Background
Cancer pain is the result of cancer growth in human tissues, or the pain produced by any of the therapies used to treat it. Adequate pain control can be achieved in the great majority of patients by means of aggressive pharmacological treatment with the use of opioids and adjuvants1,2. With these strategies, 90-95% of the patients could achieve adequate pain control3. Consequently, 5-10% of patients will need some form of invasive therapy. For the successful management of these patients it is critically important to start with a thorough assessment by means of clinical history and physical examination, and the judicious use of diagnostic testing to try to define the pathophysiological components involved in the expression of pain in order to implement optimal analgesic therapy. Although intrathecal opioids are very effective for the treatment of somatic and visceral pain, intrathecal bupivacaine and/or clonidine will be needed for the treatment of neuropathic pain. Thus, defining the specific pathophysiological component(s) will be critical for the successful management of these patients. Thus, when following specific guidelines, the great majority of patients with cancer related pain should expect adequate pain control in the 21st century. Control of pain and related symptoms is a cornerstone of cancer treatment, as it may lead to an increase in the quality of life, improved functioning, better compliance and a means for patients to focus on those things that give meaning to life4. In addition to their salutary effects on quality of life, mounting evidence suggests that good pain control may positively influence survival5,6.
Intraspinal Analgesia
Neuraxial analgesia is achieved by the epidural or intrathecal administration of an opioid alone (very rarely) or in combination with other agents such as bupivacaine, clonidine or ziconotide. With the use of neuraxial analgesia, pain relief is obtained in a highly selective fashion with the absence of motor, and sympathetic blockade, making these modalities highly adaptable to the home care environment. When first introduced, the philosophy behind neuraxial opioid therapy was that administering small quantities of opioids in close proximity to their receptors in the substantia gelatinosa of the spinal cord, one could achieve high concentrations at these sites7,8. Thus, analgesia is superior to that achieved when opioids are administered by other routes, and since the total amount of drug administered is reduced, side effects are minimized. Currently, the biggest advantage is the ability to use multiple agents to target multiple receptors resulting in better neuropathic, somatic and visceral pain control while minimizing side effects.
In general, patients with a survival expectancy greater than three months will be candidates for intrathecal therapy with a permanent intraspinal catheter and an implanted subcutaneous pump. Conversely, those patients with survival expectancy less than 3 months will require epidural therapy with an implanted system, such as the Du Pen's® epidural catheter9, or the Sims® epidural port-a-cath which will be connected to an external pump with PCA (Patient-controlled anesthesia) capabilities. When considering a patient for intrathecal therapy with a permanent intrathecal catheter and a subcutaneous pump, a trial with an epidural catheter will be necessary to: 1) Assess the need for intrathecal multimodal therapy, 2) estimate the doses of the opioid to be used, 3) confirm the best site for catheter tip positioning.
Consequently, the tip of the epidural catheter will need to be placed at the site where nociception is being processed within the spinal cord. We conduct this trial on an out-patient basis to achieve a 50% decrease in pain. If successful, we will proceed to implant the permanent device. For this purpose, we use the following protocol:
Epidural Trial
— Catheter position: dermatomal specific for the area of nociception under fluoroscopy guidance:
1. Opioids:
a) Morphine: 0.1 (60 mg) - 0.2 (120 mg) mg/ml
b) Hydromorphone: 0.03 (20 mg) - 0.12 (80 mg) mg/ml
2. Bupivacaine: 1-2 mg/ml (0.1-0.2%)
3. Total volume 600 ml.
4. If the patient's source of nociception is in the lower lumbar or sacral areas, which precludes the use of high concentrations of bupivacaine, we use a more diluted solution of bupivacaine (0.05%) to minimize the possibility of motor block and we compensate by adding clonidine: 3-5 mcg/ml.
— Determining epidural opioid doses:
1. If the patient is receiving > 300 ug/h of Fentanyl or 1200 mg/day of ¿MS? or 600 mg/day of Oxycodone or 160 mg/day of methadone, or >300 mg/day of oxymorphone:
a) Hydromorphone: 0.12 mg/ml
2. If the patient is receiving between 100 and 300 ug/h of fentanyl or an equivalent opioid dose:
a) Hydromorphone: 0.06 mg/ml
3. If the patient is receiving less than 100 ug/h of fentanyl or equivalent dose:
a) Hydromorphone: 0.03 mg/ml
— Basal infusion: 2 ml/h
— No bolus during the first 72 hours
1. Then 2 ml q 10 min
— The goal is to determine patient requirements
— Trial for 7-14 days as an outpatient.
If the patient had a successful trial, as defined above, we proceed to implant an intrathecal system. We suggest the following protocol to achieve more than 80% success rate:
— Conditions for success
a) Place the tip of the intrathecal catheter in the dermatome corresponding to the area of nociception under fluoroscopy guidance.
b) Combinations of local anesthetics and an opioid will be needed for severe somatic pain
c) For neuropathic pain:
A. If the tip of the catheter is below L3-4: Initial therapy with opioid + clonidine
B. If the tip of the catheter is above L1-2: Initial therapy with opioid + bupivacaine
The doses and drugs that we use in our practice are (82):
Thus, compounding by a trained pharmacist will be needed. The goal is to concentrate these drugs to twice the daily dose, so that the 20 ml programmable pumps may be programmed to deliver 0.5 ml/h. In this way, patients will need pump refills monthly and it will not be a burden to their quality of life by having to make visits to the pain specialist clinic. The steps that we use to implement the therapy are:
Step 1:
1. Opioid + bupivacaine:
a) MS 3-25 mg/day or hydromorphone 0.5-15 mg/day ** 6 mg of MS/day = 1 mg of hydromorphone/day
b) Bupivacaine: 6-20 mg/day
2. Opioid + clonidine:
a) Clonidine: 250-2000 ug/day
Step 2: Opioid + bupivacaine + clonidine Step 3: Ziconotide:
1. Initiate therapy with ziconotide at a dose of 2.4 ug/day (0.1 mcg/hr) and titrate to patient response
2. Rinse the pump with 2 ml of the 25 ug/ml solution three times and then fill the pump with the balance (16 ml)
a) Titration increments should not be more than 2.4 ug/ day or more frequent than once per week
b) Maximum recommended dose: 19.2 ug/day (0.8 u/hr)
3. In particular situations, the use of morphine + ziconotide may be an alternative10. However, the limitations include the following:
a) There is not the benefit of a trial, as ziconotide may not be administered in the epidural space. Consequently, the patient will need progressive titration once the implanted system is in place.
b) Patients may not allow the practitioner to carry out a titration protocol over 4-6 weeks since:
c) The starting dose for ziconotide is 2.4 ug/day with weekly increases of no more than 2.4 ug/day
— Therapeutic effects are not usually seen until a dose of 8-10 ug/day is reached.
Recently, the option to co-administer ziconotide with morphine has emerged. A phase II, open-label, multicenter study of combined intrathecal morphine and ziconotide as add on therapy in 26 patients with non-cancer pain showed that the mean improvement in pain, as judged by visual analog scale measurements was 14.5% from baseline to week 510. Moreover, there was a mean decrease in opioid therapy of 14.3% at week 5. Treatment related side effects included mental confusion, dizziness, abnormal gait, hallucinations, and anxiety. Consequently, both the mean pain improvement and the mean opioid sparing effect produced by the use of this agent where clinically insignificant. However, the maximum dose of ziconotide used in this study was 7.2 ug/day and that may explain the marginal results.
If triple therapy with an opioid, bupivacaine and clonidine at optimal doses is not working or one considers the need to implement therapy with ziconotide, then evaluation for catheter obstruction, disconnection, catheter migration, or pump malfunction is a must. In doing so, consider the following possibilities:
1. Pump: Computer program analysis for volume and the volume present within the pump needs to be within 10% of each other, otherwise pump failure is suspected due to:
a) MRI Effects (Medtronic Medical Device Correction, August 2008).
There is a potential for a delay in the return of proper drug infusion after a MRI affecting all SyncroMed pumps. Moreover, with SynchroMed II pumps, there is the potential for a delay in the logging of motor stall events after MRI. Although the reported incidence of these phenomena is very low (0.014% and 0.11%, respectively) it is important to interrogate all the pumps after the MRI, to spare patients from not receiving medication. This is particularly important for SynchroMed pumps, as a "Pump Memory Error" may be generated and the pump will NOT restart infusing unless it is reprogrammed. In contrast, the SynchroMed II may continue infusing even though the interrogation may show a stall state. In either case, the pump will alarm in the face of a stall phenomenon.
b) Missing Propellant within the pump: Synchromed® II Missing Propellant. Models Affected: 8637-20, 8637-40 (Medtronic Medical Device Recall - May 2008)
c) Synchromed® EL Pump Motor Stall Due to Gear Shaft Wear (Patient Management Information [Medtronic, August 2007].
2. Catheter: A myelogram performed through the diagnostic port of the pump will be needed to determine if there is obstruction, disconnection (Medical Device Safety Alert -June 2008: Proper Connection of Sutureless Connector Intrathecal Catheters Models Affected: 8709SC, 8731SC, 8596SC, 8578), and the position of the tip of the catheter. When performing a myelogram through the diagnostic port of the pump, remember that this only accommodates a 25 gauge Huber needle. Moreover, consider:
A. The dead space of the catheter when injecting the contrast medium: 0.196 ml [89 cm total catheter length (81.4 cm for the spinal segment + 7.6 of the catheter interface with the sutureless connector) x 0.0022 ml/cm catheter volume for the model 8709 SC]
B. The need for a bolus dose after the study is completed, as the catheter will be filled with contrast medium. Consequently, at a programmed rate of 0.5 ml/hr it will take 9.4 hours for the pump to clear all this volume resulting in inadequate pain control and possibly opioid withdrawal symptoms.
When performing pump's diagnostic port injections, one needs:
A. To withdraw enough amount of cerebrospinal fluid/ therapeutic solution prior to injecting contrast medium to remove all the volume of the drug within the catheter and avoid giving the patient a bolus of the medications in use. If this was not performed, up to 0.196 ml of solution could be pushed alone with the contrast medium. Likewise, we suggest that one should aspirate the fluid with a 3 ml syringe at a very low negative pressure to avoid turbulence and the risk of leaving medication within the catheter (cavitations phenomenon). We usually aspirate a total of 3 ml of fluid, as this should contain all the medication left in the catheter's dead space and some CSF.
B. A bolus dose should be programmed after the myelo-gram to clear the catheter's dead space containing contrast medium at this point. By doing so, one avoids leaving the patient without intrathecal treatment for periods of 16-20 hours depending of how much catheter was implanted.
Clinical Studies
A recently published multicenter prospective randomized clinical trial by Smith, et al., compared intrathecal therapy to comprehensive medical management (CMM) after 1 month of therapy in 202 cancer patients with refractory pain11. The primary outcome measure was a 20% improvement in analgesia, as measured via a 0-10 visual analog scale. Side effects changes based on the National Cancer Institute's common toxicity criteria were also recorded. There was a slight trend toward better analgesia in the intrathecal group, but this difference did not achieve statistical significance. In contrast, there was a statistical difference in the side effect profile of those patients randomized to the intrathecal group. The two side effects where the therapy had its greatest impact were constipation and level of consciousness. After a six month analysis, there was also a trend towards an increased survival in the intrathecal group (54% versus 37%). Even though the number of patients who were alive at the end of the analysis was small, this difference is about a 25% increase survival in the patients randomized to the intrathecal group when compared to the CMM group.
A longitudinal prospective analysis of 30 crossover patients who received intrathecal therapy found significant decreases in pain scores and drug toxicity (27% and 51%, respectively)12. Median survival was 103 days after crossover to an IDDS, which was similar to that of patients in the randomized controlled trial12.
The cost of implementing intrathecal therapy is initially high, because of equipment acquisition cost. In contrast, the cost of implementing long-term epidural therapy is low. Two studies evaluated the cost of implementing therapy with these two modalities. These analyses show a "break even" point at approximately 3 months13,14. Thus, epidural therapy becomes very expensive after 3 months, and is one of the reasons to limit its use in patients with survival expectations of less than 3 months.
Clinical Guidelines
A consensus panel was recently published to update recommendations on the use of intrathecal medications in chronic non-cancer pain15. Their goal was to:
1. Review the conclusions and guidelines of the Polyanalgesic Conference 2000 and Polyanalgesic Conference 2003.
2. Evaluate the current guidelines for intrathecal (IT) drug infusion.
3. Review survey responses of fellow peers in the field of IT analgesics for pain management and use the findings to guide discussion during the conference.
4. Review preclinical and clinical data relevant to IT anal-gesics published since 2000.
5. Formulate consensus opinions on critical issues for IT polyanalgesic therapy.
6. Modify and update the IT analgesic drug selection algorithm, as appropriate, based on "best evidence" from published data and expert consensus opinion.
7. Identify areas, including promising under-researched and experimental analgesic agents, for future evidence based research that will advance the clinical practice of IT drug infusion therapy.
8. Disseminate the consensus opinions and primary conclusions of the expert panelists to the medical community through data-driven articles published in appropriate peer-reviewed biomedical journals.
Although the consensus limits its conclusions to the non-cancer population, there are five issues that are important to discuss in light of the recommendations given in this review:
1. Hydromorphone equianalgesic doses
2. Hydromorphone maximum dose
3. Bupivacaine spinal cord lesions
4. Ziconotide as first line agent
5. The use of CT- Myelography for the diagnosis of granulomas at the tip of the intrathecal catheter.
1. Hydromorphone Equianalgesic Doses:
The study by Johansen et al16 quoted in the consensus (reference 36) did not study equianalgesic doses between morphine and hydromorphone. Johansen et al. simply administered hydromorphone at "a dose equivalent to the minimum intrathecal morphine dose shown to produce inflammatory masses in our sheep model (12 mg/day)". Thus, there is no basis for the authors of the consensus to conclude that "intrathecal (IT) morphine and IT hydromorphone, in a dose 20% of that of morphine, induce an equianalgesic response". Nonetheless, the discussion in the Johansen paper states that the morphine to hydromorphone conversion rate is 5-6:1: "No masses were observed at hydromorphone doses (3 and 6 mg/day) that were equianalgesic to morphine doses (18 and 36 mg/day, respectively)"16. This is the conversion rate that we have used in our clinical practice but there has not been a trial to support the validity of this conversion figure.
2. Hydromorphone Maximum Doses:
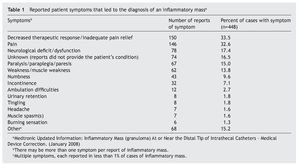
The consensus panel recommends a maximum hydromor-phone concentration of 10 mg/cc and a maximum dose of 4 mg/day for intrathecal use to prevent granuloma formation. Throughout the manuscript, there is not a single reference to support this recommendation and they acknowledge that "physicians are advised to titrate doses of these two opioids (morphine and hydromorphone) not beyond an apriori upper limit that has been determined from clinical practice"15. To date, we have treated about 60 patients with IT hydromorphone in combination with bupivacaine and/or clonidine at concentrations and doses well beyond these recommended concentrations without a single incidence of granuloma. It is noteworthy that we survey these patients with magnetic resonance imaging on yearly basis to make an early diagnosis of this condition. Moreover, we ask patients on their monthly refill visits about symptoms that may be associated with the development of these masses (Table 1).
3. Bupivacaine Spinal Cord Lesions:
The preclinical discussion on the use of IT bupivacaine in the Consensus15 begins with the following statement: "Transient neurological syndrome (TNS), defined as radicular irritation after spinal anesthesia with local anesthetics, is hypothesized to fall on the lower end of a spectrum of toxic effects caused by local anesthetics". It is noteworthy, that there is not a single report on TNS after bupivacaine spinal anesthesia. In contrast, it has been associated with the use of lidocaine and mepivacaine17. Consequently, the discussion of this syndrome in the bupivacaine section is out of contest and misleading. Additionally, there is the suggestion that bupivacaine/clonidine combinations could result in spinal cord lesions, based on a case report18. This appears as a footnote in the Recommendations section of the manuscript that states: "a spinal cord lesion has been reported with the use of bupivacaine at a concentration of 20 mg/ ml" 18. It is important to recognize that in the reported case, the neurological deficit suddenly appeared 2 years after therapy with bupivacaine and clonidine at doses of 20 mg/day and 200 ug/day respectively. The patient was a male individual who had been receiving a perfusion of this solution for about two years for a right sciatic cord compression neuropathy after a suicide attempt. The patient developed a neurological deficit 1 week after sustaining a fall and landing on his back. Neurological examination 1 week after the fall revealed gait ataxia with impaired proprioception in the left leg. No vibration sensation up to the left knee and a left foot drop was noted. Three days after these findings, he was found to have complete loss of proprioception bilaterally up to T11, hyper-reflexia in the left lower extremity and bilateral hypoesthesia of all sacral segments. The MRI showed a round cavity within the spinal cord measuring 3 mm in diameter at the T9-11 level associated with edema that extended from the T5 level to the conus medullaris. The tip of the intrathecal catheter had migrated from the T12 to T10 level. The drug infusion was stopped and the patient's neurological status improved over the following three months and he experienced improvement of the cortico-spinal signs, but only moderate improvement in the proprioception and the gait ataxia.
It is unclear if the spinal cord changes were related to drug neurotoxicity, particularly as the rate of administration was 0.5 ml/h and the edema in the spinal cord extended from the conus medularis to T5 level. The CSF spread of the intrathecal solutions administered at a rate of 0.5 ml/h has been shown to be very limited both in the animal model19, and in humans20. Consequently, it is difficult to understand how the edema in the spinal cord was so extensive. Moreover, the tip of the catheter had migrated from the T12 to the T10 level, where the lesion was found, raising the possibility that this could be the result of spinal cord catheter injury during the fall.
4. Ziconotide as First Line Agent:
The last polyanalgesic consensus recommended the use of ziconotide in chronic pain when all other options were exhausted21. At that time, the drug had not been FDA approved and the only randomized clinical trial available was the study by Staats et al22. In contrast, the panelists of the new recommendations have upgraded ziconotide to a first line agent at the same level as morphine and hydromorphone15.
Since it is acknowledged that "the medications in the current algorithm are arranged in a hierarchy based on evidence on safety, efficacy, and broad clinical parameters gleaned from previous and current consensus literature reviews, ratings of published studies, and expert opinion from three Polyanalgesic Consensus Conferences" 21, the questions is whether there is enough new data on therapeutic efficacy and safety to support that recommendation.
In the study by Staats et al22, there are two concerns:
First, the physiopathology of pain in cancer patients is disease and site specific, and may be multifactorial. Thus, treating patients without a clear description of the source of nociception (i.e., somatic, versus visceral, versus neuropathic) could be a problem.
Second, the 2 week follow-up may result in two problems. As previously discussed, ziconotide needs a significant titration window to reach a therapeutic effect and this is not normally achieved within a two week period. Thus, it is possible that the investigators were evaluating placebo effect at that time. Consequently, therapeutic responses beyond that time may have decreased and the success rate might have been lower if the follow-up was longer. Consequently, the results of this study do not fully support the use of this agent as first line.
Since the publication of the Staats et al study, five other studies addressing the use of ziconotide in severe non-cancer chronic pain have been published23-27.
Conclusion of consensus based on references 164-172
In the first study, 644 patients with severe chronic pain were studied in an open label, multi-center study with ziconotide23. In the end, 119 patients were treated for at least 1 year. Median duration of therapy was 2 months with a range of 1 to 1215 days. Mean dose was 8.4 ug/day (range 0.048-240 ug/day). Pain scores decreased from 76 mm to 68 mm after one month of therapy and to 73 mm after 2 months of therapy. Virtually all patients experienced adverse events (99.7%), of which 43.5% were mild, 42.3% moderate, and 14.2% severe. Half of those adverse events were considered non-therapy related. The most common side effects (≥ 25) were nausea, dizziness, headache, confusion, pain, somnolence, and memory impairment. The authors concluded that "long-term IT ziconotide is an option for patients with severe refractory pain". However, the high incidence of side effects and the clinically insignificant pain reduction does not support therapeutic efficacy under the present protocol design.
In the second study, in what appears to be the need to address the lack of therapeutic effects reported in the first study, the safety and efficacy of adding IT ziconotide to intrathecal morphine in patients receiving a stable IT morphine dose24. Twenty-six patients receiving doses ranging between 2-20 mg/day of morphine received 0.6 to 7.2 ug/day of IT ziconotide. The mean percentage improvement of pain in the visual analog scale was 14.5% (95% confidence interval of -9% to 38%) from baseline to week 5. The mean percentage oral opioid dose change from baseline was -14% at week 5. The investigators concluded that the co-administration of IT ziconotide and morphine may reduce pain and decrease systemic opioid use in patient receiving treatment with IT morphine alone24. However, both the mean decrease in pain intensity, as judged by the visual analog scale, and the amount of systemic opioid reduction are clinically insignificant and do not support these conclusions. Moreover, there is evidence of decreased ziconotide stability when co-administered with either morphine or hydromorphone15. Thus, at this point it is not clear what the clinical advantage of co-administering ziconotide with morphine is.
In the third study26, 255 patients were randomized to receive ziconotide (n=169) or placebo (n=86) during six days as in-patients. Patients received doses ranging from 9.6 ug/ day to 168 ug/day. But during the course of the study doses were reduced to 2.4 to 57.6 ug/day due to the high prevalence of side effects with the initial doses. The authors reported a 31% pain reduction in the ziconotide group versus a 6% reduction in the placebo group. Despite this significant pain reduction, it is noteworthy that of the 169 patients initially treated with ziconotide, only 54 patients (31%) were considered responders and were eligible for five-day outpatient treatment26. Treatment responders were defined as patients having 1) a ≥ 30% pain improvement in the VASPI compared to baseline, 2) stable or decreased concomitant opioid analgesic use, and 3) no changes in type of opioid used during the study period.
5. The use of CT- Myelography for the diagnosis of granulomas at the tip of the intrathecal catheter:
The authors of the consensus suggest that "MRI remains the gold standard for surveillance when evaluating the presence of a catheter-related inflammatory mass, although computed tomography/myelography through the pump offers a more cost-effective technique". This is true, provided that the practitioner is able to aspirate CSF from the diagnostic port prior to performing a myelography study. As noted before, if CSF is not aspirated prior to injecting the contrast medium, the catheter dead space volume will also be injected, and severe side effects may occur.
Conflict of interests
The authors have no conflict of interest to declare.
Correo electrónico:Oscar.DeLeon@RoswellPark.org
Recibido el 20 de abril de 2009;
aceptado el 25 de octubre de 2009