Breast cancer treatment encompasses a diverse range of therapeutic strategies targeting specific molecular pathways crucial for tumor growth and progression. Several exciting advancements are likely to shape the future of breast cancer treatment. Understanding the mechanisms by which FDA-approved drugs exert their effects is crucial for optimizing treatment strategies and improving patient outcomes. In this review, we aim to categorize FDA-approved drugs for breast cancer treatment based on their distinct modes of action or mechanisms These mechanisms cover several activities, such as blocking particular enzymes that are essential for the synthesis of hormones, interfering with cellular functions that are necessary for the growth and spread of tumors, altering signaling pathways that are critical for the survival of cancer cells, and taking advantage of weaknesses in DNA repair mechanisms that are specific to them. In order to effectively battle this heterogeneous disease, a customized and all-encompassing therapeutic approach is required. This multifaceted approach highlights the crucial role of therapeutic approaches and synergistic treatment modalities in maximizing efficacy and improving prognosis for individuals with breast cancer.
El tratamiento del cáncer de mama abarca una gama diversa de estrategias terapéuticas centradas en las vías moleculares específicas, que son esenciales para el crecimiento y la progresión del tumor. Es probable que los diversos e interesantes avances conformen el futuro del tratamiento del cáncer de mama. Comprender los mecanismos por los cuales ejercen sus efectos los fármacos aprobados por la FDA es esencial para optimizar las estrategias terapéuticas y mejorar los resultados del paciente. En esta revisión, nuestro objetivo fue categorizar los fármacos aprobados por la FDA para el tratamiento del cáncer de mama, basándonos en sus diferentes modos o mecanismos de acción. Dichos mecanismos cubren diversas actividades, tales como el bloqueo de enzimas particulares que son esenciales para la síntesis de hormonas, interfiriendo en las funciones celulares necesarias para el crecimiento y la diseminación tumoral, alterando las vías de señalización que son esenciales para la supervivencia de las células cancerígenas, y adquirir ventaja de las debilidades de los mecanismos de reparación del ADN específicas de las mismas. A fin de luchar de manera heterogénea contra esta enfermedad, es necesario un enfoque terapéutico personalizado e integral. Dicho enfoque multifacético destaca el papel fundamental de los enfoques terapéuticos y las modalidades de terapia sinérgica, a fin de maximizar la eficacia y mejorar el pronóstico para las personas con cáncer de mama.
Breast cancer remains one of the most prevalent and complex malignancies affecting women worldwide. Pharmacotherapy plays a pivotal role, with numerous drugs approved by the FDA demonstrating efficacy in the treatment of breast cancer. By systematically examining the mechanisms of action of these drugs, we can gain insights into the underlying biological processes targeted in the treatment of breast cancer. Categorization of drug based on their mechanism of action not only provides a comprehensive overview of the pharmacological landscape but also facilitates the identification of potential synergistic combinations and personalized treatment approaches.1 This review is an attempt to conclude the basic mechanism of action of FDA-approved drug in the treatment of breast cancer that may pave the way for more effective and tailored treatment regimens with combination therapy controlling breast cancer.
The stage of the disease often influences the choice of pharmacotherapy for breast cancer. Treatment objectives for early-stage breast cancer are mainly to remove the tumor and avoid disease recurrence. As a result, medications that target cancer cells that are actively spreading, like hormone receptor (HR) modulators and cytotoxic chemotherapeutic medicines, are frequently used. These medications try to stop cell development, cause apoptosis, or interfere with hormone signaling pathways that are essential for tumor formation. The FDA-approved drugs for breast cancer treatment can be categorized into distinct classes based on their mechanisms of action. Each class of drugs exerts its effects through unique mechanisms, targeting various aspects of cancer cell biology. By classifying these drugs according to their mechanisms of action, we can better understand the rationale behind their clinical use and facilitate the selection of optimal treatment regimens for individual patients based on the molecular characteristics of their tumors and the stage of disease.
Pharmacological therapies for breast cancer treatment encompass a wide range of approaches, each utilizing unique mechanisms to achieve therapeutic efficacy. Aromatase inhibitors (AIs) like and selective estrogen receptor modulators (SERMs) disrupt estrogen signaling pathways vital for HR-positive tumor proliferation.2 Monoclonal antibodies like target the human epidermal growth factor receptor 2 (HER-2 receptor), hindering downstream signaling cascades essential for HER-2-driven tumorigenesis.3 Kinase inhibitors interfere with intracellular signaling pathways, inhibiting key kinases involved in cancer cell proliferation and survival.4 Poly(ADP-ribose) polymerase (PARP) inhibitors exploit DNA repair mechanisms, inducing synthetic lethality in tumors harboring BRCA mutations. Microtubule inhibitors disrupt mitotic spindle formation, leading to cell cycle arrest and apoptotic cell death.5 Additionally, immune checkpoint inhibitors blocking inhibitory pathways, enhancing T-cell-mediated cytotoxicity against cancer cells.6
This review will classify FDA-approved drugs into 5 distinct categories based on their primary mechanism of action. By understanding how these drugs work at a cellular or molecular level, we can gain valuable insights into their therapeutic effects and potential side effects. This categorization will help us analyze the range of tools available in modern medicine and how they target different biological processes to achieve desired health outcomes.
Endocrine disruption: Hormone modulators and aromatase inhibitorsEstrogen modulation is an important strategy in breast cancer treatment, involving drugs that target various aspects of estrogen signaling pathways. These drugs include SERMs like tamoxifen, which block estrogen from binding to cancer cells' receptors, hindering tumor growth.7 Fulvestrant, a selective estrogen receptor degrader (SERD), promotes the degradation of estrogen receptors (ERs), reducing estrogen signaling.8 Luteinizing hormone-releasing hormone (LHRH) agonists such as goserelin decrease estrogen production by suppressing luteinizing hormone release from the pituitary gland, thereby lowering ovarian estrogen production.
Tamoxifen is a non-steroidal antiestrogen used to treat ER positive breast cancers as well as prevent the incidence of breast cancer in high risk populations.7 Its prolonged action is attributed to the active metabolite N-desmethyltamoxifen, which has a half-life of around 2 weeks.7 Tamoxifen's mechanism in breast cancer treatment involves competitive inhibition of estrogen binding to its receptor, crucial for its activity against breast cancer cells, leading to reduced levels of tumor growth factors and an elevation in sex hormone binding globulin, consequently limiting free estradiol availability and inhibiting tumor growth stimulation.7 Additionally, tamoxifen induces apoptosis in ER-positive (ER+) cells through inhibition of protein kinase C, preventing DNA synthesis, and possibly by increasing intracellular and mitochondrial calcium ion levels or inducing tumor growth factor.9
Elacestrant is a non-steroidal small molecule and an ER antagonist.5 In January 2023, it was approved by the FDA for the treatment of ER+, HER2-negative (HER2-), ESR1-mutated advanced or metastatic breast cancer.8 Elacestrant, an oral SERD, targets ER-alpha (ERα) in breast tumors that rely on estrogen-mediated growth signaling, commonly treated with endocrine therapies.10 SERDs like elacestrant antagonize ER transcriptional activity and induce its degradation. In ER+, HER2- breast cancer cells, elacestrant inhibits 17β-estradiol-mediated proliferation, induces ERα degradation via the proteasomal pathway, slows ER nuclear translocation, and disrupts downstream signaling.10
Toremifene, a nonsteroidal antineoplastic hormonal agent primarily used in advanced breast cancer treatment, demonstrates potent antiestrogenic properties by competitively binding with estrogen for target tissue receptors, particularly in breast tissues. It inhibits rat mammary carcinoma induction and induces regression of established tumors by binding to ERs.11
Fulvestrant is used to treat HR-positive (HR+) metastatic breast cancer in postmenopausal women who have progressed after antiestrogen therapy. As an ER antagonist, it downregulates and degrades the ER without exerting agonist effects. Fulvestrant competitively and reversibly binds to ERs in cancer cells, exerting antiestrogen effects through dual mechanisms.11 Firstly, it downregulates the receptors, preventing estrogen binding. Secondly, it facilitates the degradation of bound ERs.12
Goserelin is a synthetic analog of LHRH used to treat breast cancer and prostate cancer. Goserelin inhibits testosterone production in men and reduces estradiol levels in women to postmenopausal levels, thereby preventing hormone-driven cancer cell growth.13
Megestrol acetate, initially developed as a contraceptive, later, it has been evaluated in breast cancer treatment. Its precise mechanism in cancer treatment is not fully understood, but it may involve suppressing luteinizing hormone by inhibiting pituitary function, thus exhibiting progestin antitumor activity.14
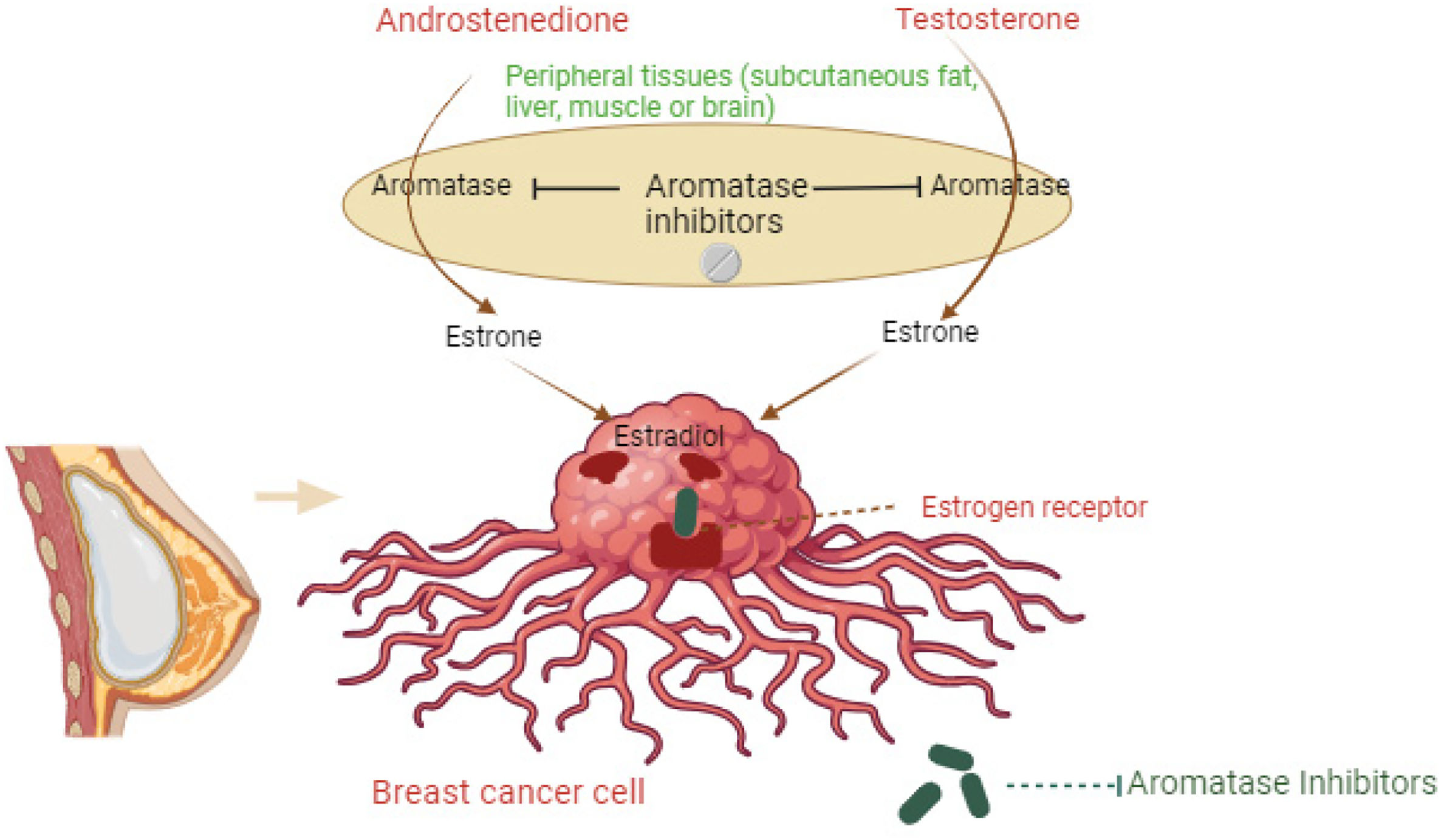
AIs represent a crucial class of drugs in the treatment of HR+ breast cancer, particularly in postmenopausal women. These agents function by inhibiting the enzyme aromatase, which is responsible for the conversion of androgens to estrogens in peripheral tissues. Fig. 1 illustrates how AIs block the conversion of androgens. By blocking estrogen synthesis, AIs effectively reduce estrogen levels in the body, thereby depriving estrogen-dependent breast cancer cells of their growth stimulus. Commonly prescribed AIs include letrozole, anastrozole, and exemestane, each exhibiting subtle differences in pharmacokinetics and side effect profiles.
Aromatase inhibitor mechanism. The figure illustrates how aromatase inhibitors block the conversion of androgens (androstenedione and testosterone) to estrogens (estrone), reducing estrogen (estradiol) levels in breast cancer cells. Aromatase inhibitors prevent aromatase activity in peripheral tissues, thereby lowering estrogen receptor activation in breast cancer cells.
These inhibitors lower estrogen levels by blocking the aromatase enzyme, which is crucial for hormone-driven breast cancer growth in postmenopausal women. By reducing estrogen, they effectively slow the growth of HR+ breast cancer cells and generally have fewer side effects compared to older treatments. Anastrozole is a non-steroidal AI, used to decrease circulating estrogen levels in the treatment of postmenopausal women with estrogen-responsive breast cancer.12 In postmenopausal women, estrogen is primarily derived from the conversion of adrenally-produced androgens into estrogens by the aromatase enzyme—by competitively inhibiting the biosynthesis of estrogen at these enzymes, anastrozole effectively suppresses circulating estrogen levels and, subsequently, the growth of HR+ tumors.12,13
Exemestane is an oral steroidal AI used in the adjuvant treatment of hormonally responsive (HR+, estrogen-responsive) breast cancer in postmenopausal women. It irreversibly binds to the active site of the aromatase enzyme, resulting in permanent inhibition of its activity.14
Letrozole is a non-steroidal type II AI.15 It blocks the active site, and therefore the electron transfer chain of CYP19A1.15 This competitive inhibition prevents the conversion of androgens to estrogen.15 Third-generation AIs do not significantly affect cortisol, aldosterone, and thyroxine levels.16
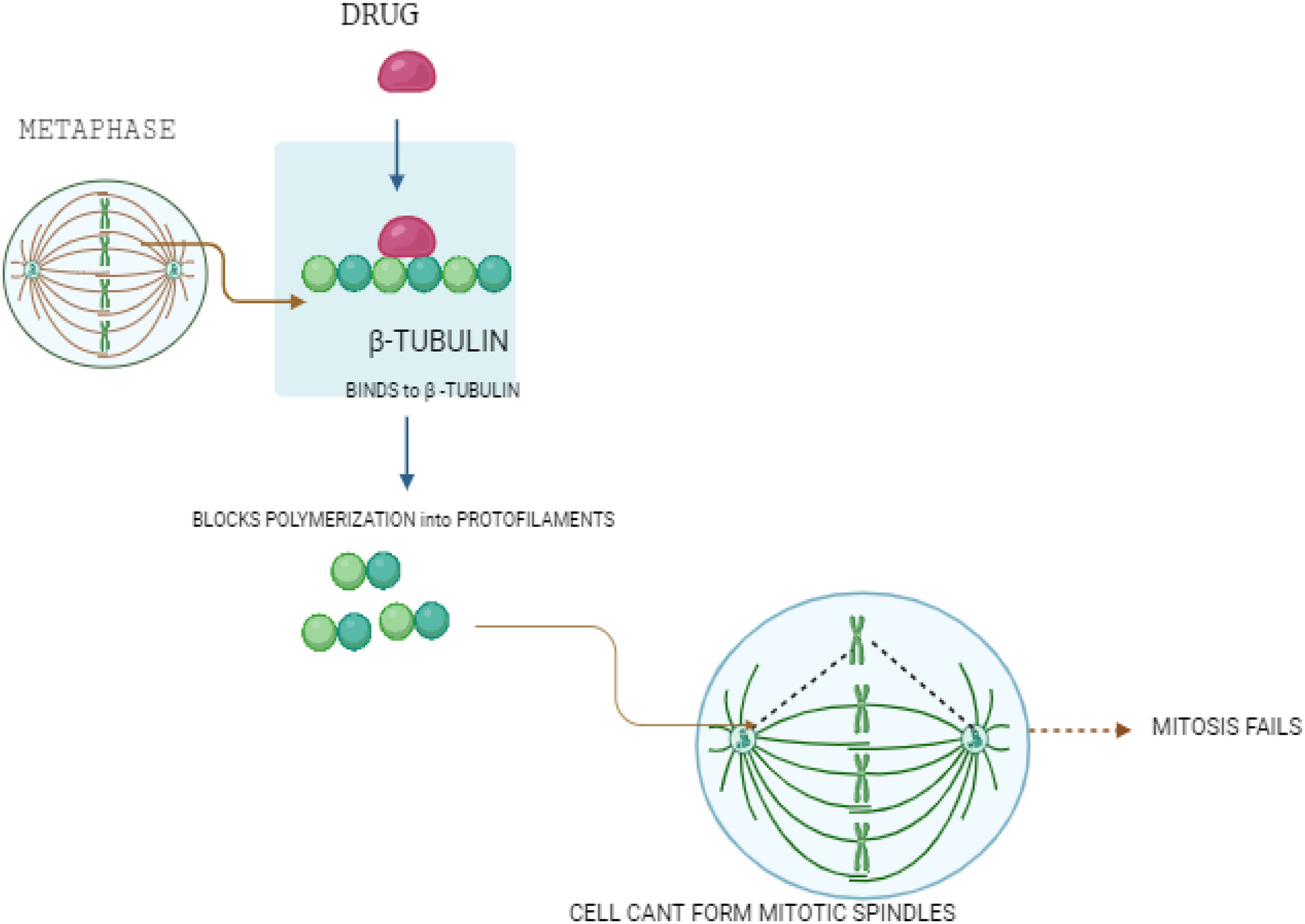
Cellular structural destabilization: Microtubule inhibitorsMicrotubule inhibitors represent a vital class of drugs in breast cancer therapy, functioning by disrupting the structure of microtubules critical for cell division and intracellular transport. Fig. 2 shows how microtubule inhibitors work. The drug binds to β-tubulin, blocking its polymerization into protofilaments. This prevents the formation of mitotic spindles during metaphase, causing mitosis to fail and stopping cancer cell division. They disrupt cell division by either stabilizing or destabilizing microtubules.
Microtubule inhibitors used in breast cancer treatment.
This figure shows how microtubule inhibitors work. The drug binds to β-tubulin, blocking its polymerization into protofilaments. This prevents the formation of mitotic spindles during metaphase, causing mitosis to fail and stopping cancer cell division.
Paclitaxel, marketed as Taxol, is a chemotherapeutic agent used to treat various cancers.16 It is a mitotic inhibitor first isolated from the bark of the Pacific yew tree, which contains endophytic fungi that produce paclitaxel.17 Paclitaxel disrupts microtubule function by hyperstabilizing their structure, preventing the normal depolymerization process. It binds to the β subunit of tubulin, locking these building blocks in place and inhibiting dynamic instability, which is crucial for cell division and intracellular transport. This interference with microtubule flexibility impedes essential cellular functions, such as chromosome movement during mitosis.18 Additionally, paclitaxel induces apoptosis in cancer cells by binding to and inhibiting the Bcl-2 protein, which normally prevents programmed cell death.19
Trastuzumab emtansine is a targeted cancer therapy for HER2+ cancer cells, combining the antibody trastuzumab with the chemotherapy drug DM1.20 Trastuzumab, produced in Chinese Hamster Ovary cells, binds to the HER2 receptor on cancer cells and is taken into the cell through endocytosis. Once inside, lysosomes break down the complex, releasing DM1, which binds to tubulin in microtubules, disrupting their function and causing cell cycle arrest and apoptosis.20,21 Additionally, trastuzumab inhibits HER2 signaling and promotes immune system attacks on the cancer cells. This combination effectively targets and kills HER2-positive cancer cells.22
Eribulin is a microtubule inhibitor used to treat metastatic breast cancer.23 Eribulin inhibits the growth phase of microtubules without affecting the shortening phase and sequesters tubulin into nonproductive aggregates.24 Eribulin exerts its effects via a tubulin-based antimitotic mechanism leading to G2/M cell-cycle block, disruption of mitotic spindles, and, ultimately, apoptotic cell death after prolonged mitotic blockage.24,25
Ixabepilone is a microtubule inhibitor that binds to beta-tubulins, such as beta-III tubulin, to stabilize microtubules.26 Since microtubules are crucial for cell division, ixabepilone disrupts this process by keeping the microtubules stable.27 By binding to the αβ-tubulin heterodimer subunit, ixabepilone decreases the dissociation rate of αβ-tubulin, leading to stabilized microtubules and preventing proper cell division.27
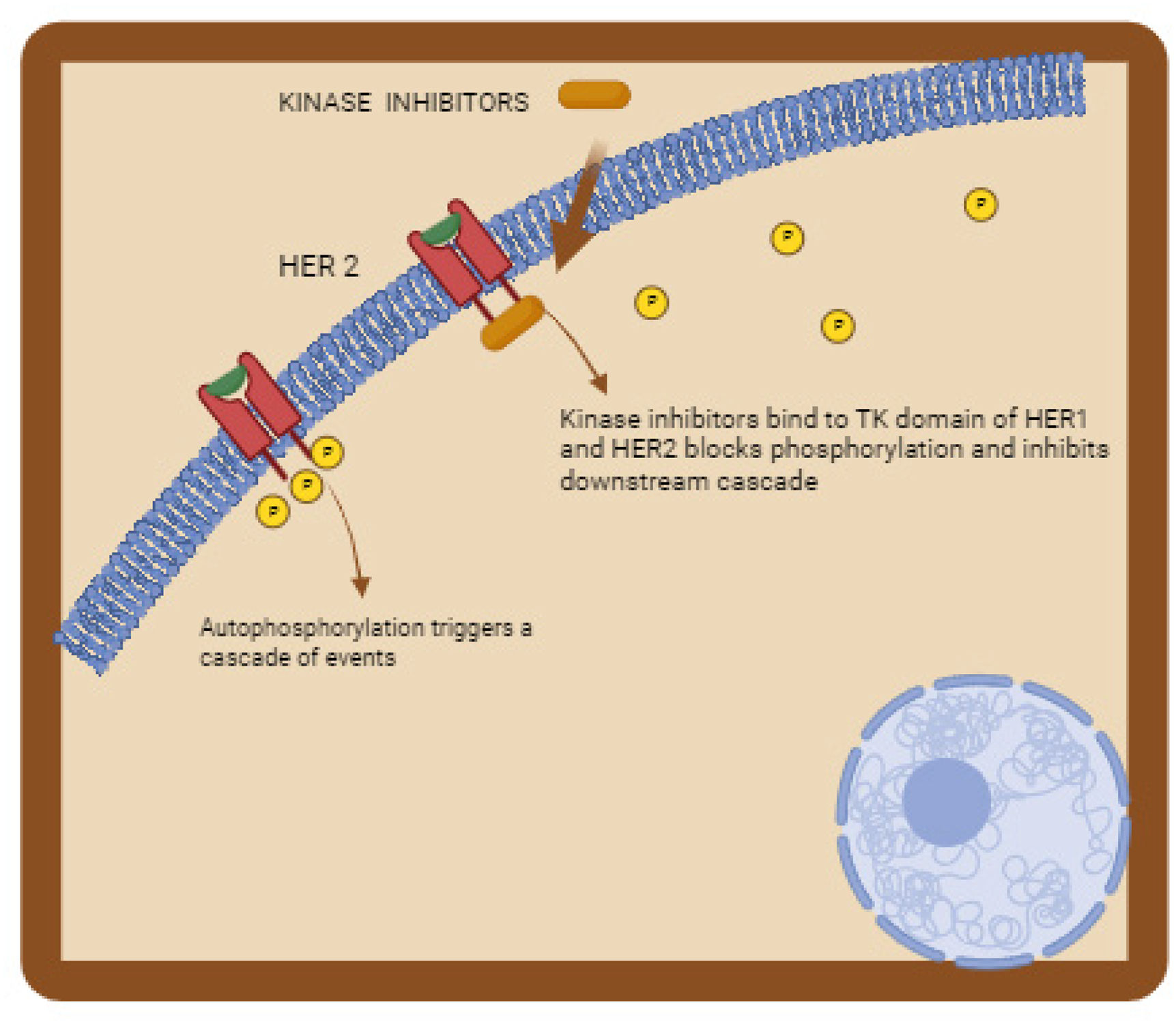
Signal transduction interference: Kinase inhibitorsKinase inhibitors have important role in breast cancer treatment, functioning by targeting specific enzymes crucial for cancer cell growth and survival. Fig. 3 illustrates how kinase inhibitors function. These inhibitors act on kinases like HER2, PI3K, AKT, mTOR, and CDK4/6, which are often overactive or mutated in breast cancer cells. By binding to these kinases, kinase inhibitors disrupt their function, preventing downstream signaling pathways that drive cell proliferation and survival.
Kinase inhibitors in cancer treatment.
This figure illustrates how kinase inhibitors function. The inhibitors bind to the tyrosine kinase (TK) domain of HER1 and HER2 receptors on the cell membrane, blocking their phosphorylation. This inhibition prevents autophosphorylation, which would otherwise trigger a cascade of downstream signaling events essential for cancer cell proliferation and survival. By blocking these pathways, kinase inhibitors effectively disrupt cancer cell growth and division.
Abemaciclib is an antitumor agent and dual inhibitor of cyclin-dependent kinases 4 (CDK4) and 6 (CDK6) that are involved in the cell cycle and promotion of cancer cell growth in case of unregulated activity.28 The cell cycle regulates proper cell growth, with dysregulation contributing to hyperproliferation and tumor formation in various cancers. The G1 to S phase transition, controlled by the retinoblastoma tumor suppressor protein (Rb)-mediated pathway, involves CDK 4 and 6 interacting with D-type cyclins to phosphorylate Rb.28,29 This phosphorylation releases the suppression of E2F transcription factors, promoting gene expression required for cell cycle progression and DNA replication initiation. Abemaciclib selectively inhibits CDK4 and CDK6, leading to Rb dephosphorylation, G1 arrest, and proliferation inhibition in Rb-proficient cells, with greater CDK4 selectivity.30
Everolimus is a mammalian target of rapamycin (mTOR) kinase inhibitor used to treat various types of malignancies.31 Everolimus, binds tightly to FKBP-12, a protein in cells. This binding forms a complex that blocks mTOR activity, preventing cells from progressing from the G1 to S phase of the cell cycle. This leads to growth arrest and sometimes cell death.32 Additionally, everolimus reduces the expression of hypoxia-inducible factor, decreasing the production of vascular endothelial growth factor and inhibiting blood vessel formation.33
Alpelisib is a phosphatidylinositol 3-kinase (PI3K) inhibitor with potent antitumor activity. It works by selectively inhibiting class I PI3K.34 Phosphatidylinositol-3-kinase-α (PI3Kα) drives cell proliferation upon activation of the growth factor-tyrosine kinase pathway.
Capivasertib, a serine/threonine kinase inhibitor, is used to treat HER+, HER2-, locally advanced, or metastatic breast cancer. It works by inhibiting all 3 isoforms of the serine/threonine kinase AKT (AKT1, AKT2, and AKT3) and blocking the phosphorylation of downstream AKT substrates. AKT activation in tumors can result from various factors, including activation of upstream signaling pathways, mutations in AKT1, loss of phosphatase and tensin homolog (PTEN) function, and mutations in the catalytic subunit alpha of phosphatidylinositol 3-kinase (PIK3CA).35
Ribociclib is a selective CDK inhibitor, that helps to slow the progression of cancer by inhibiting 2 proteins called CDK 4 and 6. Studies using knockout mice indicate that CDK4 is not necessary for normal mammary tissue development.36 However, it becomes essential for the growth of Ras-induced mammary tumors, suggesting a potential therapeutic opportunity with lower toxicity.37,38 Ribociclib, known for its high selectivity as a CDK4/6 inhibitor, demonstrated dose-dependent antitumor activity in various preclinical models.
Neratinib irreversibly inhibits EGFR, HER2, and HER4.39 This prevents auotphoshorylation of tyrosine residues on the receptor and reduces oncogenic signaling through the mitogen-activated protein kinase and Akt pathways.40
Tucatinib is a kinase inhibitor drug used to target mutations in the HER-2 gene found in certain types of breast carcinoma.41 By inhibiting the tyrosine kinase enzyme of HER-2, tucatinib blocks increased cell signaling and proliferation that lead to malignancy.41 It inhibits phosphorylation of HER-2 and HER-3, disrupting downstream MAPK and AKT signaling pathways and inhibiting cell proliferation.42
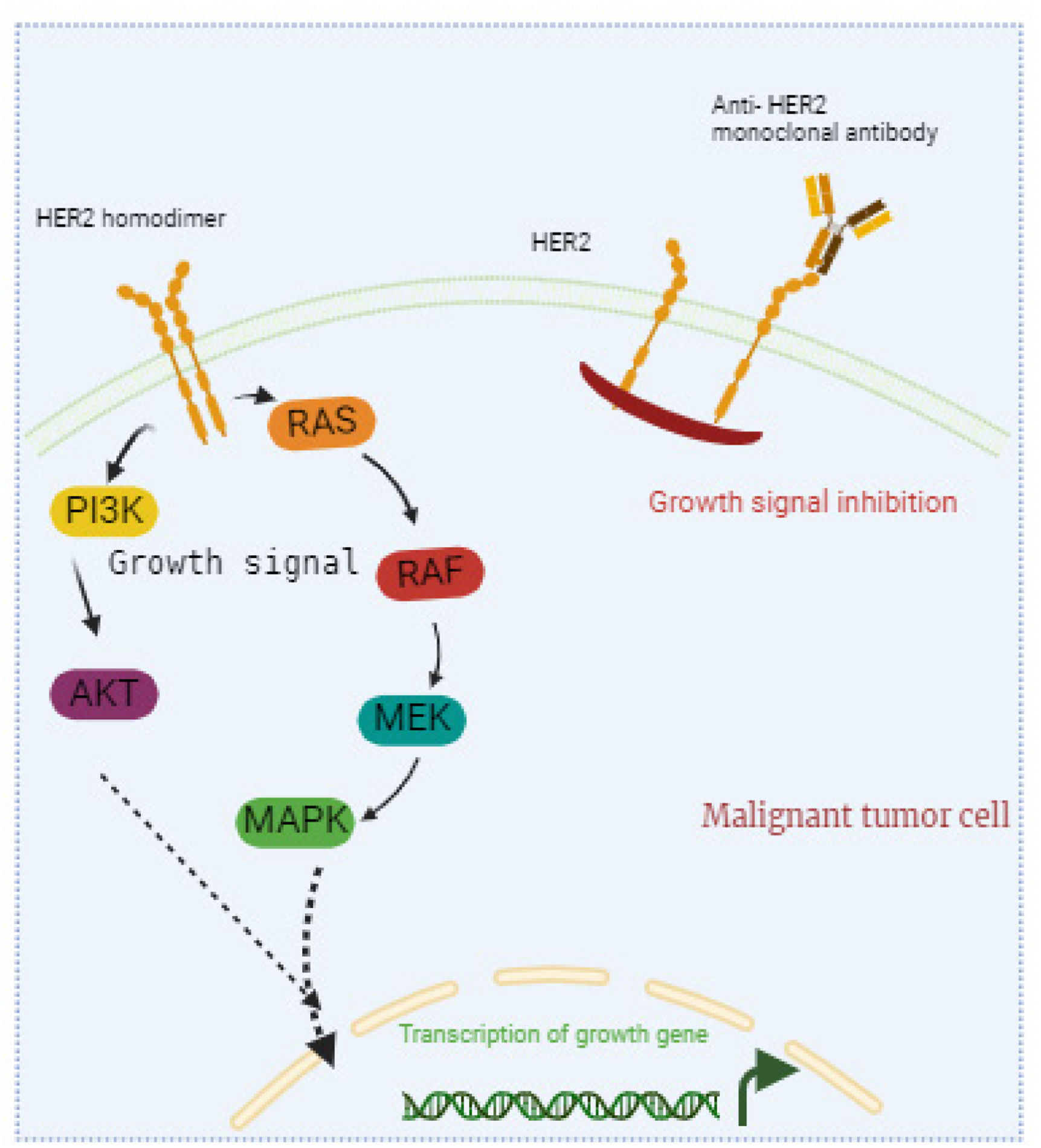
Immune system modulation: Monoclonal antibodiesMonoclonal antibodies are a significant part of breast cancer treatment, designed to target specific proteins on cancer cells and hinder their growth while enhancing the body's immune response against them. By attaching to these proteins, these antibodies disrupt signals crucial for cancer cells to proliferate and survive. Fig. 4 shows how monoclonal antibodies target cancer cells. The antibodies specifically bind to antigens on the surface of cancer cells. This binding marks the cancer cells for destruction by the immune system and can block essential growth signals, inhibit cell proliferation, and induce apoptosis. This personalized approach enhances the body's natural defenses, boosting its ability to fight cancer more effectively.
Monoclonal antibodies in cancer treatment.
This figure shows how monoclonal antibodies target cancer cells. The antibodies specifically bind to antigens on the surface of cancer cells. This binding marks the cancer cells for destruction by the immune system and can block essential growth signals, inhibit cell proliferation, and induce apoptosis. Monoclonal antibodies help the immune system identify and eliminate cancer cells effectively.
Trastuzumab deruxtecan is a HER2-directed antibody attached to a topoisomerase inhibitor that is approved for use in certain types of treatment-resistant HER2-positive cancers.43 After trastuzumab deruxtecan binds to HER2 found on malignant cells, it is internalized and linker cleavage occurs through the actions of lysosomal enzymes. After it is released through cleavage, DXd causes targeted DNA damage and apoptosis in cancer cells, due to the ability to cross cell membranes.44
Pembrolizumab binds to the PD-1 receptor, blocking its interaction with ligands PD-L1 and PD-L2.45 Normally, this interaction inhibits T-cell activity to maintain self-tolerance and minimize collateral damage. By blocking PD-1, pembrolizumab enhances immune reactivity and boosts the antitumor immune response.46
Margetuximab is a chimeric IgG1κ monoclonal antibody (mAb) directed against the extracellular domain of the HER2 cell-surface protein. Also, margetuximab has an engineered Fc region that alters its affinity for the CD16A and CD32B effector cell receptors resulting in increased antibody-dependent cell-mediated cytotoxicity.47
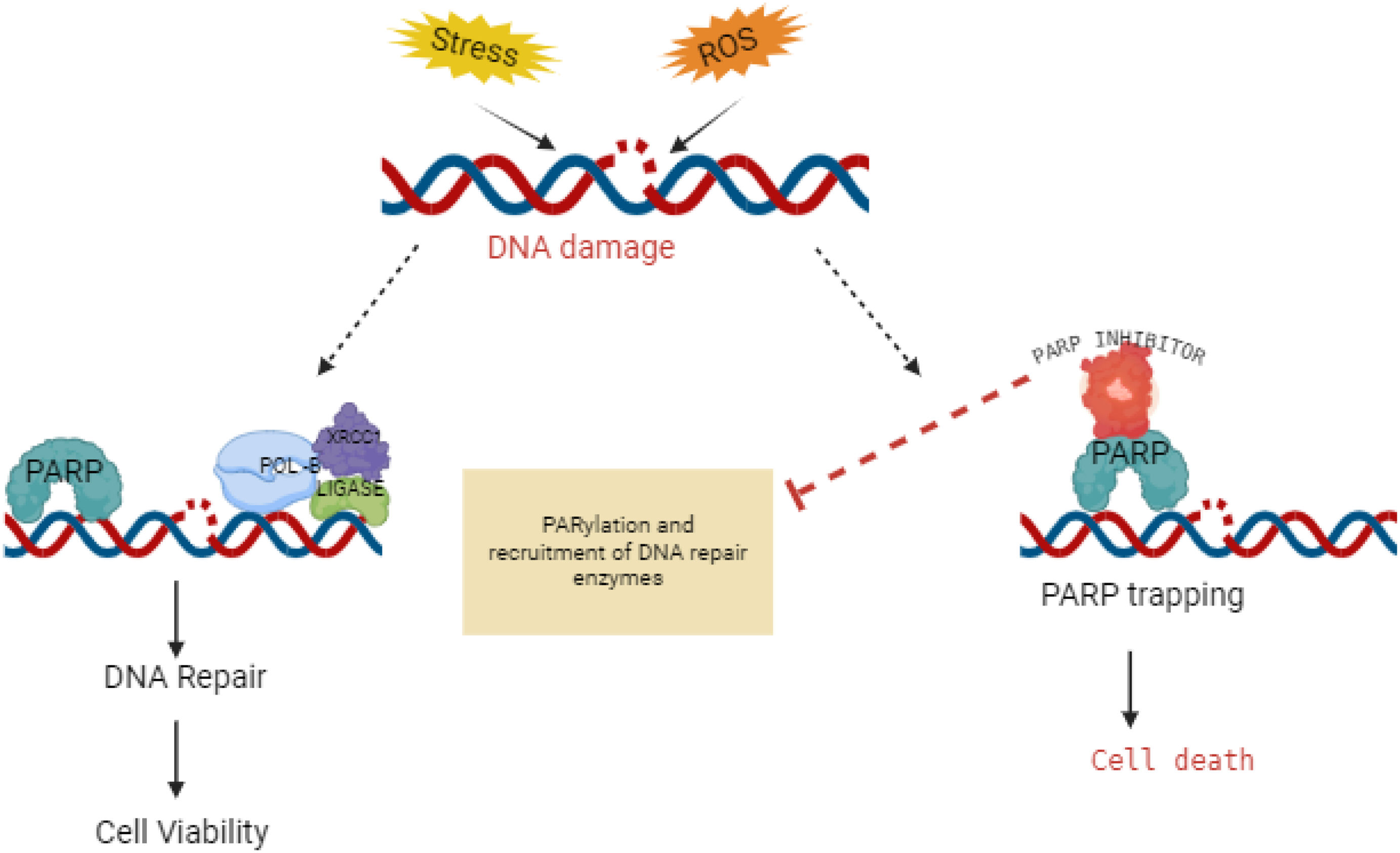
DNA damage and repair disruption: PARP inhibitors and nucleoside metabolic inhibitorsPARP inhibitors target cancer cells with BRCA mutations by blocking the PARP enzymes that repair DNA damage. This prevents cancer cells from fixing themselves, leading to their death. Fig. 5 likely illustrates the molecular mechanism or structural aspects of how PARP inhibitors interact with their target. This is especially effective in cancer cells with BRCA mutations, as they rely heavily on PARP for DNA repair due to deficiencies in other repair mechanisms. PARP inhibitors are often used as targeted therapy in combination with other treatments or as maintenance therapy to prevent cancer recurrence.
Mechanism of action of PARP inhibitors in cancer therapy.
PARP inhibitors, shown in Fig. 5, are a class of drugs that target poly (ADP-ribose) polymerase (PARP) enzymes. These enzymes play a crucial role in DNA repair mechanisms, particularly in base excision repair. By inhibiting PARP, these drugs prevent cancer cells from repairing their damaged DNA effectively, leading to cell death.
Talazoparib is an inhibitor of mammalian polyadenosine 5’-diphosphoribose polymerases (PARPs), enzymes responsible for regulating essential cellular functions, such as DNA transcription and DNA repair.48 PARPs repair DNA single-strand breaks through the base excision repair pathway. DNA double-strand breaks are repaired by homologous recombination involving tumor suppressor proteins encoded by BRCA1 and BRCA2.49,50
Olaparib is a cytotoxic antitumor agent that inhibits the growth of specific tumor cell lines and reduces tumor growth in mouse xenograft models of human cancer.49 It is effective as monotherapy or after platinum-based chemotherapy, particularly in cells and tumors with deficiencies in BRCA1/2, ATM, or other genes involved in homologous recombination repair.51
Nucleoside metabolic inhibitors are a class of medications used in cancer treatment. They work by interfering with the synthesis or function of nucleosides, which are essential building blocks of DNA and RNA. By disrupting nucleoside metabolism, these inhibitors prevent cancer cells from properly replicating their DNA and synthesizing new RNA, ultimately leading to cell death. Gemcitabine is a nucleoside analog and chemotherapeutic agent initially studied for antiviral effects but now used to treat various cancers.52 As a cytidine analog with 2 fluorine atoms replacing ribose hydroxyl groups, gemcitabine acts as a prodrug.53 It is converted into active metabolites that incorporate into DNA during elongation, halting tumor growth and inducing apoptosis in malignant cells.54
Capecitabine is converted into 5-fluorouracil (5-FU) in the body, which disrupts cancer cells through 2 mechanisms. Firstly, it inhibits thymidylate synthase, blocking DNA synthesis by depleting deoxythymidine monophosphate.55
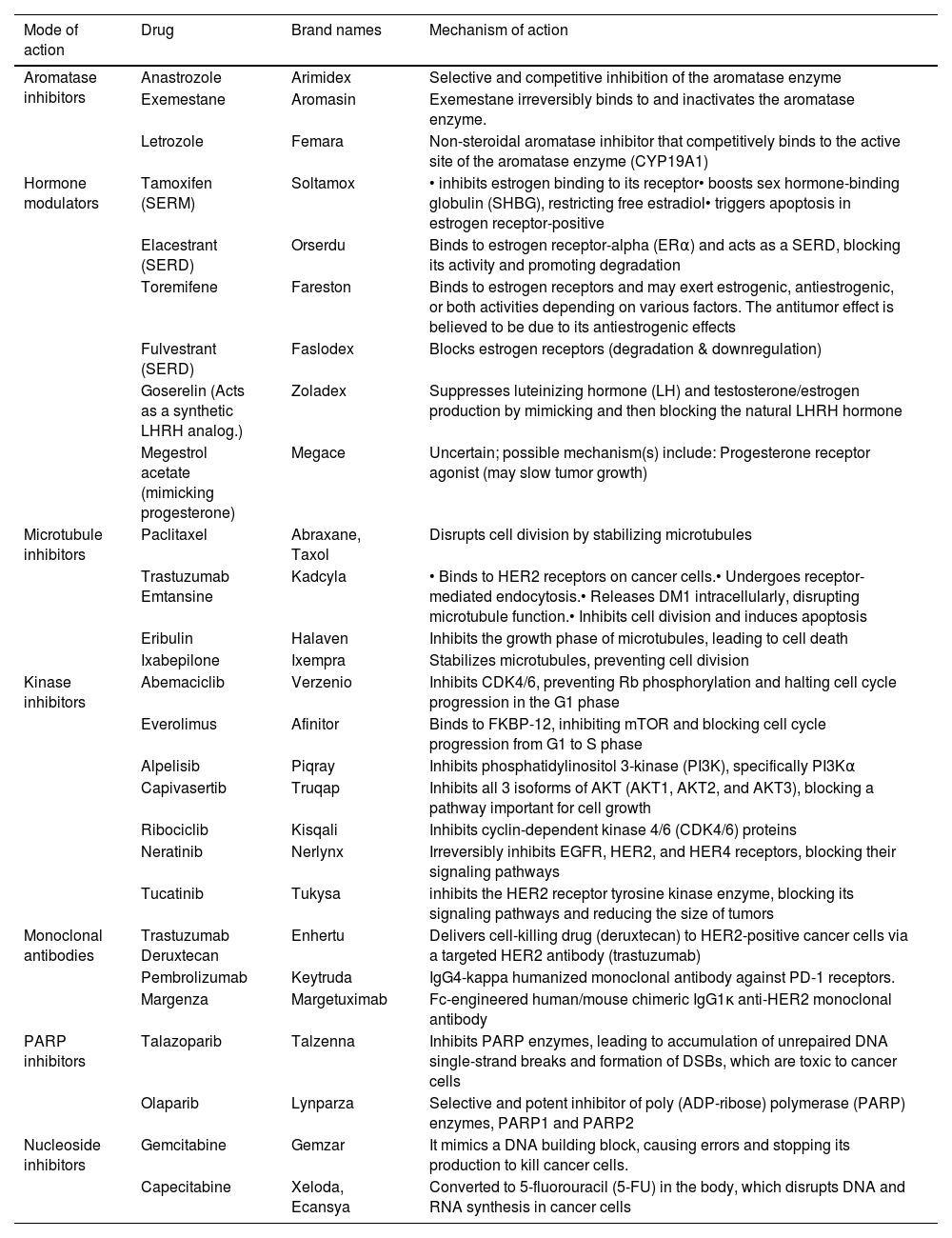
Table 1 summarizes various drugs used in the treatment of breast cancer, detailing their brand names and mechanisms of action. Each drug targets specific biological pathways involved in breast cancer progression or growth.
Drugs against breast cancer, brand names, and mechanism of action.
| Mode of action | Drug | Brand names | Mechanism of action |
|---|---|---|---|
| Aromatase inhibitors | Anastrozole | Arimidex | Selective and competitive inhibition of the aromatase enzyme |
| Exemestane | Aromasin | Exemestane irreversibly binds to and inactivates the aromatase enzyme. | |
| Letrozole | Femara | Non-steroidal aromatase inhibitor that competitively binds to the active site of the aromatase enzyme (CYP19A1) | |
| Hormone modulators | Tamoxifen (SERM) | Soltamox | • inhibits estrogen binding to its receptor• boosts sex hormone-binding globulin (SHBG), restricting free estradiol• triggers apoptosis in estrogen receptor-positive |
| Elacestrant (SERD) | Orserdu | Binds to estrogen receptor-alpha (ERα) and acts as a SERD, blocking its activity and promoting degradation | |
| Toremifene | Fareston | Binds to estrogen receptors and may exert estrogenic, antiestrogenic, or both activities depending on various factors. The antitumor effect is believed to be due to its antiestrogenic effects | |
| Fulvestrant (SERD) | Faslodex | Blocks estrogen receptors (degradation & downregulation) | |
| Goserelin (Acts as a synthetic LHRH analog.) | Zoladex | Suppresses luteinizing hormone (LH) and testosterone/estrogen production by mimicking and then blocking the natural LHRH hormone | |
| Megestrol acetate (mimicking progesterone) | Megace | Uncertain; possible mechanism(s) include: Progesterone receptor agonist (may slow tumor growth) | |
| Microtubule inhibitors | Paclitaxel | Abraxane, Taxol | Disrupts cell division by stabilizing microtubules |
| Trastuzumab Emtansine | Kadcyla | • Binds to HER2 receptors on cancer cells.• Undergoes receptor-mediated endocytosis.• Releases DM1 intracellularly, disrupting microtubule function.• Inhibits cell division and induces apoptosis | |
| Eribulin | Halaven | Inhibits the growth phase of microtubules, leading to cell death | |
| Ixabepilone | Ixempra | Stabilizes microtubules, preventing cell division | |
| Kinase inhibitors | Abemaciclib | Verzenio | Inhibits CDK4/6, preventing Rb phosphorylation and halting cell cycle progression in the G1 phase |
| Everolimus | Afinitor | Binds to FKBP-12, inhibiting mTOR and blocking cell cycle progression from G1 to S phase | |
| Alpelisib | Piqray | Inhibits phosphatidylinositol 3-kinase (PI3K), specifically PI3Kα | |
| Capivasertib | Truqap | Inhibits all 3 isoforms of AKT (AKT1, AKT2, and AKT3), blocking a pathway important for cell growth | |
| Ribociclib | Kisqali | Inhibits cyclin-dependent kinase 4/6 (CDK4/6) proteins | |
| Neratinib | Nerlynx | Irreversibly inhibits EGFR, HER2, and HER4 receptors, blocking their signaling pathways | |
| Tucatinib | Tukysa | inhibits the HER2 receptor tyrosine kinase enzyme, blocking its signaling pathways and reducing the size of tumors | |
| Monoclonal antibodies | Trastuzumab Deruxtecan | Enhertu | Delivers cell-killing drug (deruxtecan) to HER2-positive cancer cells via a targeted HER2 antibody (trastuzumab) |
| Pembrolizumab | Keytruda | IgG4-kappa humanized monoclonal antibody against PD-1 receptors. | |
| Margenza | Margetuximab | Fc-engineered human/mouse chimeric IgG1κ anti-HER2 monoclonal antibody | |
| PARP inhibitors | Talazoparib | Talzenna | Inhibits PARP enzymes, leading to accumulation of unrepaired DNA single-strand breaks and formation of DSBs, which are toxic to cancer cells |
| Olaparib | Lynparza | Selective and potent inhibitor of poly (ADP-ribose) polymerase (PARP) enzymes, PARP1 and PARP2 | |
| Nucleoside inhibitors | Gemcitabine | Gemzar | It mimics a DNA building block, causing errors and stopping its production to kill cancer cells. |
| Capecitabine | Xeloda, Ecansya | Converted to 5-fluorouracil (5-FU) in the body, which disrupts DNA and RNA synthesis in cancer cells |
Breast cancer treatment is advancing quickly, bringing new hope for better outcomes. This review looks at FDA-approved drugs, sorting them by how they work to show the variety of strategies used. Even with these advances, challenges like drug resistance remain, so ongoing research is crucial. Precision medicine and immunotherapy are particularly promising, as they use the body’s own immune system to create personalized treatments with fewer side effects. New drug delivery methods, such as nanoparticles, are being developed to target cancer cells more precisely, sparing healthy tissues. Overall, these innovations are leading to more effective and less toxic treatments, which is improving both survival rates and the quality of life for patients.
FundingThis research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical considerationAs this is a review article, no primary data was collected, and therefore, ethical approval was not required.
The figure illustrates how aromatase inhibitors block the conversion of androgens (androstenedione and testosterone) to estrogens (estrone), reducing estrogen (estradiol) levels in breast cancer cells. Aromatase inhibitors prevent aromatase activity in peripheral tissues, thereby lowering estrogen receptor activation in breast cancer cells.