Triosephosphate isomerase 1 (TPI1) plays a role in various biological processes, specifically in the development of the metastatic phenotype, and is recognized as a significant enzyme in the glucose metabolism pathway. TPI1 has been proposed as a potential oncogene, with several types of cancer including breast cancer (BC).
MaterialThis research aims to assess activity of TPI1 in serum samples obtained from female subjects with BC. The levels of TPI1 were evaluated in the serum samples of four different BC groups, with 54 women included as the control group.
ResultsBased on the findings, the average TPI levels were (41.01 ± 7.92) and (11.91 ± 2.68) in BC patients and healthy controls, respectively. The mean level higher in BC patients compared to healthy controls (P<.001). Additionally, the serum TPI1 levels in group IV showed a significant increase (P<.0001) compared to groups I, II, and III. Analysis of both univariate and multivariate models indicated that age (odds ratio [OR] = 1.12, 95% confidence interval [CI] = 0.76–8.12, P<.01) and TPI1 levels (OR = 4.1, 95% CI = 4.31–26.71, P=.001) were identified as independent predictors for the presence of malignancy. Furthermore, receiver operating characteristic analysis demonstrated a high diagnostic accuracy for TPI concentrations, with an area under the curve value of 0.999.
ConclusionThe levels of serum TPI1 have been observed to increase in patients diagnosed with BC. There is a correlation between the concentration of TPI1 and the presence of breast malignancy.
Triosa fosfato isomerasa 1 (TPI1) juega un papel en diversos procesos biológicos, y específicamente en el desarrollo del fenotipo metastásico, estando reconocida como una enzima significativa en la vía metabólica de la glucosa. TPI1 ha sido propuesta como oncogén potencial, con diversos tipos de cáncer que incluyen el cáncer de mama (BC).
MaterialEl objetivo de esta investigación fue evaluar la actividad de TPI1 en las muestras séricas obtenidas de mujeres con cáncer de mama. Se evaluaron los niveles de TPI1 en las muestras séricas de cuatro grupos diferentes de cáncer de mama, incluyendo 54 mujeres en el grupo control.
ResultadosSobre la base de estos hallazgos, los niveles medios de TPI fueron de (41,01 ± 7,92) y (11,91 ± 2,68) en las pacientes con cáncer de mama y los controles sanos, respectivamente. El nivel medio fue superior en las pacientes con cáncer, en comparación con los controles sanos (P < 0,001). Además, los niveles séricos de TPI1 en el grupo IV reflejaron un incremento significativo (P < 0,0001), en comparación con los grupos I, II y III. El análisis de los modelos univariante y multivariante indicó que la edad (odds ratio [OR] = 1,12, intervalo de confianza [IC] del 95% = 0,76-8,12, p < 0,01) y los niveles de TPI1 (OR = 4,1, 95% IC = 4,31-26,71, p = 0,001) fueron identificados como factores predictivos independientes de la presencia de neoplasia. Además, el análisis de la curva ROC demostró una alta precisión diagnóstica para las concentraciones de TPI, con un valor de área bajo la curva (ABC) de 0,999.
ConclusiónSe ha observado que los niveles séricos de TPI 1 se incrementan en los pacientes diagnosticados de BC. Existe una correlación entre la concentración de TPI1 y la presencia de neoplasia en mama.
Breast cancer (BC) is a prevalent form of cancer in women and ranks as the second leading cause of death due to cancer among women.1 It affects approximately 2.09 million women annually, resulting in 627 000 deaths. Among Iraqi women, it is the most commonly diagnosed cancer and the primary cause of death.2 Cancer is characterized by dysregulated metabolism, making glycolysis a promising therapeutic approach for BC.3 Triosephosphate isomerase 1 (TPI1) plays a role in numerous biological processes, and is recognized as a crucial enzyme within the glucose metabolism pathway.4 TPI1 has been proposed as a potential oncogene with elevated expression observed in various cancer types such as intrahepatic cholangiocarcinoma (ICC),5 gastric,6 lung,6,7 and prostate cancer.8 Furthermore, TPI1 has been identified as a serum biomarker in cancer patients, including those with BC,9,10 lung squamous cell carcinoma6 and hepatocellular carcinoma (HCC).11 TPI1 is known to act as a tumor suppressor gene. Our study aims to demonstrate the involvement of TPI1 in the progression of BC.
Material and methodsInclusion criteriaCancer groupsThis study included 126 patients diagnosed with malignant breast tumors. Group I consisted of 30 patients with stage I BC, while Group II comprised 54 patients at stage II. Additionally, Group III included 20 patients at stage III, and Group IV consisted of 22 BC patients at stage IV. All patients received medical care at AL-baquba Teaching Hospital and were recently diagnosed, histologically confirmed, and had not undergone any treatment.
Healthy control (HC)Fifty-four female patients who did not have benign or malignant breast tumors were selected from the specialized center for early detection of BC (Baquba Hospital) between November 2023 and May 2024. Their ages ranged from 25 to 72 years. Comprehensive histories, including their ages, family history of BC (presence or absence), social status, menstrual history (age of menstruation, duration of cycles, and any menstrual disorders associated with symptoms of infertility or miscarriage), weight, and height, were obtained from the healthy women. All participants were free from medications that affect hormone levels.
Exclusion criteriaPregnant female patients with BC, patients with mixed treatment and individuals with any condition that could interfere with the research were excluded from participation.
Blood collectionVenous blood samples were aspirated following a 12-h fasting into plain tubes and centrifuged to obtain serum for the measurement of TPI1 level in the fasting state as well as other biochemical tests.
Estimation of TPI1The level of TPI1 in the serum was measured using a quantitative sandwich enzyme immunoassay technique with a kit provided by Cusabio, China. The process involved coating the microplate with a specific TPI1 antibody, then adding assay samples and standards to the tests. Any TPI1 present in the samples would bind to the immobilized antibody. Any unbound substances were removed, and a specific conjugated antibody for TPI1 was added to the samples. Horseradish peroxidase was then added to the samples after a washing process. Another washing process was performed to remove any unbound enzyme reagent, and the color of the end product was developed after a substrate solution was added. The intensity of the color was determined after stopping the color development.
Determination of cancer-related parametersThe liver enzymes (AST, ALT, and ALP), urea, and creatinine were determined by auto analyzer ARCHITECT c4000. The ARCHITECT c4000 clinical chemistry analyzer performs diagnostic tests that monitor patients' levels of biochemical variables.
Ethical approvalBefore being included in the study, each participant provided informed written consent, and the research adhered to the principles outlined in the Helsinki Declaration. The study received approval from the ethics committees of Baquba Teaching Hospital, University of Diyala, and the National Center for Training and Human Development of the Iraqi Ministry of Health.
Statistical analysisStatistical analysis was conducted using SPSS (version 25). For numerical variables with a normal distribution, the data were presented as the mean ± standard error. The ANOVA test and independent t-test were employed to assess significant differences between these numerical variables, with a significance level set at P < .05. The t-test was utilized to determine the significance of the Pearson correlation for the relationship between 2 quantitative variables. The identification of independent predictors for ongoing data involved employing linear regression models. Significant indicators of quantitative results were identified through both univariate and multivariate logistic regression analyses. To evaluate the diagnostic potential of biomarkers, multiple assessments were performed within healthy subjects and 4 different stages of cancer groups.
ResultsDemographic characteristics of patients with breast cancer (BC) and healthy control subjectsThe average age of individuals diagnosed with BC was 53.85 years with a standard deviation of 10.97, and a range of 23–72 years. In comparison, the average age of the HC group was 50.59 years with a standard deviation of 12.27, and a range of 25–72 years. Importantly, there was no statistically significant difference in mean age between the patients and the HC group (P = .215). Table 1 displays the frequency distribution of patients with BC and control subjects by age group. There was no significant difference in the distribution of patients and control subjects by age group (P = .502). The majority of patients with BC in the study were over 50 years old, comprising 65.1% of the total. Of these patients, 65.1% resided in urban areas and 34.9% in rural areas, while among the control group, 48.1% resided in urban areas and 51.9% in rural areas. In terms of residency, there was no noticeable variation in the occurrence rate of patients and control subjects (P = .133). The average weight of patients was 66.65 ± 10.56 and that of control subjects was 66.18 ± 11.27 years, with no significant variance between patients and control subjects in terms of weight mean (P = .851). With respect to BMI, the current findings indicate non-significant distinctions between BC patients and control subjects based on the average BMI (P = .923). The current study revealed that there was no significant variation in the distribution of individuals in both groups with respect to age and place of residence.
Demographic characteristics of patients with breast cancer (BC) and healthy control and levels of some biochemical parameters in patients with BC and healthy control subject.
| Characteristic | Patients with BC | Healthy control | P |
|---|---|---|---|
| n = 126 | n = 54 | ||
| Age (years) | |||
| Mean ± SD | 53.85 ± 10.97 | 50.59 ± 12.27 | .215†NS |
| Range | 23–72 years | 25–72 years | |
| <40, n (%) | 8 (12.7%) | 6 (22.2%) | .502 ¥NS |
| 40–49, n (%) | 14 (22.2%) | 6 (22.2%) | |
| ≥50, n (%) | 41 (65.1%) | 15 (55.6%) | |
| Residency | |||
| Urban, n (%) | 41 (65.1%) | 13 (48.1%) | .133 ¥NS |
| Rural, n (%) | 22 (34.9%) | 14 (51.9%) | |
| Weight (kg) | |||
| Mean ± SD | 66.65 ± 10.56 | 66.18 ± 11.27 | .851 ¥NS |
| Range | 49–88 Kg | 48–89 Kg | |
| Height (cm) | |||
| Mean ± SD | 162.93 ± 4.98 | 162.14 ± 5.35 | .703 ¥NS |
| Range | 150–175 cm | 150–171 cm | |
| Body mass index (BMI) kg/m2 | |||
| Mean ± SD | 25.07 ± 3.67 | 25.15 ± 4.05 | .923†NS |
| Range | 19.14–34.41 | 19.23–34.39 | |
| Blood urea | |||
| Mean ± SD | 50.44 ± 7.40 | 26.30 ± 6.98 | <.001†HS |
| Range | 22.10–88.20 | 17.00–44.00 | |
| Serum creatinine | |||
| Mean ± SD | 1.36 ± 0.32 | 0.69 ± 0.088 | <.001†HS |
| Range | 0.23–2.91 | 0.60–0.95 | |
| Alanine transaminase (ALT) | |||
| Mean ± SD | 52.35 ± 12.42 | 26.51 ± 4.74 | <.001†HS |
| Range | 18.30–104.30 | 18.20–35.50 | |
| Aspartate aminotransferase (AST) | |||
| Mean ± SD | 45.15 ± 10.85 | 21.71 ± 5.01 | <.001†HS |
| Range | 13.20–98.10 | 13.20–29.30 | |
| Alkaline phosphatase (ALP) level | |||
| Mean ± SD | 72.79 ± 12.12 | 65.92 ± 9.94 | .156†NS |
| Range | 32.0–138.0 | 48.0–83.0 | |
n: number of cases; SD: standard deviation; †: independent samples t-test; ¥: Chi-square test; HS: highly significant at P≤.001; NS: not significant at P > .05.
A comparative analysis of certain biochemical parameters was conducted between patients diagnosed with BC and HC subjects. The findings have been presented in Table 1. The average blood urea levels were 50.44 ± 7.40 and 26.30 ± 6.98 in patients with BC and HC subjects, respectively, signifying a statistically significant elevation in the patient group compared to the HC group (P > .001). Similarly, the mean serum creatinine levels were 1.36 ± 0.32 and 0.69 ± 0.088 in the BC patient group and HC group, respectively, demonstrating a highly significant difference (P > .001). Furthermore, the study revealed a significantly higher mean alanine transaminase (ALT) level in BC patients compared to HCs, with values of 52.35 ± 12.42 versus 26.51 ± 4.74, respectively (P > .001). The mean levels of ALT and aspartate aminotransferase (AST) were also significantly higher in patients with BC compared to HC subjects, with respective P-values of <.001 for both parameters. However, there was no significant difference in the mean alkaline phosphatase (ALP) levels between the 2 groups despite being slightly higher in patients with BC (P < .001).
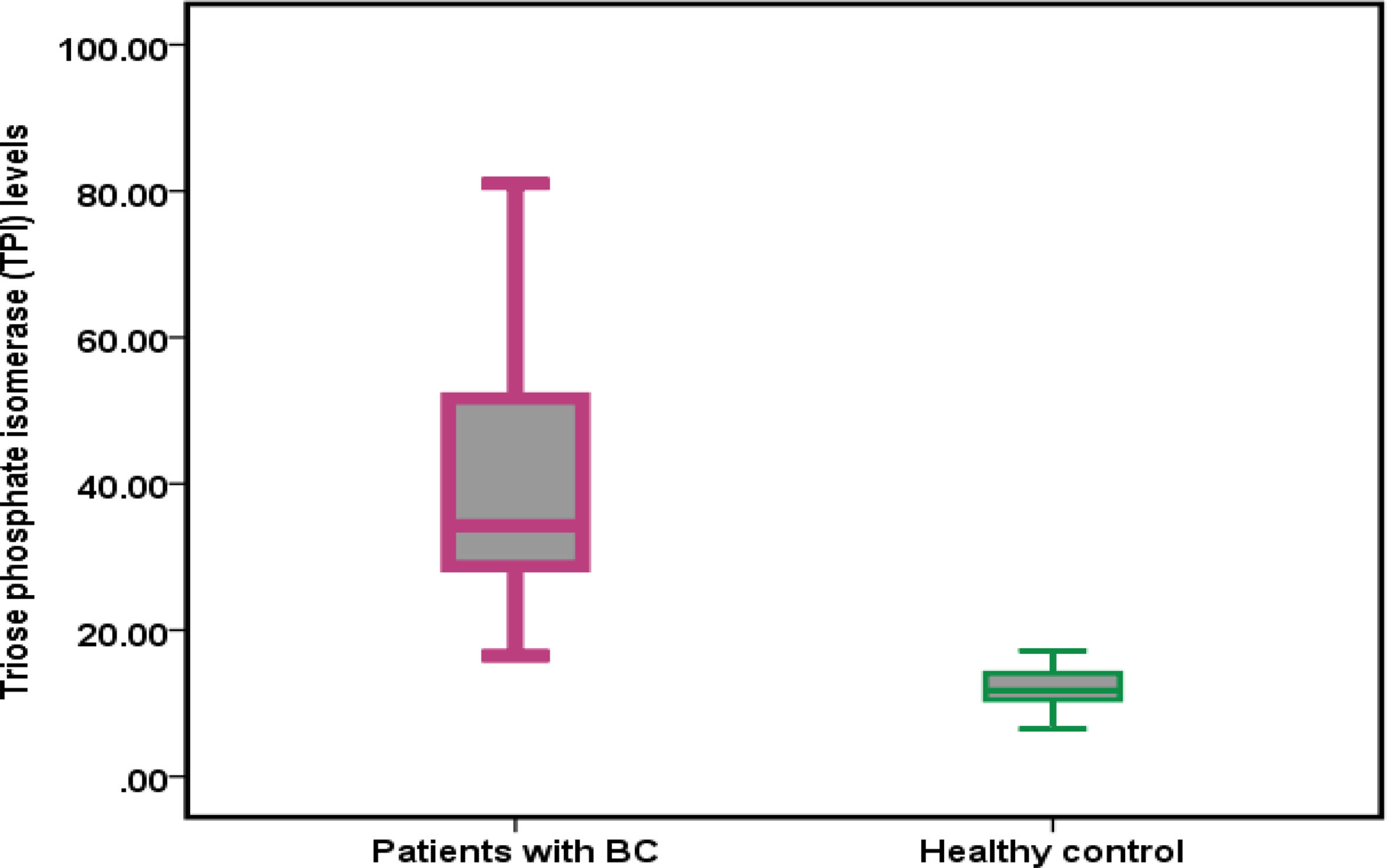
Triose phosphate isomerase (TPI) level in patients with BC and healthy controlA comparison was conducted to determine the levels of TPI in patients with BC and healthy individuals. The findings are presented in Fig. 1. The mean TPI levels were 41.01 ± 7.92 and 11.91 ± 2.68 in patients with BC and HC subjects, respectively. The difference in mean TPI levels between patients with BC and HCs was found to be statistically significant (P < .001).
Frequency distribution of TPI1 levels according to BC stageTPI1 levels were compared across different stages of BC, and the findings are shown in Table 2. The mean TPI1 levels were 25.63 ± 5.14, 39.90 ± 8.71, 44.58 ± 7.33, and 58.19 ± 9.03 in patients with stage I, II, III, and IV, respectively. The mean TPI1 level was notably higher in patients with stage IV compared to the other groups, and this difference was statistically significant (P = .001).
Frequency distribution triose phosphate isomerase (TPI) levels according BC stage.
| Characteristic | Stage I (n = 30) | Stage II (n = 54) | Stage III (n = 20) | Stage IV (n = 22) | P |
|---|---|---|---|---|---|
| Triose phosphate isomerase (TPI) | |||||
| Mean ± SD | 25.63 ± 5.14 | 39.90 ± 8.71 | 44.58 ± 7.33 | 58.19 ± 9.03 | .001†NS |
Different letters denote to the significant differences at P<.05
n: number of cases; SD: standard deviation; †: One way ANOVA; NS: non-significant at P<.05.
Several studies identified independent factors associated with the presence of malignancy through the use of both univariate and multivariate models. When comparing individuals undergoing treatment for known BC and those recently diagnosed, age (odds ratio (OR) = 1.12, 95% confidence interval (95% CI) = (0.76–2.12), P < .05) and TPI1 levels (OR = 4.1, 95% CI = (4.31–26.71), P = .001) were the sole independent predictors in the combined group analysis (Table 3).
Independent predictors for the presence of cancer.
| Characteristic | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age | 1.07 (0.61–7.47) | 0.482 | 1.12 (0.76–8.12) | .501 |
| Weight | 1.007 (0.61–6.44) | 0.498 | – | – |
| Height | 1.005 (0.17–6.2) | 0.583 | – | – |
| BMI | 0.99 (0.059–4.83) | 0.499 | – | – |
| Urea | 1.92 (0.15–11.9) | 0.322 | – | – |
| Creatinine | 1.97 (0.35–18.6) | 0.269 | – | – |
| ALT level | 1.99 (0.18–12.82) | 0.214 | – | – |
| AST level | 2.08 (0.24–14.84) | 0.201 | – | – |
| ALP level | 1.13 (0.067–7.98) | 0.347 | – | – |
| TPI level | 3.44 (2.01–19.71) | 0.001 | 4.1 (4.31–26.71) | .001 |
The study aimed to evaluate the diagnostic accuracy of using TPI concentrations to distinguish between patients with BC and HC participants through receiver operating characteristic (ROC) analysis. The findings, presented in Fig. 2, revealed that an optimal TPI cut-off value exceeding 16.85 yielded an AUC value of 0.999 (95% CI, 0.997–1.000, P < .001), with a sensitivity of 98.4%, specificity of 96.3%, PPV of 98.4%, and NPV of 96.3%. These results indicate that TPI can be considered an outstanding diagnostic marker for BC.
DiscussionIn this study, it was showed that the activities of liver enzymes and renal function tests were elevated in the sera of BC patients when compared to a matched HC group. Liver damage and the resulting biochemical abnormalities are common in cancer patients, possibly due to metastasis, concurrent drugs, or prior liver damage such as cirrhosis.12 When it comes to kidney function, the increased concentration of serum creatinine may not always be a reliable indicator of kidney function in certain clinical situations. It can be influenced by various patient factors that are not directly related to kidney function, such as age, sex, nutritional status, overall muscle mass, and tubular secretion.13 In the context of cancer, tumor growth is often linked to a significant decrease in body mass, which can be further worsened by chemotherapy. As a result, these fluctuations in body weight can lead to significant variations in creatinine levels.14 Also, serum urea level was significantly increased in malignant BC patients than in healthy women. This finding is similar with the study conducted in India which showed that the level of urea was significantly increased in BC cases compared with controls.15
Metabolic enzymes play a role in the conventional processes of cancer cells by facilitating the reconfiguration of their metabolism to meet the cellular demands. Nevertheless, several metabolic enzymes found in cancerous growths also perform unorthodox or non-metabolic “moonlighting” functions.16,17 These non-canonical roles of metabolic enzymes, contribute significantly to tumor development due to their involvement in various cellular processes.18,19 In this study, it was observed that the activities of TPI1 were elevated in the sera of BC patients when compared to a matched HC group. These findings suggest that TPI1 may play a role in the oncogenesis of BC. Nonetheless, although no statistically significant difference was observed between the 2 groups, previous studies did not explore the correlation between TPI expression and patient characteristics or clinicopathological factors likely due to limited sample size. Consistent with similar publications, our study's results indicated that TPI was upregulated in the sera of BC patients. After controlling for potential covariates, we observed notably elevated serum TPI1 levels in patients with stage III and IV BC compared to those newly diagnosed with the disease. Moreover, we identified a positive correlation between TPI1 concentrations and tumor behavior. Our findings indicate that TPI1 could be implicated in the initiation and advancement of BC, possibly during the early phases of tumorigenesis. This implies that TPI1 might serve as a valuable indicator for diagnosing and predicting the course of breast tumors; additional prospective research is essential to validate this potential. There are various favorable aspects highlighted in our study. Initially, the groups were matched based on age. The study encompassed newly diagnosed BC patients, with a majority lacking access to chemotherapy. This may be attributed to abnormal TPI1 levels secreted by -cells during the early stages of BC. Moreover, patients with BC received only a singular form of treatment. Lastly, the patients were categorized into subgroups based on the disease stages. This study has certain limitations. Firstly, the sample size was relatively small. Also, a more comprehensive investigation into TPI1 levels at the time of diagnosis and during the course of BC treatment could enhance researchers' understanding of the role of TPI1 as a potential marker or therapeutic target.
ConclusionThe findings indicate a significant association between serum TPI1 levels and newly diagnosed patients. Therefore, we suggest that serum TPI1 could serve as an early diagnostic tool in BC patients, with a cut-off value of 16.85 ng/ml being the most effective in distinguishing patients from controls.
FundingNone.
Ethical considerationsThe Baquba Teaching Hospital Committee of Ethics, and the Iraqi Ministry of Health's National Centre for Training and Human Development all permitted this study. The research was carried out in accordance with the Helsinki Declaration.
Informed consent1.All Participation by individuals capable of giving informed consent as subjects in medical research voluntary. and to consult family members or community leaders, no individual capable of giving informed consent enrolled in this research study unless he or she freely agrees.
2. This medical research involved human subjects capable of giving informed consent, each subject was adequately informed of the aims, methods, sources of funding, any possible conflicts of interest, institutional affiliations of this researcher.
3. When I sought informed consent for participation in my research study the subject was in a no relationship with the physician and no consent under duress.
4. All subjects who are incapable of giving informed consent, not included in a research study.
The researchers express their gratitude to all those who have remained involved in this study.