Major depressive disorder (MDD) is a serious psychiatric condition. Its treatment remains a challenge nowadays. Vortioxetine is a novel antidepressant with a unique profile, as it acts as a multimodal serotoninergic agent. Its efficacy in MDD has been established in many short- and long-term studies, with 7 positive, 4 negative and 1 failed randomized controlled trials. Moreover, its ability to modulate a wide range of neurotransmitters (serotonin, dopamine, norepinephrine, histamine, glutamate or GABA) confers vortioxetine pro-cognitive effects. Side effects are also different from conventional antidepressants, according to its low incidence of sexual dysfunction, weight gain or cardiovascular alterations. The aim of this systematic review is to describe the pharmacology, clinical efficacy and safety profile of vortioxetine, as well as its potential effectiveness in improving cognitive symptoms.
El trastorno depresivo mayor (TDM) es una enfermedad psiquiátrica grave cuyo tratamiento sigue siendo un reto en la actualidad. Vortioxetina es un nuevo fármaco antidepresivo con acción multimodal, lo que le confiere un perfil único. Su efectividad antidepresiva ha sido demostrada en varios estudios a corto y largo plazo, con 7 estudios aleatorizados positivos, 4 negativos y uno nulo. Además, su capacidad para modular varios neurotransmisores (serotonina, dopamina, noradrenalina, histamina, glutamato y GABA) le permite actuar sobre dominios como la cognición. Su perfil de efectos adversos es también distinto al de otros antidepresivos convencionales, dado que se relaciona con una baja incidencia de disfunción sexual, aumento de peso o alteraciones cardiovasculares. En esta revisión sistemática se describirán las características farmacológicas de vortioxetina y se detallará la evidencia disponible respecto a su eficacia clínica, su tolerabilidad y su prometedor efecto sobre los síntomas cognitivos.
The lack of response to available antidepressant treatments remains one of the great challenges in psychiatry. Up to 1/3 of patients with major depressive disorder (MDD) do not achieve a complete response, and even in patients in remission, residual cognitive symptoms are commonly observed.1–4 With this need in mind, and thanks to the advance in knowledge of the neural circuits involved in MDD,5 molecules with a mechanism of action that goes beyond serotonergic activity are emerging.6 The monoaminergic system, which includes the serotonergic, noradrenergic and dopaminergic pathways, is widely spread across the brain and it is known that this system and its multiple interconnections play an important role in mood disorders. This more integrative concept has motivated the search for drugs that will modulate the neurotransmitters involved in regulation of the monoaminergic system, such as glutamate or γ-aminobutyric acid (GABA).7 Thus, the antidepressant, Vortioxetine, has recently been introduced on to the market. This drug is characterized precisely by its multimodal mechanism of action. In this systematic review, the pharmacological profile of Vortioxetine will be described; the available information regarding its clinical efficacy and safety will be detailed; and existing evidence will be thoroughly examined, focussing on its promising effect on cognitive symptoms.
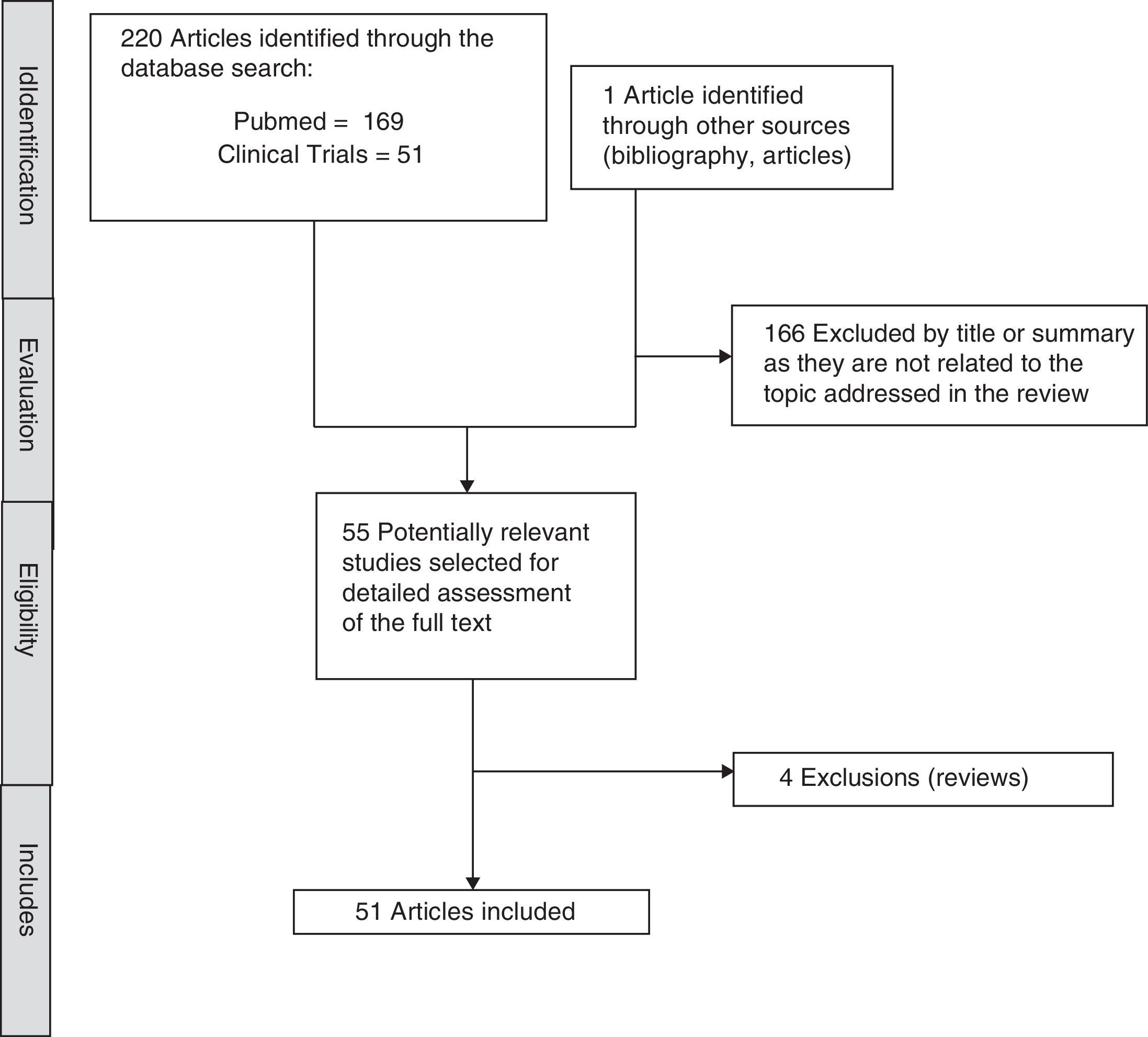
MethodIn order to identify studies focussed on the antidepressant, Vortioxetine, a systematic search of the literature was run. The search was conducted in November 2016 in Pubmed and ClinicalTrials.gov databases. The search terms used were “Vortioxetine AND (“depression” or “depressive” or “action” or “effects” or “cognition”)” for the Pubmed database and “Vortioxetine AND depression” for ClinicalTrials.gov. Preclinical articles were included to assess the effect of Vortioxetine in adults with MDD, published in English, with no date limit. The search was completed by manually reviewing the references of the first selected articles and review articles on the subject, which finally identified 221 articles of which 51 have been included. The selection process is described in detail in Fig. 1.
Pharmacological profilePharmacokineticsVortioxetine is absorbed slowly and has an absolute bioavailability of 75% after oral administration. Food does not influence its pharmacokinetics. The maximum plasma concentration is reached at 7–11h and its half-life is 57h.8 It has a high plasma protein binding rate (96%)9 and is widespread in peripheral tissues (volume of distribution: 2600l).10 Vortioxetine is metabolized in the liver through multiple isoenzymes of cytochrome P-450 (CYP450), such as CYP2D6, CYP3A4/5, CYP2C9, CYP2C19, CYP2A6, CYP2C8 and CYP2B6, with linear kinetics.9,11 Its main metabolite is pharmacologically inactive and it is mostly excreted through the kidneys.8 Stable plasma concentrations are reached at approximately 2 weeks and dose adjustment is not required for age, gender, renal disorders or in the case of mild-moderate hepatic dysfunction.12
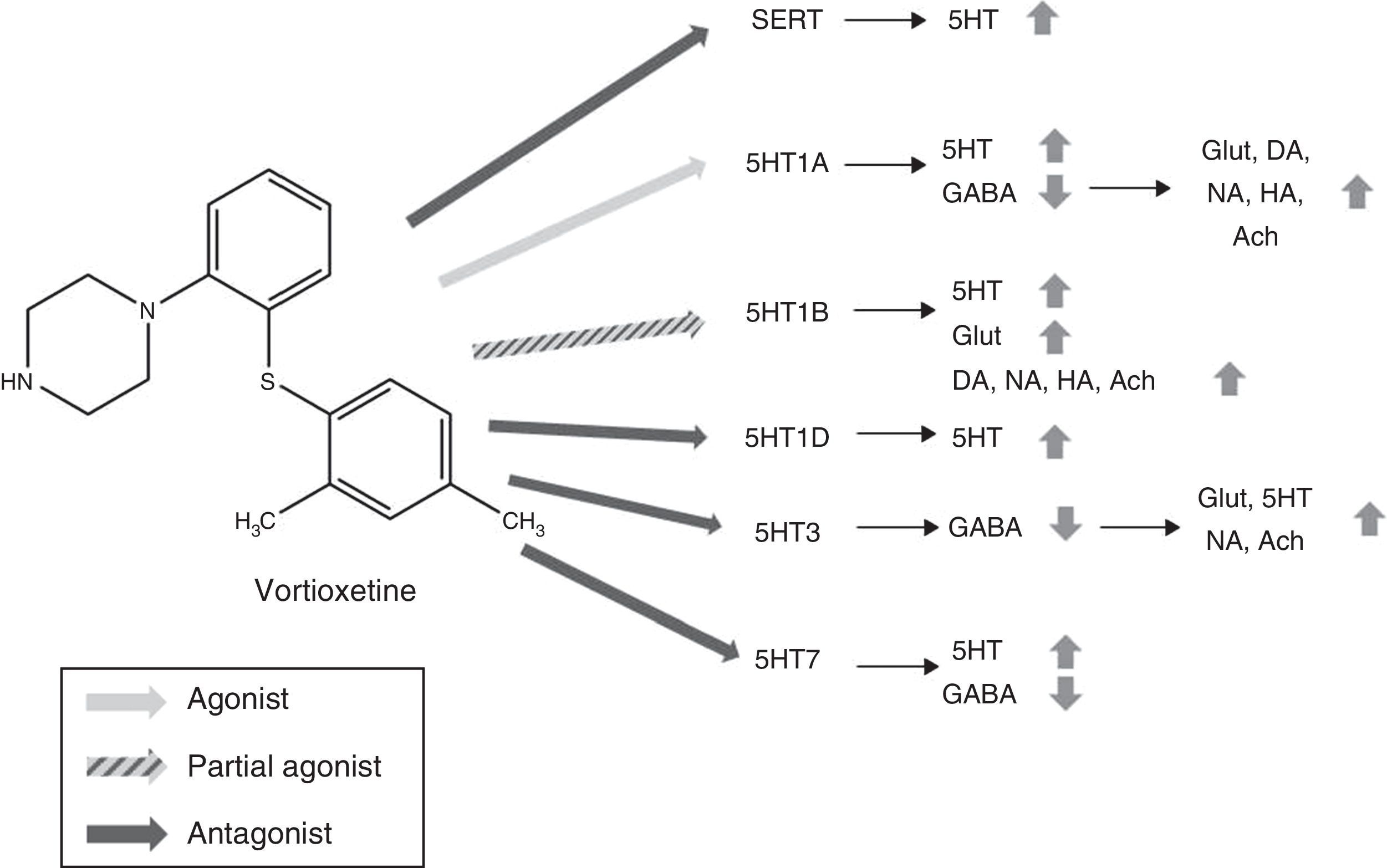
PharmacodynamicsVortioxetine acts as an inhibitor of serotonin transporters (SERT) (Ki=1.6nM) and regulates multiple subtypes of serotonin receptors (5HT): it acts as an agonist of 5HT1A receptors (Ki=15nM), partial agonist of 5HT1B receptors (Ki=33nM) and 5HT1D receptor antagonist (Ki=5.5nM), 5HT3 (Ki=3.7nM) and 5HT7 (Ki=19nM).13–17 As reflected by affinity constants, Vortioxetine has a high affinity for SERTs18 and 5HT3 receptors, so it must be taken into account that these receptors will be inhibited with low doses of Vortioxetine but high doses will be required to occupy all the receptors.9
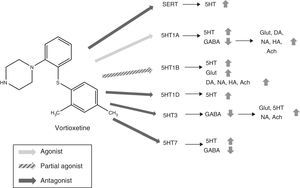
Mechanism of actionVortioxetine presents a unique and complex mechanism of action. Like conventional selective serotonin reuptake inhibitors (SSRIs), it is able to increase serotonin levels through the inhibition of serotonin transporters (SERTs). However, its action on the various subtypes of 5HT receptors gives it characteristic properties and has led to it being considered as a multimodal antidepressant. Fig. 2 summarizes the mechanism of action of Vortioxetine and its effects on various neurotransmitters.
Mechanism of action proposed for Vortioxetine and its effects on various neurotransmitters. SERT antagonist: blocking SERT causes an increase of 5HT levels in all 5HT presynaptic nerve ends. 5HT1A agonist: stimulation of the presynaptic autoreceptors of 5HT1A causes their desensitization, interrupting negative feedback and thus inducing an increase in the release of serotonin. The stimulation of postsynaptic 5HT1A receptors, on the other hand, inhibits GABAergic interneurons, which favours the release of Glut, NA, DA, Ach and HA in the prefrontal cortex. 5HT1B partial agonist: blocking 5HT1B autoreceptors due to its action as a partial agonist (or functional antagonist) increases the concentration of 5HT. The partial agonist action of Vortioxetine on the postsynaptic 5HT1B heteroreceptors, located in the GABAergic interneurons increases the release of Glut in the hippocampus and prefrontal cortex. Blocking these postsynaptic 5HT1B heteroreceptors also increases the release of Ach, NA, DA and HA. 5HT1D antagonist: blocking the autoreceptors of 5HT1D increases the release of 5HT. 5HT3 Antagonist: the potent blocking of 5HT3 receptors is the main mechanism by which the release of Glut is favoured in the hippocampus and prefrontal cortex, thanks to the interruption of a large population of GABA interneurons. This, in turn, leads to an increase in NA and Ach levels. 5HT7 Antagonist: Blocking the 5HT7 receptors located in the GABAergic neurons of the raphe nuclei increases the release of 5HT. 5HT: serotonin; Ach: acetylcholine; DA: dopamine; GABA: γ-aminobutyric acid; Glut: glutamate; HA: histamine; NA: noradrenaline; SERT: serotonin reuptake transporters.
5HT neurons abound in the brain, especially in the prefrontal cortex and the hippocampus—areas of the brain involved in both affective and cognitive symptoms of TDM22—and interact with a large number of glutamatergic and GABAergic neurons.19 It has been proposed that Vortioxetine could inhibit the release of GABA, the main inhibitory neurotransmitter in the body, through the stimulation of 5HT1A receptors, and this would cause, secondarily, the release of dopamine (DA), noradrenaline (NA), histamine (HA) and acetylcholine (Ach) in the prefrontal cortex.20 Blocking the 5HT1B heteroreceptors is considered another mechanism through which Vortioxetine would increase the release of DA, NA, Ach and HA.20 Preclinical studies support these hypotheses, since it has been observed that Vortioxetine increases the levels of Ach, HA, DA and NA in the hippocampus and medial prefrontal cortex measured by microdialysis.23–25 Likewise, blocking the 5HT3 receptor, which is expressed selectively in GABAergic interneurons,26 interrupts GABAergic inhibition and increases the release of glutamate and secondarily of 5HT,19,26 NA and Ach.21 On the other hand, Vortioxetine blocks self-regulating systems (5HT1B, 5HT1D, 5HT7),17 which halts the negative feedback that drugs like the SSRIs suffer from and which limit their action.9 In summary, Vortioxetine's multiple sites of action would succeed in increasing the levels of 5HT, DA, NA, Ach and HA in various neural networks in the brain. Likewise, its ability to increase the release of serotonin through the modulation of several receptors, not only acting on SERTs. This enables therapeutic doses to block SERTs at a much lower level than in the case of SSRIs. This would favour better tolerance.
EfficacyPreclinical studies have shown that Vortioxetine produces anxiolytic and antidepressant effects,14,27 this effect being more pronounced than with fluoxetine.27
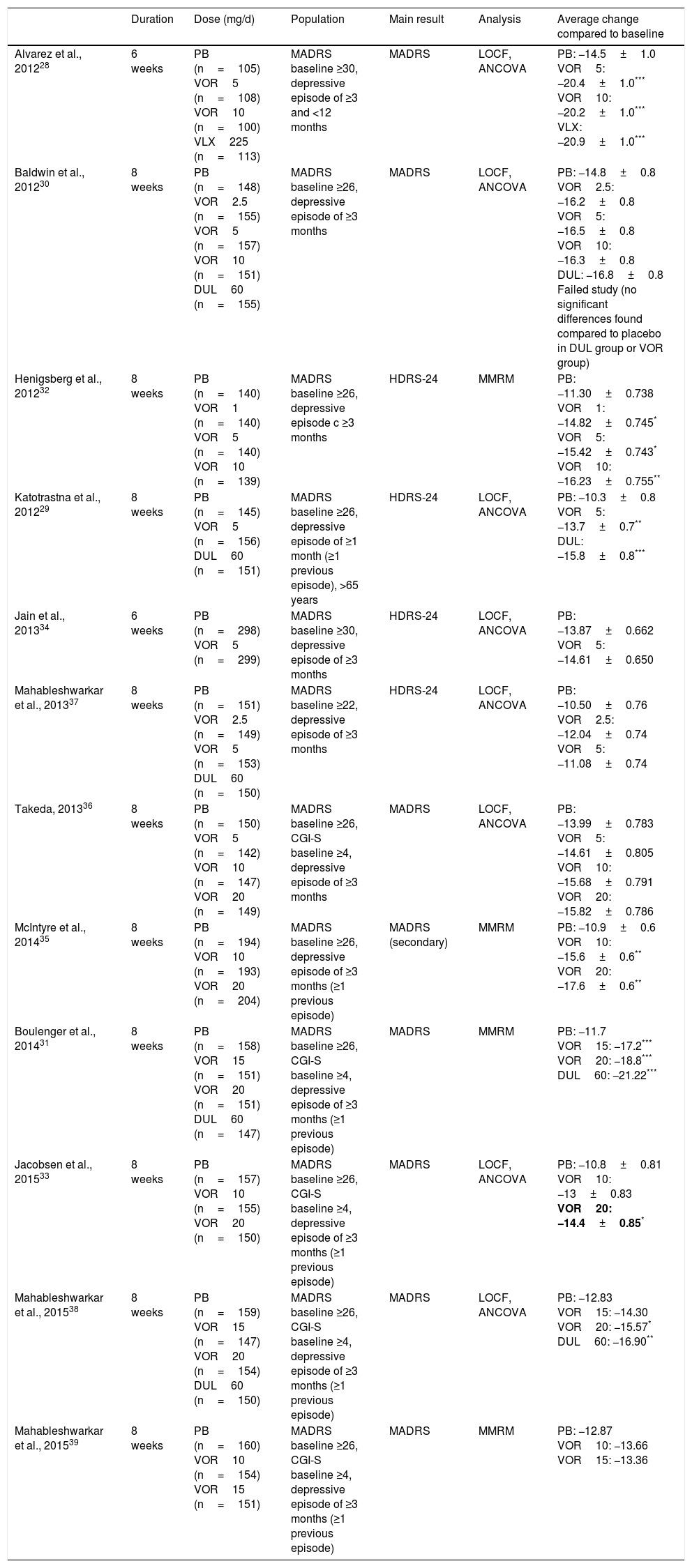
To date,12 randomized, double-blind, placebo-controlled studies have been published assessing as a primary or secondary outcome the changes in depressive symptoms in adult patients with MDD, treated with Vortioxetine. The main characteristics and results of these studies are summarized in Table 1. The average duration of these studies was 6–8 weeks and the range of doses used was between 2.5 and 20mg/d.28–39 In 8 of the 12 studies, the main result chosen was the difference in the total final score on the Montgomery-Asberg Depression Scale (MADRS) with respect to the baseline.28,30,31,33,35,36,38,39 Seven of the 12 studies were positive28,30–33,35,38 while 5 did not find any significant differences when compare to the placebo.30,34,36,37,39 Of these 5 studies, one was considered unsuccessful, given that neither Vortioxetine nor duloxetine (active comparator) showed significant differences compared to the placebo in reducing the MADRS score.30 When interpreting this data, however, we must bear in mind that it is common to find negative results in clinical trials in MDD, even with drugs considered effective in the treatment of depression, as indicated by the analysis carried out by Khin et al.40 A recent meta-analysis of 11 of the 12 clinical trials mentioned above supports the short-term efficacy of Vortioxetine vs placebo, with the effect of the treatment being greater with higher doses.41 Likewise, a higher response and remission rate was found in patients treated with Vortioxetine, as compared to placebo, as well as a more pronounced improvement in scales such as the Clinical Global Impression Scale (CGI-I, CGI-S). This same meta-analysis carried out an exploratory analysis and demonstrated a significant response to treatment for all doses of Vortioxetine. There has been only one randomized clinical trial carried out on an elderly population,29 not included in the previous meta-analysis, where patients received Vortioxetine at a dose of 5mg/d for 8 weeks. In line with the meta-analysis results of Thase et al.,41 both the reduction in the MADRS score and remission and response rates were significantly higher in the group treated with Vortioxetine than in the group treated with placebo. Vortioxetine has also been shown not to be inferior to other active reference drugs,42 such as venlafaxine,43 escitalopram44 or agomelatine,45 although this data should be interpreted with caution due to the design of the studies. In cost-efficacy studies, this drug was superior to venlafaxine XR46 and in cost-benefit studies it has shown itself to be superior to agomelatine, bupropion, sertraline and venlafaxine.47 Regarding the long-term efficacy of Vortioxetine, there are open studies where Vortioxetine (2.5–20mg/d) has been shown to maintain efficacy after 52 weeks of treatment48,49 and to be effective in the prevention of relapse in MDD.50
Efficacy of Vortioxetine vs placebo in randomized, double blind, placebo controlled clinical trials on TDM.
| Duration | Dose (mg/d) | Population | Main result | Analysis | Average change compared to baseline | |
|---|---|---|---|---|---|---|
| Alvarez et al., 201228 | 6 weeks | PB (n=105) VOR5 (n=108) VOR10 (n=100) VLX225 (n=113) | MADRS baseline ≥30, depressive episode of ≥3 and <12 months | MADRS | LOCF, ANCOVA | PB: −14.5±1.0 VOR5: −20.4±1.0*** VOR10: −20.2±1.0*** VLX: −20.9±1.0*** |
| Baldwin et al., 201230 | 8 weeks | PB (n=148) VOR2.5 (n=155) VOR5 (n=157) VOR10 (n=151) DUL60 (n=155) | MADRS baseline ≥26, depressive episode of ≥3 months | MADRS | LOCF, ANCOVA | PB: −14.8±0.8 VOR2.5: −16.2±0.8 VOR5: −16.5±0.8 VOR10: −16.3±0.8 DUL: −16.8±0.8 Failed study (no significant differences found compared to placebo in DUL group or VOR group) |
| Henigsberg et al., 201232 | 8 weeks | PB (n=140) VOR1 (n=140) VOR5 (n=140) VOR10 (n=139) | MADRS baseline ≥26, depressive episode c ≥3 months | HDRS-24 | MMRM | PB: −11.30±0.738 VOR1: −14.82±0.745* VOR5: −15.42±0.743* VOR10: −16.23±0.755** |
| Katotrastna et al., 201229 | 8 weeks | PB (n=145) VOR5 (n=156) DUL60 (n=151) | MADRS baseline ≥26, depressive episode of ≥1 month (≥1 previous episode), >65 years | HDRS-24 | LOCF, ANCOVA | PB: −10.3±0.8 VOR5: −13.7±0.7** DUL: −15.8±0.8*** |
| Jain et al., 201334 | 6 weeks | PB (n=298) VOR5 (n=299) | MADRS baseline ≥30, depressive episode of ≥3 months | HDRS-24 | LOCF, ANCOVA | PB: −13.87±0.662 VOR5: −14.61±0.650 |
| Mahableshwarkar et al., 201337 | 8 weeks | PB (n=151) VOR2.5 (n=149) VOR5 (n=153) DUL60 (n=150) | MADRS baseline ≥22, depressive episode of ≥3 months | HDRS-24 | LOCF, ANCOVA | PB: −10.50±0.76 VOR2.5: −12.04±0.74 VOR5: −11.08±0.74 |
| Takeda, 201336 | 8 weeks | PB (n=150) VOR5 (n=142) VOR10 (n=147) VOR20 (n=149) | MADRS baseline ≥26, CGI-S baseline ≥4, depressive episode of ≥3 months | MADRS | LOCF, ANCOVA | PB: −13.99±0.783 VOR5: −14.61±0.805 VOR10: −15.68±0.791 VOR20: −15.82±0.786 |
| McIntyre et al., 201435 | 8 weeks | PB (n=194) VOR10 (n=193) VOR20 (n=204) | MADRS baseline ≥26, depressive episode of ≥3 months (≥1 previous episode) | MADRS (secondary) | MMRM | PB: −10.9±0.6 VOR10: −15.6±0.6** VOR20: −17.6±0.6** |
| Boulenger et al., 201431 | 8 weeks | PB (n=158) VOR15 (n=151) VOR20 (n=151) DUL60 (n=147) | MADRS baseline ≥26, CGI-S baseline ≥4, depressive episode of ≥3 months (≥1 previous episode) | MADRS | MMRM | PB: −11.7 VOR15: −17.2*** VOR20: −18.8*** DUL60: −21.22*** |
| Jacobsen et al., 201533 | 8 weeks | PB (n=157) VOR10 (n=155) VOR20 (n=150) | MADRS baseline ≥26, CGI-S baseline ≥4, depressive episode of ≥3 months (≥1 previous episode) | MADRS | LOCF, ANCOVA | PB: −10.8±0.81 VOR10: −13±0.83 VOR20: −14.4±0.85* |
| Mahableshwarkar et al., 201538 | 8 weeks | PB (n=159) VOR15 (n=147) VOR20 (n=154) DUL60 (n=150) | MADRS baseline ≥26, CGI-S baseline ≥4, depressive episode of ≥3 months (≥1 previous episode) | MADRS | LOCF, ANCOVA | PB: −12.83 VOR15: −14.30 VOR20: −15.57* DUL60: −16.90** |
| Mahableshwarkar et al., 201539 | 8 weeks | PB (n=160) VOR10 (n=154) VOR15 (n=151) | MADRS baseline ≥26, CGI-S baseline ≥4, depressive episode of ≥3 months (≥1 previous episode) | MADRS | MMRM | PB: −12.87 VOR10: −13.66 VOR15: −13.36 |
ANCOVA: analysis of covariance; CGI-S: Clinical Global Impressions-Severity of Illness Scale; DSST: Digit Symbol Substitution Test; DUL: duloxetina; HDRS: Hamilton Depression Rating Scale; LOCF: last observation carried forward; MADRS: Montgomery-Asberg Depression Scale; MMRM: modelo mixto para mediciones repetidas (mixed model for repeated measurements); PB: placebo; RAVLT: Rey Auditory Verbal Learning Test; TDM: trastorno depressive major; VLX: venlafaxina; VOR: vortioxetine.
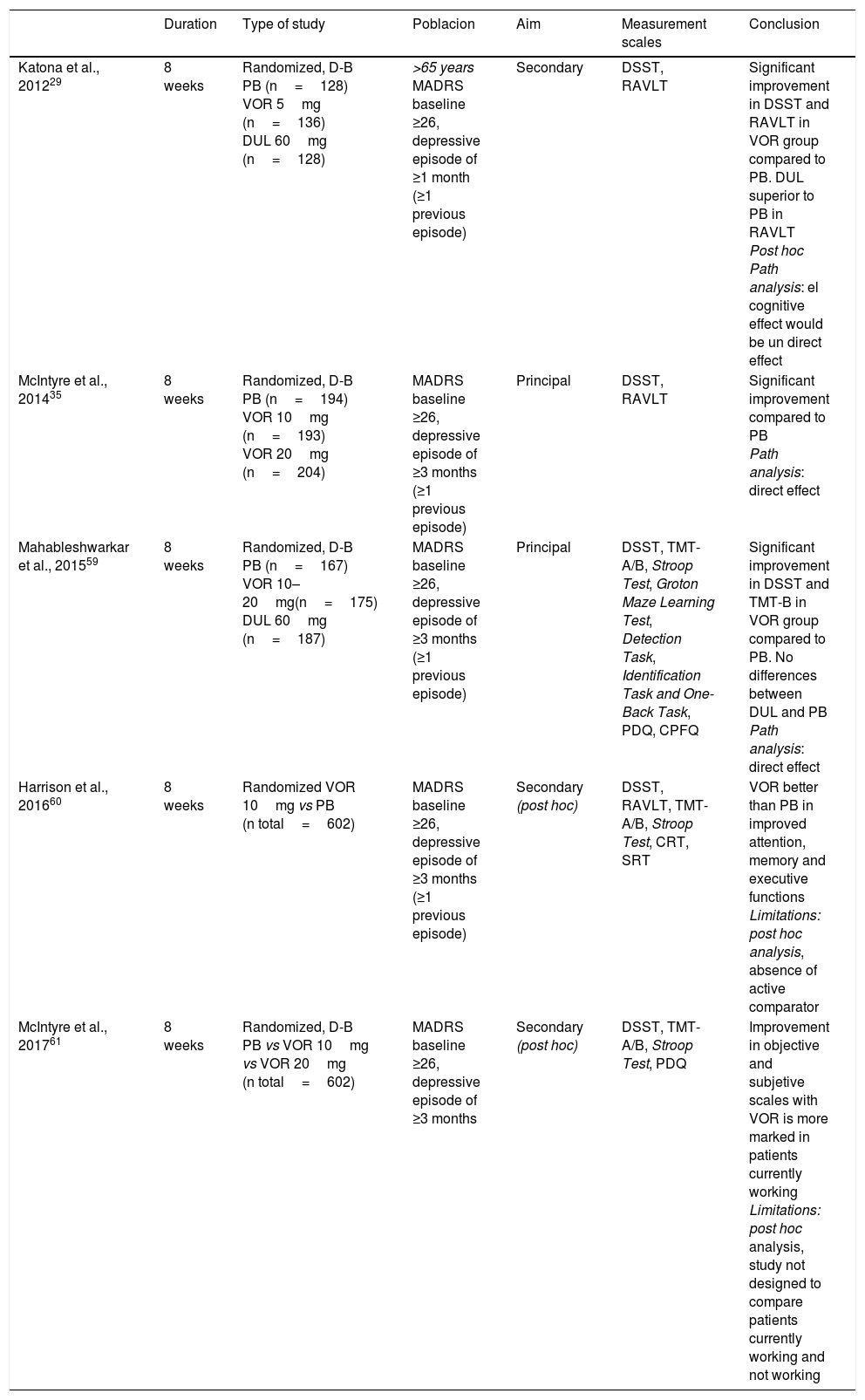
Vortioxetine se Vortioxetine is a good alternative in the treatment of neurocognitive deficits in MDD, according to preclinical and clinical studies, mainly thanks to its antagonist action of 5HT3 and 5HT7 receptors and as a 5HT1A receptor agonist through which it modulates glutamatergic transmission.51 In preclinical studies, Vortioxetine improves learning difficulties and prevents deficits in learning induced by stress.52 This suggests a positive effect for Vortioxetine on deficits in cognitive flexibility, one of the main cognitive symptoms in MDD. A positive effect on memory has also been observed,23,53 greater than with escitalopram or duloxetine,54 through its action as an 5HT1A agonist, and as a 5HT353 antagonist. In addition, Vortioxetine would favour neuroplasticity55 more rapidly than other SSRIs.16,56,57 In clinical studies, Vortioxetine has been shown to have no negative effects on cognition.58 A randomized clinical trial assessed as a main result the effect of 8 weeks of treatment with Vortioxetine 10mg, Vortioxetine 20mg or placebo on cognition in people with moderate to severe major depressive episodes.35 The group treated with Vortioxetine, regardless of the dose, obtained significantly higher scores in the Digit Symbol Substitution Test (DSST), which assesses processing speed, executive functions and attention, and in the King Auditory Verbal Learning Test (RAVLT), which assesses learning and memory. Similarly, in the study carried out by Mahableshwarkar et al.59 Vortioxetine, at a dose of 10–20mg/d was superior to placebo in the DSST; in addition, the group treated with Vortioxetine showed a marked improvement in functional capacity. The active reference drug, duloxetine, did not show any superiority to placebo either in the DSST or on the functional capacity scale. In elderly patients with MDD, Vortioxetine was similarly superior to placebo in DSST and RAVLT. Again, duloxetine, used as an active reference drug, did not show differences in comparison with placebo in the DSST scores but did in the case of RAVLT.29 In view of these results, it has been suggested that Vortioxetine exerts positive effects in more cognitive domains than other antidepressants.8 In fact, post hoc analyses indicate that Vortioxetine does not seem to act on a specific cognitive domain but rather on a wide variety of domains,60 and that it is more useful in patients who are actively employed.61 The main characteristics and results of studies evaluating the effect of Vortioxetine on cognition are summarized in Table 2. Importantly, a recent meta-analysis has shown that the favourable effects of Vortioxetine on cognition are independent of its antidepressant effects.62
Efficacy of Vortioxetine vs placebo on cognitive domains in randomized, placebo-controlled clinical trials on MDD.
| Duration | Type of study | Poblacion | Aim | Measurement scales | Conclusion | |
|---|---|---|---|---|---|---|
| Katona et al., 201229 | 8 weeks | Randomized, D-B PB (n=128) VOR 5mg (n=136) DUL 60mg (n=128) | >65 years MADRS baseline ≥26, depressive episode of ≥1 month (≥1 previous episode) | Secondary | DSST, RAVLT | Significant improvement in DSST and RAVLT in VOR group compared to PB. DUL superior to PB in RAVLT Post hoc Path analysis: el cognitive effect would be un direct effect |
| McIntyre et al., 201435 | 8 weeks | Randomized, D-B PB (n=194) VOR 10mg (n=193) VOR 20mg (n=204) | MADRS baseline ≥26, depressive episode of ≥3 months (≥1 previous episode) | Principal | DSST, RAVLT | Significant improvement compared to PB Path analysis: direct effect |
| Mahableshwarkar et al., 201559 | 8 weeks | Randomized, D-B PB (n=167) VOR 10–20mg(n=175) DUL 60mg (n=187) | MADRS baseline ≥26, depressive episode of ≥3 months (≥1 previous episode) | Principal | DSST, TMT-A/B, Stroop Test, Groton Maze Learning Test, Detection Task, Identification Task and One-Back Task, PDQ, CPFQ | Significant improvement in DSST and TMT-B in VOR group compared to PB. No differences between DUL and PB Path analysis: direct effect |
| Harrison et al., 201660 | 8 weeks | Randomized VOR 10mg vs PB (n total=602) | MADRS baseline ≥26, depressive episode of ≥3 months (≥1 previous episode) | Secondary (post hoc) | DSST, RAVLT, TMT-A/B, Stroop Test, CRT, SRT | VOR better than PB in improved attention, memory and executive functions Limitations: post hoc analysis, absence of active comparator |
| McIntyre et al., 201761 | 8 weeks | Randomized, D-B PB vs VOR 10mg vs VOR 20mg (n total=602) | MADRS baseline ≥26, depressive episode of ≥3 months | Secondary (post hoc) | DSST, TMT-A/B, Stroop Test, PDQ | Improvement in objective and subjetive scales with VOR is more marked in patients currently working Limitations: post hoc analysis, study not designed to compare patients currently working and not working |
CPFQ: Cognitive and Physical Functioning Questionnaire; CRT: Choice Reaction Time Test; D-B: double blind; DSST: Digit Symbol Substitution Test; DUL: duloxetina; MADRS: Montgomery-Asberg Depression Scale; PB: placebo; PDQ: Perceived Deficits Questionnaire; RAVLT: Rey Auditory Verbal Learning Test; SRT: Buschke Selective Reminding Test; TMT-A/B: Trail Making Test A/B; VOR: vortioxetine.
Baldwin et al.63 conducted a meta-analysis to analyze the safety and tolerability of Vortioxetine in MDD, where they included a total of 11 short-term randomized, double-blind, placebo-controlled studies and 5 open-label studies with a follow-up time of up to 52 weeks In short-term studies, adverse events were generally classified as “mild or moderate”. The most frequent were nausea and vomiting, with a transitory, dose-dependent effect. The proportion of patients with side effects classified as severe was 4.6% for the placebo group, 5.8% for the Vortioxetine group, 8.2% for the duloxetine group and 11.5% for the venlafaxine XR group. Similarly, the number needed to harm (NNH), based on the number of patients who discontinued treatment due to an adverse event, was markedly higher in the 5–10mg Vortioxetine group than for the active comparators, duloxetine and venlafaxine XR. Regarding serious adverse events, the incidence in patients treated with Vortioxetine (5–20mg) was similar to that of placebo (0.6% vs 0.5%), regardless of the dose. No significant differences were found in the incidence of suicidal ideation or suicidal behaviour in the Vortioxetine group, as compared to placebo. Treatment with Vortioxetine was also not associated with greater weight gain or with an increased incidence of insomnia, akathisia, dyskinesia, hostility or aggressive behaviour, compared to placebo. The incidence of sexual dysfunction was similar to that of placebo for all doses of Vortioxetine (5–20mg). Vortioxetine presented a cardiovascular safety profile comparable to that of placebo.
The incidence of sexual dysfunction was similar to that of placebo for all doses of Vortioxetine (5–20mg) and Vortioxetine presented a cardiovascular safety profile comparable to that of placebo. The incidence of discontinuation symptoms was also comparable to that of placebo, probably due to the long half-life of Vortioxetine. Of the total of 3018 patients treated with Vortioxetine, one single patient presented hypomanic symptoms and none had a clear manic episode. In long-term open studies, the most common adverse events for both dose ranges were again nausea (16.3 and 24.2%), followed by headache (13.0 and 12.5%), diarrhoea (6.4 and 7.3%), nasopharyngitis (10.9 and 6.4%), weight gain (5.7 and 5.9%) and insomnia (5.0 and 7.1%). The proportion of patients with sexual dysfunction was 1.7% in the Vortioxetine 5–10mg group and 2.3% in the Vortioxetine 15–20mg group. The changes in cardiovascular parameters with respect to the baseline were not clinically relevant. No adverse events of a new type were observed either. The incidence of serious adverse events was 2.9% for Vortioxetine 5–10mg and 2.2% for Vortioxetine 15–20mg.
InteractionsVortioxetine is metabolized mainly through CYP450 isoenzymes, which also intervene in the metabolism of many other molecules. However, Vortioxetine has little or no effect on these isoforms (that is, it does not induce or inhibit their activity), so it would not be expected to interfere in the plasma concentrations of other drugs metabolized by these enzymes.64 Several studies have analyzed the possible interactions of Vortioxetine. Chen et al.65 demonstrated that co-administration of Vortioxetine with ethanol, diazepam or lithium was well tolerated and did not present clinically relevant pharmacokinetic or pharmacodynamic interactions. No pharmacokinetic interactions were found with the antifungals, fluconazole or ketoconazole, both isoenzyme inhibitors of CYP45066, or with aspirin, warfarin,67 the contraceptives, levonorgestrel/ethinyl estradiol or omeprazole.66 However, these studies suggest that dose adjustments may be required when administered with bupropion (CYP2D6 inhibitor and CYP2B6 substrate) or rifampicin (CYP inducer).66
ConclusionsThis review, focussed on available evidence on Vortioxetine, supports its antidepressant efficacy and tolerability in the short and long term, with a dose-dependent antidepressant effect. Long-term studies also show that the number of relapses decreases, compared to placebo. In terms of side effects, the most frequent are nausea and vomiting, with a transient and dose-dependent effect. Vortioxetine does not appear to produce cardiovascular changes or induce significant weight gain; In addition, it would be associated with fewer sexually-related side effects, at least at low doses, so this would be an alternative to consider in patients with inadequate adherence to treatment due to complaints of sexual dysfunction.9 Furthermore, the prolonged half-life of Vortioxetine facilitates administration in a single daily dose and decreases the risk of discontinuation syndrome.68 Its combined action as a serotonin reuptake inhibitor and as a modulator of a wide variety of serotonergic receptors gives it a broader pharmacological profile than other antidepressants, making Vortioxetine an attractive alternative in the case of lack of response to conventional antidepressants. Nevertheless, more studies would be required in patients with resistant MDD to assess its true usefulness in this population. Interestingly, several preclinical and clinical studies suggest that Vortioxetine also has a procognitive action, which appears to be independent of its effect on depressive symptoms. This may be due to its indirect action on the release of glutamate, exclusive to Vortioxetine, which would increase neuronal plasticity and improve memory.19 Thus, Vortioxetine would be a useful therapeutic option for patients with MDD and marked cognitive symptoms,69 which usually affect the patient's psychosocial functioning.70,71 In addition, cost-efficacy and cost-benefit studies have been run in recent years to support the superiority of Vortioxetine in the increase in life years, adjusted for quality of life (QALY) and functionality.46,47 Despite its promising action on cognition72 and its sex-related side effects,73 more studies are needed to confirm the available findings and to assess the mechanisms through which Vortioxetine would produce this effect. In addition, it would be interesting to have studies designed specifically to compare the efficacy of Vortioxetine, as compared to other antidepressants.68 Future studies will shed some light on the relationship between Vortioxetine and cognition,74,75 as well as its efficacy and tolerability in the short and long term.76–81 In conclusion, taking into account the receptor and side effect profile of Vortioxetine, this drug appears to be a great ally of personalized psychiatry.82,83 Thus, Vortioxetine would be particularly indicated for specific patient profiles, such as those with poor response to conventional antidepressants, patients with complaints of cognitive dysfunction or patients with poor tolerance to other antidepressant treatments due to sexually related side effects, such as has been noted in previous reviews.9,68,69,84 In addition, its procognitive action and the low incidence of somnolence or heart-related disorders make it an alternative to consider in the elderly population,68,84 although only one clinical trial has been conducted on this population, with positive results and good tolerability.29 In the future, it should undoubtedly be tested for other indications beyond TDM and anxiety disorders, and especially for indications where antidepressant and eventually procognitive effects may be a key advantage over existing alternatives, such as in bipolar depression and attention deficit hyperactivity disorder (ADHD).
Conflict of interestDr. Íria Grande has received a Juan Rodés JR15/00012 research contract and a Healthcare Research Project PI16/00187 grant from the Carlos III Health Institute, Spanish Ministry of Economy, Industry and Competitiveness and has worked as a consultant for Ferrer and as speaker for AstraZeneca, Ferrer and Janssen-Cilag.
Dr. Eduard Vieta has received grants and collaborated as an advisor or speaker for the following companies: AB-Biotics, Actavis, Allergan, AstraZeneca, Bristol-Myers Squibb, Ferrer, Forest Research Institute, Gedeon Richter, Glaxo-Smith-Kline, Janssen, Lundbeck, Otsuka, Pfizer, Roche, Sanofi-Aventis, Servier, Shire, Sunovion, Takeda, Telefónica, the Brain and Behaviour Foundation, the Spanish Ministry of Science and Innovation (CIBERSAM), the Seventh European Framework Programme (ENBREC), and the Stanley Medical Research Institute.
Dr. Íria Grande would like to thank the Carlos III Health Institute, the Spanish Ministry of Economy, Industry and Competitiveness for the Juan Rodés JR15/00012 research contract and the Health Research Project PI16/00187 grant; the Centre for Biomedical Research into the Mental Health Network (CIBERSAM); the Grups Consolidats de Recerca 2014 (SGR 398); and the Seventh European Framework Programme (ENBREC). Dr. Eduard Vieta would like to thank the Carlos III Health Institute, the Spanish Ministry of Economy, Industry and Competitiveness (PI 12/00912) within the National R&D&I plan and co-financed by the ISCIII-General Sub-directorate for Assessment and the European Regional Development Fund (ERDF); the Centre for Biomedical Research into the Mental Health Network (CIBERSAM); the Secretary for Universities and Research within the Department of Economics and Knowledge Transfer (2014_SGR_398); the Seventh European Framework Programme (ENBREC); the CERCA Programme/the Catalonian Regional Government; and the Stanley Medical Research Institute for their support.
Please cite this article as: Salagre E, Grande I, Solé B, Sanchez-Moreno J, Vieta E. Vortioxetina: una nueva alternativa en el trastorno depresivo mayor. Rev Psiquiatr Salud Ment (Barc.). 2018;11:48–59.