Ductal carcinoma in situ (DCIS) accounts for 20% of new breast cancer diagnoses. Treatment includes breast-conserving surgery (BCS) with or without radiation therapy (RT), mastectomy, and sentinel lymph node biopsy. Surgical decisions depend on the centre's policy and patient choice. We aimed to compare outcomes and quality-of-life (QoL) between treatment with BCS+RT or mastectomy in patients with breast DCIS.
MethodsWe conducted a retrospective study of 155 patients with DCIS from January 2009 to December 2018. Among them, 83 were treated with mastectomy and 72 with BCS+RT. Disease-free survival (DFS), local-recurrence-free survival, and overall survival (OS) were recorded. Statistics: Kaplan–Meier, log rank.
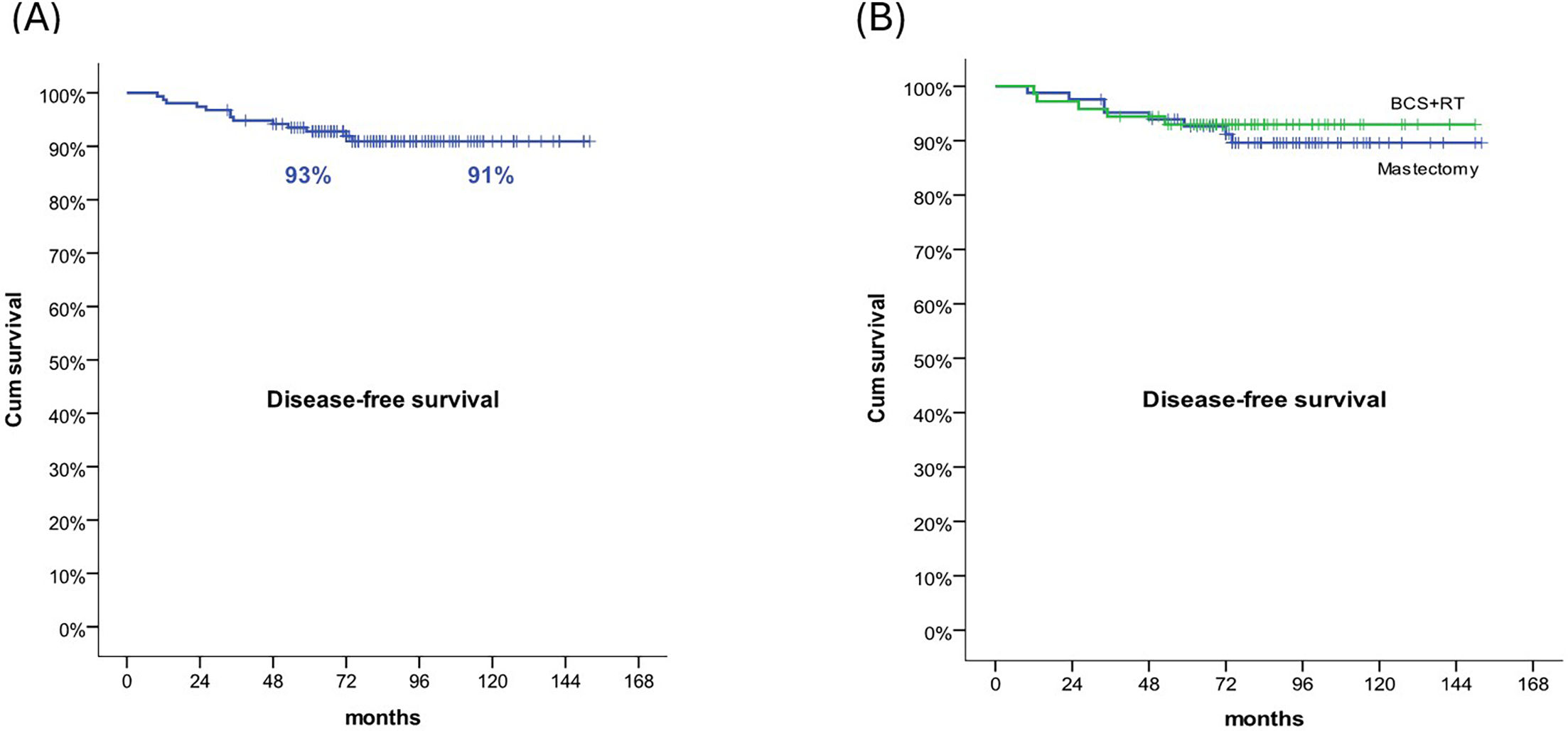
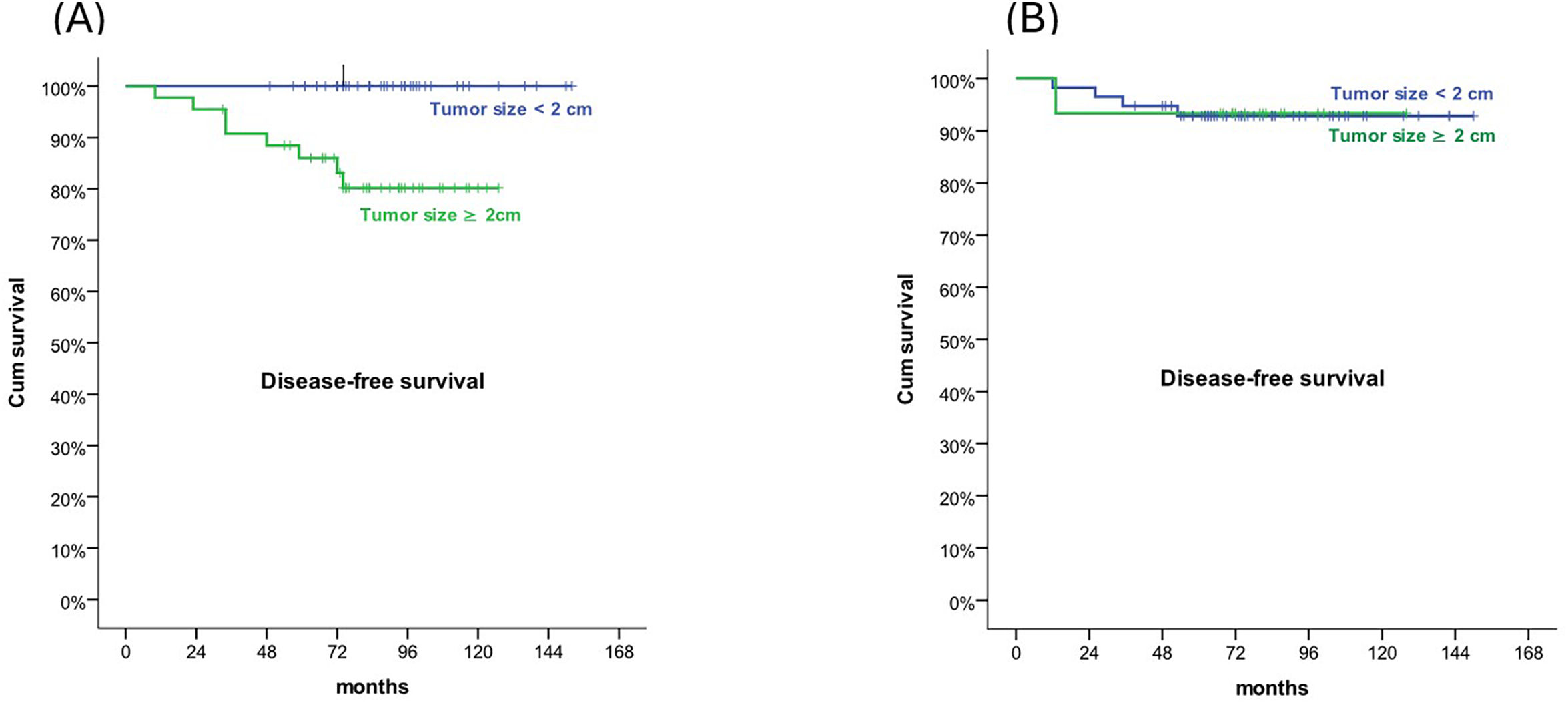
ResultsMedian follow-up was 82 months (33–152). Five- and 10-year DFS were 93% and 91%, respectively. There were no differences in DFS rate between the 2 groups (9.6% vs 6.9%, p=.38). OS rates at 5- and 10-years was 100% in both groups. Larger tumours were associated with a higher contralateral DCIS relapse (47.3 mm vs 19.3 mm, p=.004). In mastectomy group, patients with tumour size >2 cm had a worse 5- and 10-years DFS than patients with smaller tumours (p=.019). In terms of QoL, there was no difference between the 2 treatment groups.
ConclusionBCS+RT and mastectomy offer similar clinical outcomes and QoL. Our results demonstrate that BCS is a viable option even for patients with larger tumours. These findings serve as a guide for clinical decision-making to optimise the management of DCIS.
El CDIS representa el 20% de los nuevos diagnósticos de cáncer. El tratamiento incluye cirugía conservadora de la mama (CCM) con o sin radioterapia (RT), mastectomía y biopsia del ganglio centinela. Las decisiones quirúrgicas dependen de la política del centro y de la elección del paciente. Nuestro objetivo fue comparar los resultados y la calidad de vida (CdV) entre tratamiento con CCM más RT o mastectomía en las pacientes con DCIS.
MétodosRealizamos un estudio retrospectivo de 155 pacientes con DCIS de enero de 2009 a diciembre de 2018. De entre ellas, 83 fueron tratadas con mastectomía y 72 con CCM + RT. Se registraron la supervivencia libre de enfermedad (SLE), la supervivencia sin recaída local (SSRL) y la supervivencia global (SG). Estadísticas: Kaplan–Meier, log rank.
ResultadosEl seguimiento medio fue 82 meses (33–152). Las SLE a cinco y diez años fueron del 93 y el 91%, respectivamente. No se produjeron diferencias en cuanto a la tasa de SLE entre los dos grupos (9,6% vs 6,9%, p = 0,38). Las tasas de SG a cinco y diez años fueron del 100% em ambos grupos. Los tumores de mayor tamaño estuvieron asociados a una recaída de CDIS contralateral (47,3 mm vs 19,3 mm, p = 0,004). En el grupo de mastectomía, las pacientes con tamaño tumoral >2 cm tuvieron una peor SSE a cinco y diez años que las pacientes con tumores más pequeños (p = 0,019). En términos de CdV, no se produjo diferencia entre los dos grupos de tratamiento.
ConclusiónCCM + RT y mastectomía ofrecen resultados clínicos y CdV similares. Nuestros resultados demuestran que la CCM es una opción viable incluso para pacientes con tumores de mayor tamaño. Estos hallazgos sirven como guía para la toma de decisión clínica, de cara a optimizar el manejo de CDIS.
Ductal carcinoma in situ (DCIS) of the breast comprises a heterogeneous group of breast lesions confined to the ducts and lobules. Histologically, it is characterised by the proliferation of malignant epithelial cells delimited by the basement membrane of the mammary ducts. They can be low- to high-grade lesions that may contain foci of invasive cancer. DCIS is usually classified according to architectural pattern (solid, cribriform, papillary, and micropapillary), tumour grade (high, intermediate, and low), and the presence or absence of comedonecrosis.
DCIS accounts for about 20% of new breast cancer diagnoses.1 Before the widespread use of screening mammography, it was diagnosed by surgical removal of a suspicious breast mass. Nowadays, most of them are diagnosed after identifying microcalcifications in a screening mammogram, which are removed surgically. For this reason, the natural history of unresected DCIS is not widely known. Pending validated progression markers, treatment is based on data such as the patient's age, tumour location and diameter, presence of comedonecrosis, and Van Nuys index (tumour size, margin width, histopathological classification, age).2 Treatment options for DCIS include breast-conserving surgery (BCS) alone, BCS followed by radiation therapy (RT), mastectomy (with or without primary reconstruction), sentinel lymph node biopsy (SLNB), and endocrine therapy. The types of treatment are constantly evolving to reduce the risk of local in situ or invasive relapses and improve aesthetic results and quality-of-life (QoL) for patients.3 In general, the standard of care is surgical resection, either BCT or mastectomy. Both treatments confer similar results in terms of cause-specific mortality at 10 years. Data from population studies indicate that the 10-year breast cancer mortality in patients with DCIS is less than 2% after excision or mastectomy.4,5 There are no randomised studies comparing mastectomy with BCS. Mastectomy achieves excellent local control, with 10-year disease-free survival (DFS) rates of around 96%.6–11 Randomised trials to analyse the benefit of RT to the whole breast irradiation (WBI) after BCS have demonstrated a low risk of local recurrence after its administration.12–16 The benefit of adjuvant WBI on overall survival (OS) has not yet been established,17 so there may be doubts regarding the benefit it provides. The clinical benefit of early detection and treatment for DCIS remains unclear, so the treatment of patients with DCIS is widely debated.
Currently, the decision of the type of surgery is made based on the policy of the centre and the patient's choice. Centres with more experience in breast reconstruction tend to perform mastectomies more frequently. A better knowledge of patient-reported outcomes is necessary to assist the specialist and the patient in decision-making, especially in the case of DCIS, which has very high disease control rates.
This study aimed to retrospectively compare the clinical outcomes and QoL between BCS plus WBI and mastectomy in patients diagnosticated with DCIS in our centre.
MethodsStudy designThe study was a retrospective analysis of patients who were treated with mastectomy or BCS plus adjuvant RT in the management of DCIS in the period between January 2009 and December 2018 in our centre. The study was approved by the Local Ethical Committee.
Patient selectionAll female patients with a DCIS diagnosis by core needle biopsy who underwent breast surgery were retrospectively reviewed and considered for the study. Exclusion criteria were male patients, lobular carcinoma in situ, and presence of microinvasion or invasive carcinoma. A total of 155 patients were finally enrolled after implementing the inclusion and exclusion criteria.
Type of treatmentBased on radiographic characteristics, size of the breast and patient's choice, the treating physician chose for mastectomy or BCS with adjuvant RT. Radiotherapy schemes included 50 Gy, 2 Gy/fx or 42.56 Gy, 2.66 Gy/fx to the whole breast. Based on age and risk factors, some patients received a radiotherapy boost of 10–16 Gy at the lumpectomy site.
Health-related quality of lifeThe European Organisation for Research and Treatment-QoL questionnaire and breast cancer specific module (EORTC QLQ-C30 and QLQ-BR23) were used to measure the health-related quality of life (HRQoL). The EORTC QLQ-C30 is a cancer-specific measure of HRQoL. It consists of 30 items to assess physical, role, emotional, cognitive, and social functioning, global health status or QOL scales, fatigue, pain, nausea and vomiting, dyspnoea, insomnia, appetite loss, constipation, diarrhoea, and financial difficulties.18 The EORTC QLQ-BR23 is a breast-specific module that comprises of 23 questions to assess body image, sexual functioning, sexual enjoyment, future perspective, systemic therapy side effects, breast symptoms, arm symptoms, and upset by hair loss. The letter of invitation and consent forms for the QoL questionnaires were sent to all patients between June 2021 and October 2021. Patients completed QoL questionnaires and returned them to the hospital by post.
Follow-upFollow-up consisted of clinical check-up every 6 months including an annual mammogram, up to at least 5 years after treatment.
Ipsilateral breast tumour recurrence (IBTR) was defined as a recurrent DCIS (DCIS-IBTR) or invasive (invasive-IBTR) carcinoma that occurred after treatment in either the skin or parenchyma of the ipsilateral breast without clinical–radiological evidence of regional or distant disease.
Nodal recurrence was defined as recurrence of DCIS or invasive breast cancer in the ipsilateral regional lymph nodes.
Contralateral breast tumour recurrence (CBTR) is defined as a recurrent DCIS (DCIS-CBTR) or invasive (invasive-CBTR) carcinoma that occurred after treatment in either the skin or parenchyma of the contralateral breast without clinical–radiological evidence of regional or distant disease.
The follow-up time was defined as the time between oncological treatments for DCIS until last follow-up date.
Data analysisDFS, local-recurrence-free survival (LRFS), and OS were recorded. OS was defined as the time from surgery to death or the last follow-up, whereas DFS was defined as the time from surgery to IBRT, CBRT, regional lymph node metastasis, distant metastasis, death, or the last follow-up.
Continuous variables are described using medians. LRFS, DFS, and OS were calculated by the Kaplan–Meier method and comparison of curves by log-rank statistic. p-values <.05 were considered statistically different. All statistical analyses were completed using SPSS version 27.0 (IBM Corp, Armonk, NY).
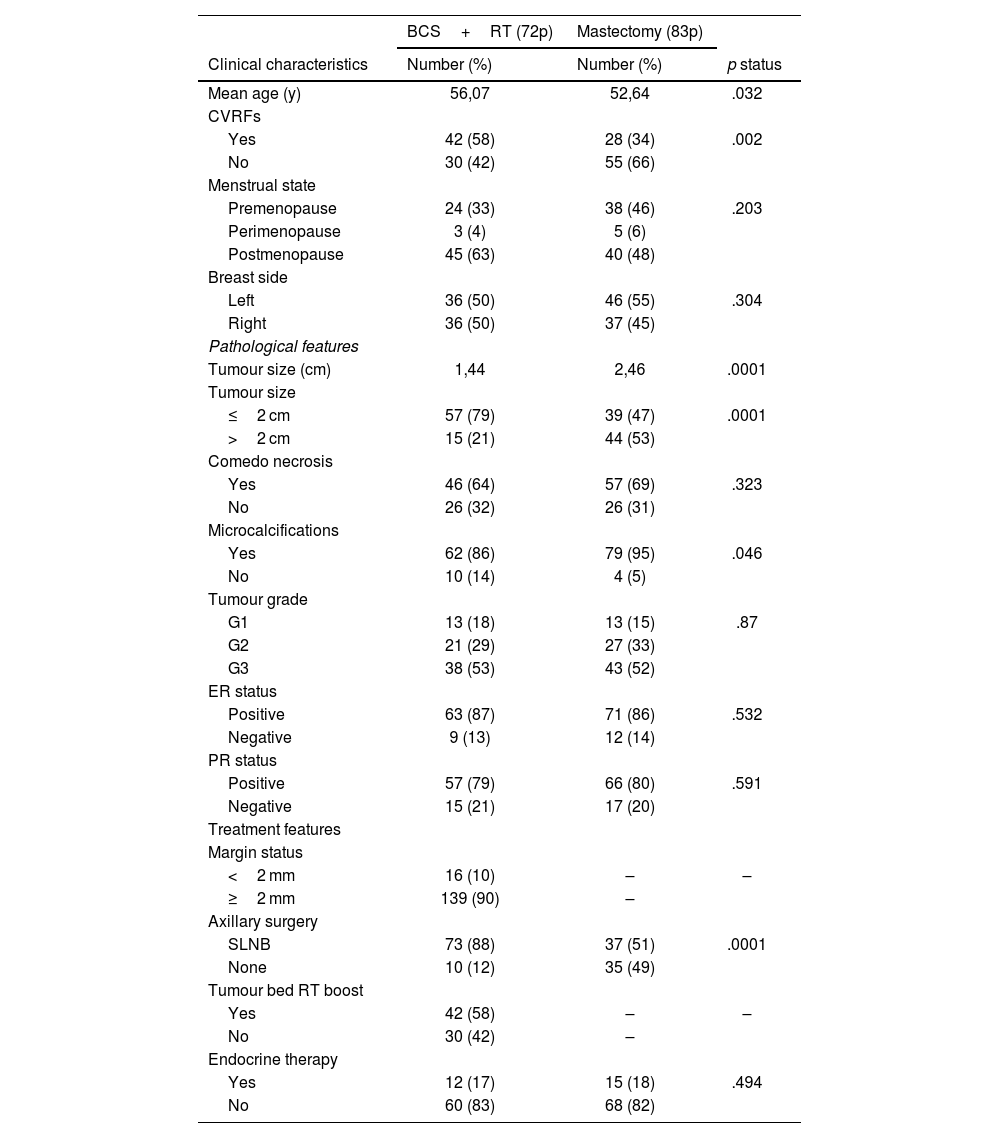
ResultsPatient populationA total of 155 patients with DCIS were identified between January 2009 and December 2018. The median age of the patients was 53 years (range 30–85). Additional clinical, pathological, and treatment characteristics are detailed in Table 1. The 2 groups were not balanced due to a treatment selection bias. Most patients younger and with higher tumoral size underwent mastectomy.
Clinical characteristics and pathological and treatment features.
| BCS+RT (72p) | Mastectomy (83p) | ||
|---|---|---|---|
| Clinical characteristics | Number (%) | Number (%) | p status |
| Mean age (y) | 56,07 | 52,64 | .032 |
| CVRFs | |||
| Yes | 42 (58) | 28 (34) | .002 |
| No | 30 (42) | 55 (66) | |
| Menstrual state | |||
| Premenopause | 24 (33) | 38 (46) | .203 |
| Perimenopause | 3 (4) | 5 (6) | |
| Postmenopause | 45 (63) | 40 (48) | |
| Breast side | |||
| Left | 36 (50) | 46 (55) | .304 |
| Right | 36 (50) | 37 (45) | |
| Pathological features | |||
| Tumour size (cm) | 1,44 | 2,46 | .0001 |
| Tumour size | |||
| ≤2 cm | 57 (79) | 39 (47) | .0001 |
| >2 cm | 15 (21) | 44 (53) | |
| Comedo necrosis | |||
| Yes | 46 (64) | 57 (69) | .323 |
| No | 26 (32) | 26 (31) | |
| Microcalcifications | |||
| Yes | 62 (86) | 79 (95) | .046 |
| No | 10 (14) | 4 (5) | |
| Tumour grade | |||
| G1 | 13 (18) | 13 (15) | .87 |
| G2 | 21 (29) | 27 (33) | |
| G3 | 38 (53) | 43 (52) | |
| ER status | |||
| Positive | 63 (87) | 71 (86) | .532 |
| Negative | 9 (13) | 12 (14) | |
| PR status | |||
| Positive | 57 (79) | 66 (80) | .591 |
| Negative | 15 (21) | 17 (20) | |
| Treatment features | |||
| Margin status | |||
| <2 mm | 16 (10) | – | – |
| ≥2 mm | 139 (90) | – | |
| Axillary surgery | |||
| SLNB | 73 (88) | 37 (51) | .0001 |
| None | 10 (12) | 35 (49) | |
| Tumour bed RT boost | |||
| Yes | 42 (58) | – | – |
| No | 30 (42) | – | |
| Endocrine therapy | |||
| Yes | 12 (17) | 15 (18) | .494 |
| No | 60 (83) | 68 (82) | |
CVRFs: cardiovascular risk factors; ER: oestrogen receptors; PR: progestagen receptors; SLNB: sentinel lymph node biopsy.
In both groups, surgical procedure included breast surgery±axillary lymph node surgery (ALNS). A total of 83 patients (53.5%) underwent a mastectomy, of whom 78.3% (65/83) underwent breast reconstruction. In total, 72 (46.5%) patients underwent BCS, among whom 100% (72/72) underwent post-operative adjuvant WBI. Out of 155 patients, 110 (71%) underwent SLNB. In 45 patients (29%), ALNS was not performed.
Regarding RT treatment, three-dimensional conformal RT technology was used in 100% of the patients. Radiotherapy schemes included 50 Gy, 2 Gy/fx or 42.56 Gy, 2.66 Gy/fx; tumour bed boost consisted of 10–16 Gy, 2 Gy/fx sequentially. Some treatment features are shown in Table 1.
Analysis of survival outcomes and prognostic factorsMedian follow-up was 82 month (32–155). OS was 100% in both groups of treatment.
IBTR was present in 3 out of 155 patients (2%) of which 2 were DCIS and 1 was invasive. DCIS-IBRT was the same in both groups (1 patient in BCS+RT and 1 patient in mastectomy group). None of the prognostic factors analysed (tumour size, comedonecrosis, presence of microcalcifications, tumour grade, hormone receptor status) influenced IBTR or OS. Five- and 10-year ipsilateral DCIS and invasive breast recurrence free survival was 99% in both groups of treatment.
There were 10 cases of CBTR, all of which were pathologically confirmed: 3 cases were DCIS and 7 were invasive carcinomas. For the overall population, 5- and 10-year contralateral DCIS and invasive breast recurrence free survival was 99% and 97%, and 95% and 95%, respectively. Only 2 patients presented with nodal relapse (1 nodal relapse in the mastectomy group and 1 in the BCS group). Nodal relapse-free survival was 99% in both groups. Only 1 patient presented distant metastases.
Five- and 10-year DFS were 93% and 91%, respectively. There were no differences in DFS rate between the 2 groups (9.6% vs 6.9%, p=.38). No differences were found between groups regarding 5- and 10-years DFS (93% for both groups) (Fig. 1). Patients with recurrences had a higher tumour size (31 mm vs 18.89 mm, p=.016). Larger tumours were associated with a higher contralateral DCIS relapse (47.3 mm vs 19.3 mm, p=.004). In mastectomy group, patients with tumour size >2 cm had a worse 5- and 10-year DFS than patients with smaller tumours (86% and 80% vs 100% and 100%, respectively, p=.019) (Fig. 2). In the mastectomy group, 5- and 10-year DFS was 88% in patients treated with SLNB and 100% in patients with no ALNS. In BCS+RT group, 5- and 10-year DFS was 92% in SLNB and 94% in patients with no ALNS (no significant differences).
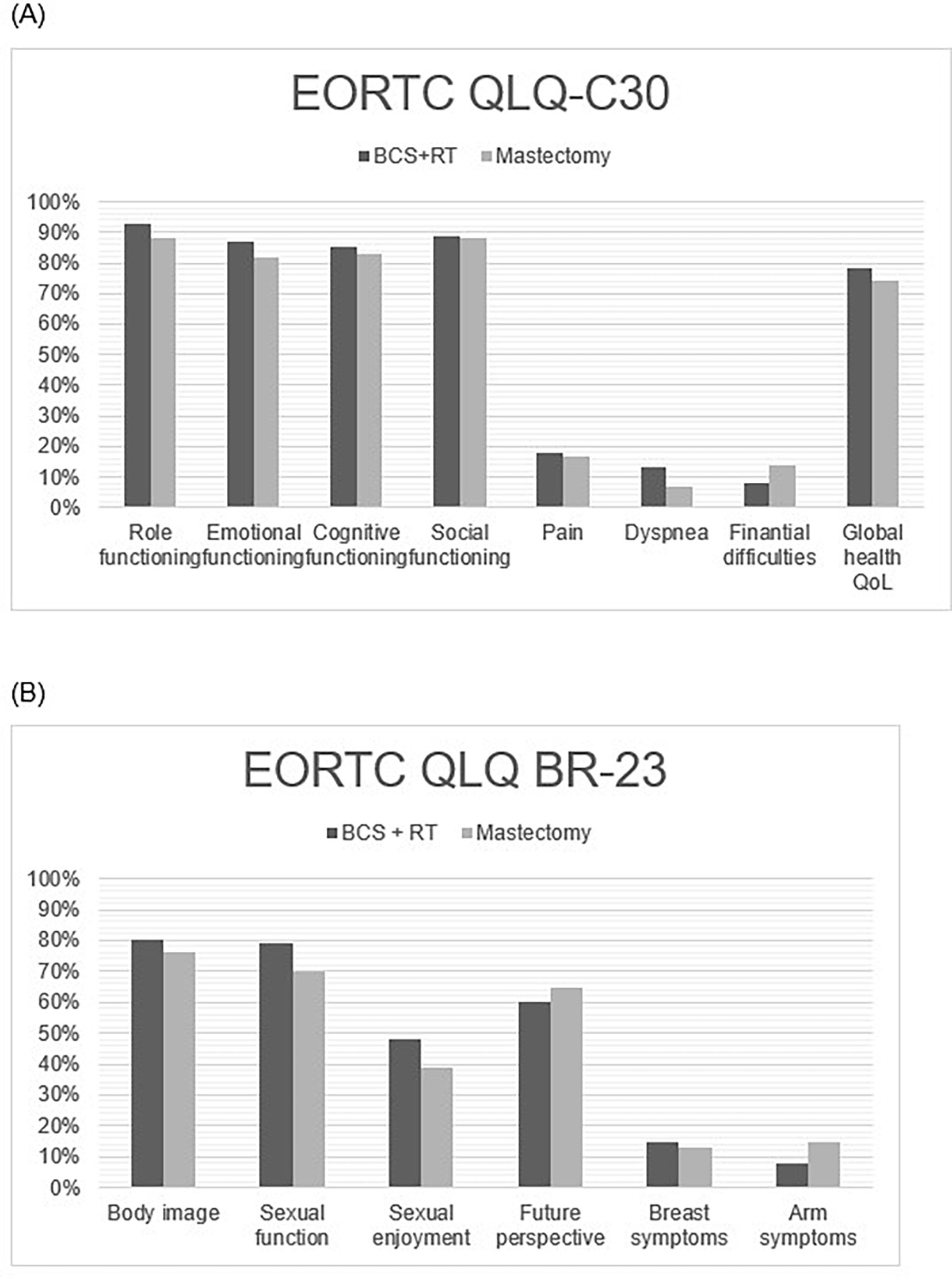
Sixty-five out of 155 patients (35 from the mastectomy group and 30 from the BCS+RT group) agreed to answer the QoL questionnaires (EORTC_QLQ_C30 and BR-23 module). QoL survey response rate was 42%. EORTC-QLQ-C30 Global Health Index was similar for BCS+RT and mastectomy groups (78% vs 74%). There was no significant difference between treatments in any item of the EORTC_QLQ_C30 or BR-23 module questionnaires (Fig. 3). Median global health status/QoL was 83.33 in both groups. There was a non-significant trend on item 21 (did you feel nervous?) of EORTC_QLQ_C30 in favour of conservative treatment (p=.056).
DiscussionUntil the 1990s, mastectomy was the standard of care for patients with DCIS with a low recurrence rate. In a meta-analysis of the Boyages´ group, Stuart et al. evaluated long-term outcomes of DCIS treatment from 5 prospective and 21 retrospective studies, with a median follow-up of 10 years. The study showed a 10-year local relapse rate of 2.6% after mastectomy.19 Nowadays, treatment options for DCIS include BCS alone, BCS followed by RT, mastectomy (with or without primary reconstruction), sentinel lymph node biopsy, and endocrine therapy. To date, no prospective randomised controlled studies have compared mastectomy with BCS±RT in patients with DCIS. Both treatments confer similar results in terms of cause-specific mortality at 10 years. Data from population studies indicate that the 10-year breast cancer mortality in patients with DCIS is less than 2% after excision or mastectomy.4,5
The presence of different surgical treatment options for breast cancer highlights that care is preference-sensitive, with decisions typically made through a shared decision-making process between the patient and the healthcare team. In the early years of immediate reconstruction mastectomies, there was an increase in these procedures. However, in recent years, especially following the publication of a large observational study and recent meta-analyses suggesting that BCS+RT may provide more benefit compared to mastectomy for early breast cancer, there has been a shift.20,21 Additionally, the rate of BCS among eligible patients is a quality measure used by the Commission on Cancer for accredited facilities. As the relative benefits of each surgery type continue to be evaluated in DCIS, it is imperative to monitor temporal trends in treatment to ensure all groups are receiving treatment based on contemporary standards of evidence.
According to the US National Cancer Database, 63% of 88 083 patients with DCIS underwent BCS from 1998 to 2011. Furthermore, the UK Sloane Project's long-term follow-up of 9938 DCIS cases detected by screening from 2003 to 2012 showed a BCS rate of 70%.22 Meanwhile, the results of the French National DCIS Survey revealed 74.8% BCS and 25.2% total mastectomies.23 Compared with the published series, our study had a less proportional number of patients treated with BCS. The improvement in immediate reconstruction techniques may have influenced the trend towards a surge in mastectomies over breast-conserving surgeries at our centre in previous years. The prevalence of mastectomy was generally higher for women younger than 65 compared to older women, potentially reflecting a trend to de-escalate breast cancer therapy in older women or a preference for a surgery perceived as more aggressive among younger women. Additionally, young patients (<40 years), those with high-grade tumours, and those with multicentric lesions are more likely to be treated with mastectomy due to higher recurrence rates.23,24
In a questionnaire-based QoL survey of 3233 patients with stage I and II breast cancer at Memorial Sloan Kettering Cancer Center, breast satisfaction and QoLscores were higher for BCS compared with mastectomy with implant reconstruction.25 In our series, patients had similar QoLscores when comparing the 2 treatment options. Therefore, the choice of treatment must consider the clinicopathological characteristics of the tumour as well as the personal choice of the patients.
Five randomised trials have studied the benefit of adding WBI after BCS in DCIS. The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-17,17 the SweDCIS,14 the European Organisation for Research and Treatment of Cancer 10 853 trial,13 the UK Coordinating Committee on Cancer Research trial12 and more recently the RTOG 980415 have demonstrated a significant 50% reduction in the risk of local recurrence in all subgroups of patients (from 28.1% to 12.9%). A review of the first 4 trials confirmed a 15.2% absolute reduction in local recurrence at 10 years, also in all patient subgroups. To avoid local recurrence, treatment of 9 patients was necessary. Its administration did not show a significant effect on overall mortality or breast cancer-specific mortality at 10 years of follow-up.26 In our study, all patients received RT after BCS.
Absence of ER, Her2 positivity, high percentage of Ki67 expression, age ≤40 years, high histological grade, larger tumour size, presence of more than 2 lesions, necrosis, and positive or close margins have been described as factors affecting local relapse.27,28 However, there is controversy regarding the definition of negative margin for women who are treated with BCS for DCIS. According to a meta-analysis of retrospective studies of patients treated with BCS and WBI, a surgical-free margin of 2 mm has been identified as the minimum requirement for acceptable surgical treatment of patients with DCIS.29 In terms of tumour size, a multinational-pooled cohort study showed that patients with large DCIS (≥50 mm) more often developed stage III and IV ipsilateral invasive breast cancer when compared to patients with DCIS <2 cm.30 In our study, none of the clinical or pathological factors were significantly associated with ipsilateral in situ or invasive relapse, except for tumour size greater than 2 cm.
Our study had limitations as it is a retrospective series of a single institution. Moreover, the inclusion period is from 2009 to 2018 to allow for a longer follow-up period, so there is some restriction on the completeness of the data and the use of early diagnostic methods.
ConclusionIn summary, the findings support equivalence of BCS+RT and mastectomy plus reconstruction regarding clinical outcome and QoL. Our results show that BCS is a good option even in patients with tumour size >2 cm. These findings provide a reference for clinical decision-making to optimise the management of DCIS.
FundingNone declared.
Ethical considerationsThe work was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments on human subjects. The work was approved by the Research Ethics Committee of the Hospital Universitario Puerta de Hierro. The centre's protocols on the publication of patient data were followed and patient privacy was respected.
Patients consentThe patients' consent for participation and for publication have been obtained.