144 positions using very suprathreshold stimuli (threshold×6; threshold×8, en UL), with a lower acute cognitive effect and at 6 months,54,145–148 although the improvement appears after more sessions.149–151 The initial fear of an increased risk of asystole has been ruled out.152 The data on the threshold is contradictory; in general, it is indicated that it is lower with ultra-brief pulses,73,149,153 although this increases further during the sessions.148 Ultra-brief pulses mean more total stimulation time, which, together with the positions of electrodes, can be a confounding factor in the analysis of results,125,154 in addition to the lack of randomization144 or retrospective design.150 Nevertheless, experimental data on effectiveness and risk are contradictory: using stimuli well above the threshold (threshold×8), Spaans et al. find brief pulses more effective, without major cognitive impairment,155 concluding that the available literature does not justify the systematic use of ultra-brief pulses,156 a position that is maintained in the updated guidelines.21 The controversy, nevertheless, remains open,35,157 and the individualization of the decision based on greater effectiveness of brief pulses, but less cognitive impairment with ultra-brief pulses, appears to be the desirable option.158 Finally, we would point out that, although the practice that available commercial stimulators permit is based on bi-directional square-pulse stimuli, other waveforms and other parameters of the stimulus, such as the directionality of the pulses and their unidirectional polarity, have been investigated at some point in time, suggesting lower thresholds of convulsion and, therefore, fewer charges with unidirectional pulses.13
Summary and conclusionsThe great advances made in the knowledge of the technique of stimulation in ECT, enables us to individualize action taken on a patient and their specific circumstances, and before the session to foresee the ingredients that will provide the best risk-benefit equilibrium.
No tools have been described to predict the number of sessions that will be necessary; the treatment is assessed and reconsidered after each session, and it is terminated when no further improvement is obtained. Although the usual practice regarding the interval between sessions in most countries is still to apply 3 sessions per week, individual adjustments of the decision are extended to between 2 and 3 sessions per week, depending on the technique used; the urgency of the response; and the risk of cognitive involvement.
Regarding the placement of the electrodes, it is a common practice to start using BFT placement with modest charges (threshold×1.5–2.5 or age×2.5), producing greater overall effectiveness, although at the cost of accepting a greater risk of negative cognitive effects than with UL. However, some solid lines of investigation show that using high enough charges adjusted to suit the patient, the UL stimuli can be as effective as BFT, preserving the cognitive advantage. The data on the practice in Spain suggests the need to identify this decision more often by proposing UL positions in situations of risk or cognitive impairment, always with the appropriate adjustment of the stimulus (threshold×4–6, or age×5). Other positions such as BF are not currently considered a usual alternative. Whether we consider the stimulus as a way to provoke the crisis which produces the therapeutic result, or if we defend the principle that this provokes effects directly, the scale and quality of the electrical stimulus is enormously significant. Although insufficient, the overarching measurements of the scale of the stimulus (at this point, the charge) are still used as a simplification to calculate the “dose” that we will use. Currently there are 2 strategies to decide on the charge that we will use on a patient: firstly, direct calculation in advance, based on the variables with greatest weight, and secondly, progressive titration, seeking the individual patient threshold. Direct calculation of the charge is based on the age variable to calculate the stimulus that is required. By multiplying the age by a different constant, depending on the position of the electrodes (2.5 in BFT, 5 in UL), the charge that is supposed to be clinically effective for the patient will be obtained directly. This calculation is performed automatically by certain models of stimulator (Thymatron), designed in the light of this method, where the percentage of charge corresponding to the age for UL stimulation will be indicated directly on the dial, and half that for bilateral stimulation. Great variability of the precise charge in order to obtain a crisis in a particular patient at a given time and under specific conditions seems evident. This is influenced by a large number of factors, in addition to age. As a consequence, the empirical or titration method was developed to determine the necessary stimulus, testing low charges until finding the appropriate one to obtain a minimum EEG crisis of 15s, which will be termed threshold charge. This charge will in principle be insufficient to achieve a therapeutic effect; it will be necessary to multiply this, in following stimulations, by a constant, which is higher in UL (4–6), and lower in BFT/FB (1.5–2.5). This will be the “clinically effective” charge, individualized for that patient at that point in time.
The indisputable logic of the proposal to individualize the charge by titration, as opposed to calculation based on a single variable such as age, has gradually prevailed in the main official guides on the practice of ECT, although this is an open topic and sometimes controversial, and both options are legitimate on the basis of our current knowledge, but always dependent upon the particular circumstances of the patient and the local environment where it is applied.
Finally, the general measures for the scale of the stimulus, such as the charge, are insufficient. It seems evident, and has been demonstrated, that supplying the same charge at different times, or with different intensity, amplitude or frequency of pulses, produces different results as regards the crisis obtained, its efficacy and its side effects. Research on these parameters is scanty, and the clinical significance of the findings is not well defined. To sum up, it seems possible to exceed convulsion thresholds with lower charges, lengthening the total duration of the stimulus. As regards intensity, there is little or no leeway for settings in the case of commercially available stimulators: all evidence, however, points to the fact that the area of the stimulus could be more focussed with lower levels of intensity, requiring fewer overarching charges at a higher intensity. Regarding the frequency variable, the data points to greater effectiveness with low frequencies, which would obtain crises with lower charges. The findings on pulse amplitudes point in the same direction, where there is interest in researching the possible cognitive advantage of ultra-brief stimuli: less than 0.5ms, as opposed to brief pulses of 0.5–2ms. Although this line is open, the systematic use of ultra-brief pulses does not appear to be recommended due to less effectiveness, although it could be considered as an alternative in situations of clear cognitive risk. Altogether, we find a progressive sophistication in the knowledge of stimulation used in ECT that, together with other fields within this technique, it is necessary to a reach consensus on, disseminate and adopt in our regular practice. This will be one of the bases of the process of dignifying and standardizing the treatment we are committed to.
FundingNo funding was given for this study.
Conflict of interestThe author has no conflict of interest to declare.
Please cite this article as: Sanz-Fuentenebro FJ. Características del estímulo en terapia electroconvulsiva. Una revisión pragmática. Rev Psiquiatr Salud Ment (Barc.). 2018;11:36–47.