Breast cancer is the most common cancer in women in the European Union (EU) and it is estimated that it represents 13.3% of all new cancer cases. The last available estimates predicted large variations in incidence and mortality rates across EU countries. In Spain, the estimated incidence and mortality rates were 132 and 24 per 100,000 women, respectively.1
In some countries higher rates of mortality from breast cancer may reflect a higher incidence, while in others they may indicate lower survival. These differences in mortality could be explained, at least partially, by inequalities in access and quality of health services.
It is not within the mandate of the EU to define health policies, nor to organise and deliver health services and healthcare, but to complement or incentivise national policies and to support cooperation between member states in the field of public health. The 2008 Council Conclusions on reducing the burden of cancer suggested the Commission ‘to explore the potential for the development of voluntary European accreditation schemes for cancer screening and appropriate follow-up of lesions detected by screening, such as a European pilot accreditation scheme for breast cancer screening and follow-up based on the European guidelines for quality assurance in breast cancer screening and diagnóstico’.2
In response to it, the European Commission Initiative on Breast Cancer (ECIBC)3 has been established that includes the development of a European quality assurance scheme (QA scheme) for healthcare services to guarantee the use of the most appropriate and updated procedures for breast cancer screening and care.
The Joint Research Center (JRC) manages and organises the scientific technical aspects of ECIBC. The JRC works independently and transparently under the auspices of the Directorate General for Health and Food Safety (DG SANTE). The ECIBC is built around two main pillars, the: i) the development of European guidelines on screening and diagnosis of breast cancer; and ii) an associated quality assurance scheme that shall guarantee equal access to high quality care in breast cancer services.
DG SANTE appointed two expert working groups to support the ECIBC: the Guidelines Development Group (GDG) and the Quality Assurance Development Group (QASDG). The experts were selected based on an open call for expression of interest to participate in the initiative. The members of these groups work on a voluntary basis. Possible conflicts of interest are evaluated annually and prior to each meeting.
The ECIBC guidelines developed by the GDG, an international and multidisciplinary panel, refer to organised population-based screening programs. The involvement of patients and patient advocates, who participate in all working group activities, ensures that the patient views and needs are properly considered during every step of the discussions. The developed recommendations are evidence-based and are regularly updated.
All recommendations are published on the ECIBC website1 together with the synthesised evidence underpinning the final conclusions. Summary information in lay language for women and patients is also made available online.
The European QA scheme defines a common set of quality and safety requirements for breast cancer services in Europe. This scheme covers all areas relevant to the provision of care for breast cancer patients and is the result of the cooperative work of the QASDG. The group is constituted by a panel of international experts in all areas of breast cancer care and quality assurance, as well as patients.
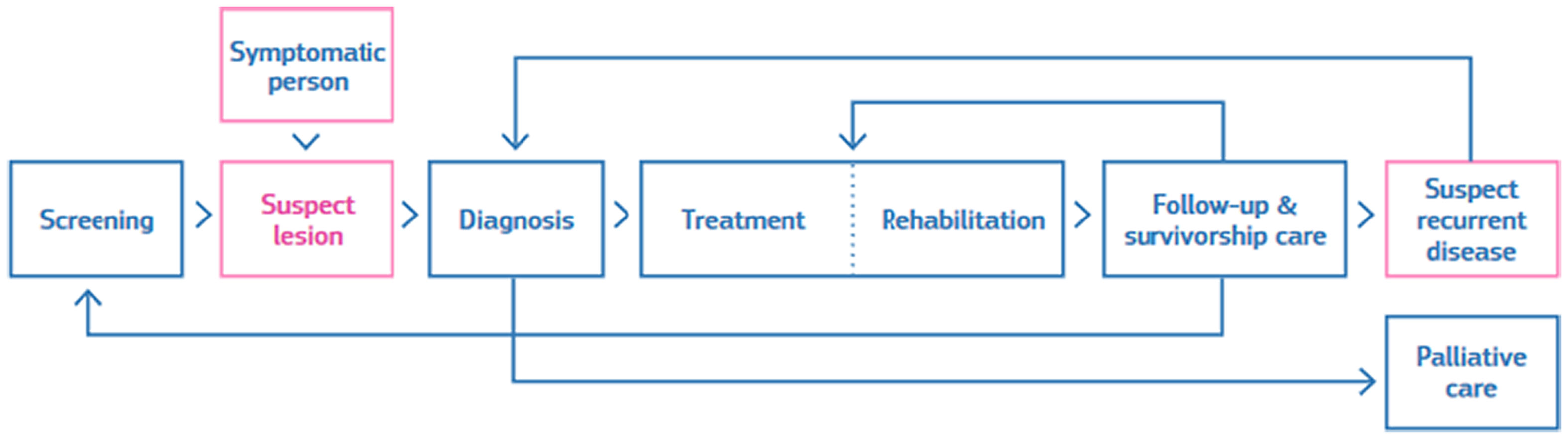
Developing such a scheme is a complex task. The first step was the definition of the scope and the process. The breast cancer pathway defined in the scheme encompasses the different stages followed by a patient, including possible entry and end points and the services involved (see Fig. 1).
Breast cancer screening and care involve different health services, depending on the individual course of the disease. The scheme includes a list of requirements for breast cancer services that assess the quality of care. Each requirement is described in a standardised way.
The requirements were developed based on reviews of existing literature, indicators and other quality assurance schemes. In addition, new requirements were formulated in areas where the expert group identified a need for quality improvement. The requirements were prioritised and scored first for relevance and understandability and after for feasibility. Relevance relates to the importance of the requirements for person-oriented care. Feasibility relates to the ability of the requirements to be implemented.
A survey published in 2014 by the EU4 showed differences in the organisation of breast cancer care between countries and regions. Therefore, the scheme has been developed as a modular scheme, allowing different legal entities or geographically separated services to participate in the different stages of breast cancer care. However, it is essential to ensure that when modules, or processes and sub-processes within modules, are offered by different entities, all entities involved in the process must take responsibility for meeting the requirements and coordinate for continuity of patient care.
The adoption of the European QA scheme is voluntary, but when an entity decides to implement it and requests certification, all the requirements of the scheme must be met. The scheme follows the accredited certification (ISO 17065). Certification is the formal recognition by an independent and impartial organisation that a breast cancer service meets all the requirements of the scheme.
The scheme contains 86 requirements covering screening, diagnosis, treatment (surgery, systemic treatment, and radiotherapy), rehabilitation, follow-up and palliative care.
Manuals describing the Scheme are freely available on the web https://healthcare-quality.jrc.ec.europa.eu/breast-quality-assurance-scheme/manuals and are detailed in Tresserra F, 2022.5
Currently, the feasibility of the requirements developed is being checked in real settings in breast cancer services/ units through a self-assessment. The next step will be to verify the accredited certification process that consists of the audit by independent third parties of the services/units. Both testing phases (feasibility and piloting) shall ensure the implementability of the scheme considering different healthcare infrastructures. Therefore, when all components are tested, improvements will be made and the QA scheme will be updated for use by Member States.
FundingThe development of the European Commission Initiative on Breast Cancer is funded by the European Commission.
EthicsThe editorial does not involve patients. The involvement of patients and patient advocates (who participate in all working group activities) in the development of the ECIBC ensures that the patient views and needs are properly considered during every step of the discussions.
Conflicts of interestThe authors are employed by the European Commission and declare no conflict of interest.