The purpose of neurophysiological monitoring during surgery is to identify damage or functional neurological disturbances as soon as possible, ensuring spinal cord functionality and avoiding neurological complications.
However, the lack of standardisation of the methodology, together with some inconsistencies in the studies on its usefulness, limit the establishment of universal recommendations for its use.
The present paper intends to review neurophysiological monitoring procedures during surgery, including their strength and weaknesses, as well as to assess the convenience of their use during spinal surgery.
It is concluded that, in general, the lack of its use in this surgery does not legally contravene the standard of care, as there is no evidence it could result in reverting neurological damage.
Nevertheless, it can be used as evidence both in detecting the time and kind of neurological injury, and for increasing the defensibility. Protocols of use, provided either by scientific societies or hospitals themselves, are required.
El objetivo de la monitorización neurofisiológica intraoperatoria es la detección de daño o alteración funcional en el sistema nervioso lo más precozmente posible, asegurando la funcionalidad medular, evitando las complicaciones neurológicas.
Sin embargo, la falta de estandarización en la metodología, junto con cierta inconsistencia en los estudios sobre su utilidad, limita el establecimiento de unas recomendaciones universales para su utilización.
El presente trabajo pretende revisar los procedimientos de monitorización neurofisiológica, sus fortalezas y debilidades, así como la conveniencia de su empleo en cirugía espinal.
Concluimos que, con carácter general, su falta de uso en dicha cirugía no contraviene la «lex artis», pues no existe evidencia de que pueda ayudar en revertir el daño neurológico. Sin embargo, puede emplearse como elemento de prueba tanto para detectar el momento y el tipo de daño neurológico como para aumentar la defensibilidad. Se requieren protocolos de uso, bien por las sociedades científicas o por los propios centros hospitalarios.
The main objective of intraoperative neurophysiological monitoring (IONM) is to detect neurological damage or functional disturbances as soon as possible,1 ensuring spinal cord functionality and enabling reduction of complications.2,3
There can be a risk of neurological complications in spinal surgery, principally in osteotomies, kyphosis correction, combined spinal approaches, congenital scoliosis, Cobb angle above 90°, revision surgeries, distractions, instrumentation with sublaminar wire and reduction in spinal perfusion pressure due to hypotension or haemorrhage.4–6
However, the lack of standardisation of IONM techniques means that recommendations for use in spinal surgery are not clear. Added to this are inconsistencies in the studies on their efficacy and usefulness in spinal surgery.7–9
The aim of this paper is to review the types of IONM, their strengths and weaknesses, and to establish the suitability of use in spinal surgeries.
Similarly, we will review the medico-legal aspects of IONM, as the standard of care is not clear, with the obvious medico-legal implications that this entails.
Types of neurophysiological monitoringThere are different techniques, which complement each other, depending on whether any of the following parameters have been checked:
- 1.
Integrity of motor pathway. Usually measured with motor evoked potentials (MEPs) or with direct wave recording (D waves) with epidural electrodes.
- 2.
Functional integrity of the sensory pathways. Usually performed with somatosensory evoked potentials (SSEPs).
- 3.
Integrity of nerve routes. Monitored by spontaneous or stimulated electromyogram (EMG).
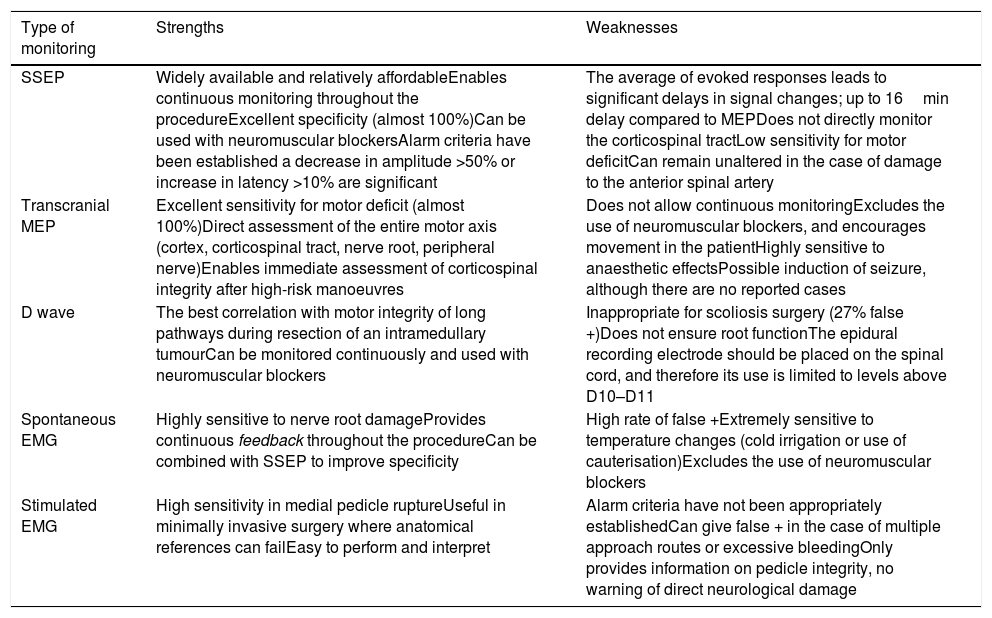
The combination of several procedures (multimodal monitoring), will optimise recording, since the isolated use of each could lead to false negatives10,11 (Table 1).
Strengths and weaknesses of the different types of monitoring.
| Type of monitoring | Strengths | Weaknesses |
|---|---|---|
| SSEP | Widely available and relatively affordableEnables continuous monitoring throughout the procedureExcellent specificity (almost 100%)Can be used with neuromuscular blockersAlarm criteria have been established a decrease in amplitude >50% or increase in latency >10% are significant | The average of evoked responses leads to significant delays in signal changes; up to 16min delay compared to MEPDoes not directly monitor the corticospinal tractLow sensitivity for motor deficitCan remain unaltered in the case of damage to the anterior spinal artery |
| Transcranial MEP | Excellent sensitivity for motor deficit (almost 100%)Direct assessment of the entire motor axis (cortex, corticospinal tract, nerve root, peripheral nerve)Enables immediate assessment of corticospinal integrity after high-risk manoeuvres | Does not allow continuous monitoringExcludes the use of neuromuscular blockers, and encourages movement in the patientHighly sensitive to anaesthetic effectsPossible induction of seizure, although there are no reported cases |
| D wave | The best correlation with motor integrity of long pathways during resection of an intramedullary tumourCan be monitored continuously and used with neuromuscular blockers | Inappropriate for scoliosis surgery (27% false +)Does not ensure root functionThe epidural recording electrode should be placed on the spinal cord, and therefore its use is limited to levels above D10–D11 |
| Spontaneous EMG | Highly sensitive to nerve root damageProvides continuous feedback throughout the procedureCan be combined with SSEP to improve specificity | High rate of false +Extremely sensitive to temperature changes (cold irrigation or use of cauterisation)Excludes the use of neuromuscular blockers |
| Stimulated EMG | High sensitivity in medial pedicle ruptureUseful in minimally invasive surgery where anatomical references can failEasy to perform and interpret | Alarm criteria have not been appropriately establishedCan give false + in the case of multiple approach routes or excessive bleedingOnly provides information on pedicle integrity, no warning of direct neurological damage |
Modified from Lall et al.7
Described by Nash et al.12 in 1977, these check functional integrity from the peripheral nerve to the sensory cortex.
The tibial posterior and peroneal nerves are stimulated in the lower extremities, and the median and ulnar nerves in the upper extremities, with cortical recording at Cz. Pre-surgical recording is performed to establish baseline amplitudes and latencies.
SSEPs enable continuous monitoring, but do not record motor activity.
These techniques do not have 100% sensitivity on their own. Therefore, post-operative paraplegia has been reported with preserved SSEPs.13–15 This procedure is very sensitive to anaesthetics (halogenated gases). Its acquisition time is prolonged (minimum: 1min) (Table 1).
Motor evoked potentialsDescribed by Merton and Morton16 in 1980, the papers by Taniguchi and Taylor allowed their use in spinal surgery.17,18
After a transcranial stimulus, the response is recorded with electromyography (muscular EMG) or by epidural electrodes that collect D waves.
Thus, depending on the form of recording, we refer to:
- a.
Combined muscle action potentials (muscular MEPs).
These monitor the entire motor axis: motor cortex, corticospinal tract, nerve root and peripheral nerve. Recording is done in different muscle groups. Its use does not require the use of muscle blockers (MB).
As it is not continuous monitoring, it could delay recognition of damage. It must be agreed, prior to surgery, how often the motor pathway should be recorded, as well as the alarm criteria to follow. Table 1 shows the strengths and weaknesses of this recording.
It is considered the “gold standard” for detecting motor deficits, providing faster detection of medullary ischaemia (if compared with SSEPs and D waves).
- b.
D waves. Generated by transcranial stimulation and an epidural electrode located caudally to the risk zone. They are relatively resistant to anaesthetics.
Considered very useful as support for the resection of medullary tumours (“gold standard”), provided they are above vertebral level D10–11 (since the recording electrode must be positioned on the spinal cord). Of little use in scoliosis surgery because of the percentage of false positives.19
- c.
Neurogenic MEP. The stimulus is generated by an epidural electrode located cranially to the risk zone. Recording is performed either with an epidural electrode positioned caudally to the zone, or on a peripheral nerve. Some believe that it does not measure the motor pathway, but the dorsal spine in an antidromic way.20
Lall et al.7 advise against their use as the only method of monitoring the motor pathway. Other authors, such as Gavaret, consider them very useful to determine the medullary lesion level.
Given their non-specificity, it is proposed that neurogenic MEPs should be used as additional methods to monitoring with SEPs and MEPs.15,21
There are 2 modalities:
- a.
Spontaneous EMG. Does not require stimulation, enabling continuous recording. Performed on pre-selected muscle groups. One muscle group per nerve root is sufficient, but in cervical surgery, some prefer to monitor 2 groups.22–25
In the baseline situation, a healthy root should not show muscular activity. During surgery, any root irritation (traction, electrocoagulation, etc.) will cause neurotonic discharges. The amplitude and frequency of these shocks will increase in relation to the damage received by the root.
The disadvantages are: MB must not be used. It is a technique that is very sensitive to thermal changes (cold serum, electro-scalpel). False positives are also given.
- b.
Stimulated EMG. Described by Calancie et al.26 in 1992. Based on the fact that a recording will be achieved if we stimulate a pedicle screw, even at low voltage, if it contacts the root.27,28 Some highlight that the stimulus should be increased to 20mA if there is no EMG response at a lower intensity.29 Stimulus trains have recently been being used to detect whether the screw is touching or is near the medulla.30
The disadvantages are that it appears that relocation of a screw could result in false positives. Similarly, if MB have been used, at least 30min should be waited before provoking the EMG.
This modality is of special interest in minimally invasive surgery, which depends on the quality of the radiological equipment. It goes without saying that any preoperative root damage to the problem roots must be established. If there is risk of root damage, spontaneous or stimulated EMG can be useful for roots S1 and L5. All spinal roots can now be monitored without problems.7,9,24
These seek to compensate for weaknesses or limitations of procedures used in isolation.31,32 The combined use of spontaneous and stimulated EMG increases the detection of root damage. Gavaret et al. use the combination of SSEPs, neurogenic MEPs and D waves for spinal deformity surgery.
Epstein33 establishes the need to combine MEPs, SSEPs and EMG in cervical spine surgery. Kaliya-Perumal et al.34 suggest that, for pedicle screws, palpation, radioscopy and provoked EMG should be combined. This significantly reduces the number of reinterventions.
Indications for monitoringAccording to the literature, it has been reported as a useful procedure principally for: thoracolumbar spine deformity surgery (not so much for lumbosacral surgery)35; medullary tumour surgery, both intra and extramedullary35,40; prevention of neurological damage while positioning the patient40 and herniated thoracic disc.35
It has also been pointed out that it does not make sense for pre-existing medullary lesions and in revision surgeries with abnormal reference potentials.2
In degenerative cervical spine surgery, some support its use,36 whereas for others it is of little use, as very few true positives are identified.40
In cervical disc surgery without myelopathy, some papers support the use of MEPs and SSEPs.37 Weinzierl et al.38 indicate that loss of MEPs indicates a motor deficit, that unaltered MEP indicates preservation of the motor pathway, and that changes in MEPs may or may not coincide with a motor deficit.
Others point out that indications in spinal surgery can be39: cranio-cervical junction, spinal cord procedures, including cauda equina, spinal deformity correction, vertebral fracture surgery, spinal tumour resections and anterior cervical discectomy.
Thus, the routine use of IONM in all spinal surgeries remains controversial.40
Modification of anaesthesiaIt is important to highlight that, if IONM is decided, anaesthetic techniques must be adapted, which is not without drawbacks.
In the literature, we find publications contraindicating the use of many anaesthetics, mainly neuromuscular blockers and halogenated anaesthetics (HA) (both fundamental in general anaesthesia with orotracheal intubation). This is in addition to the influence that these recommendations may have on the anaesthetic procedure and its safety.
Thus, HA are contraindicated, since both SSEPs and MEPs are affected, MEPs being much more sensitive. However, there are publications that allow their use at low doses (<.5 minimum alveolar concentration).5,41,42
MB should be excluded from use in any EMG or MEP modality. If they were to be used, monitoring should be undertaken with a 100% train of 4.43 For this reason, their use should be restricted to the time of orotracheal intubation, using very low doses or reversing their effect.44
Due to these limitations, the suggested gold standard would be total intravenous anaesthesia based on propofol and remifentanil.3,39,45
Although both drugs barely alter IONM, their use has certain limitations.
Thus, haemodynamic control may be more unstable than with HA. There is a greater risk of intraoperative awakening,46 especially in obese patients. This constitutes a serious complication, forcing level of consciousness monitoring, which, in turn, is distorted by lack of muscle relaxation and stimulation of potentials.
Remifentanil allows early anaesthetic education and early neurological assessment. Propofol, however, can lead to prolonged awakenings.47
In addition, the fact that sedoanalgesia is mainly based on these 2 drugs, requires a higher plasma concentration, with the risk of overdose and side effects, such as hyperalgesia syndrome (opioid-induced hyperalgesia), and propofol infusion syndrome (rare but lethal).
Therefore, although it is important to provide adequate conditions for correct neurophysiological monitoring, it is also necessary to find anaesthetic levels that allow adequate haemodynamic control and anaesthetic depth.
Not all patients are equally sensitive to drugs, nor do they need the same intensity of stimulation. For this reason, the exclusion of some drugs which, at low doses, allow correct IONM in some patients5,41,42 may be premature, there being the possibility of defining the pharmacological levels admissible for obtaining an adequate recording at the start of surgery.
Methodology of actionThis is not limited to the intervention but begins beforehand.
Before the surgical procedure, ideally, an anaesthesiologist, neurophysiologist and surgeon should establish a common plan of action, defining which monitoring techniques to use and how to use them.
Thus, consensus should be reached on:
- 1.
The type of monitoring for each case, based on expected neurological deficits.
- 2.
The type of anaesthesia, dose and other factors according to the risk/benefit.
- 3.
The start of monitoring (premobilization or preoperative).
- 4.
If medullary deficits are anticipated, combined SSEP and MEP recording would be advisable.
- 5.
Alarm and response criteria.
- 6.
If new techniques are used, defining the mode of action.
Some have proposed monitoring without a neurophysiologist, although recent reviews consider this possibility less reliable than when carried out by experienced neurophysiologists.6
It is the role of the surgeon to establish a clear plan of action in the event of any alarm. The steps to be taken in the event of a warning are as follows.
The first thing to be discounted are false positives, which include: hypotension, hypothermia, use of HA or migration of stimulation and recording electrodes
Once discounted, real neurological damage must be considered, reverting any risky manoeuvre and ensuring recovery of the amplitude or latency of the lost signal7 (Table 2).
Alarm criteria in spinal IONM.
| Type | Criteria |
|---|---|
| SSEP | Decrease in amplitude >50%Increase in latency >10% |
| MEP | Not definedDecrease in amplitude >50% |
| Stimulated EMG | Not definedThreshold value of 5mA |
EMG: electromyogram; IONM: intraoperative neurophysiological monitoring; MEP: motor evoked potential; SSEP: somatosensory evoked potentials.
We shall look at the following.
Non-use of IONMThe assessment of possible medical negligence includes (STS 19 7 2002):
- 1.
A voluntary action or omission.
- 2.
The creation with it of a predictable and avoidable risk situation.
- 3.
Breach of a standard of care.
- 4.
A harmful outcome deriving from careless conduct.
- 5.
There is adequate causal relationship between the conduct and the harm.
With respect to the first point, non-use of IONM would be considered.
The second point refers to whether the omission of use would imply a foreseeable and avoidable risk to the life or health of the patient.
In short, IONM is useful as48:
- 1.
Adjunct diagnosis. In this regard, there is evidence that IONM is a valid and sensitive means of detecting neurological damage during spinal cord and spinal column surgery.
- 2.
Therapeutic tool. It has not been established as useful in reducing perioperative neurological impairment, or in improving neurological outcomes in spinal surgery.
There are even publications (level of evidence II) that refute the usefulness of IONM as a therapeutic adjunct in spinal surgery. Several scientific societies agree on the usefulness of IONM in spinal surgery, but do not qualify it as a standard of care.49,50 Nor has its efficacy as a neuroprotective element been proven in most degenerative cervical spine surgical procedures.40 Other authors have found no justification for its use based on cost-benefit.7
It follows that, in general, not using IONM does not per se create a predictable and avoidable risk situation, and therefore its use during rachimedullary surgery cannot be considered a standard of care, i.e. as an obligation of the lex artis ad hoc legal criterion.48,51
With regard to the fourth point, the lack of use of IONM cannot be considered synonymous with causing damage. Moreover, there is currently no evidence that the detection of such damage by means of IONM leads, in rachimedullary surgery, to its avoidance or reduction, therefore no causal relationship can be established between damage and not using IONM (point 5).
In other words, according to the scientific evidence, not using IONM in spinal surgery does not per se constitute a breach of the standard of care, nor does it infringe any of the other elements giving rise to an appraisal of careless conduct in the medico-legal sense.
Failure in intra-operative neurophysiological monitoring surveillance/interpretationInitially, this is the responsibility of the neurophysiologist, but there are many potential problems. For example, good practice in patients with severe cervical stenosis could be to perform it from the start, specifically before intubation, to avoid harm while the neck is being hyperextended.52
Consequently, close collaboration between the surgeon, the anaesthetist and Neurophysiology is required with permanent flow of information between them, as well as appropriate baseline recordings before any manoeuvre derived from the intervention, including those performed by Anaesthesiology.
Use of intraoperative neurophysiological monitoring as an element of evidenceIf the surgeon can prove that there have been no disturbances that would indicate neurological damage, this is evidence for possible claims.
IONM can also demonstrate the type or severity of resulting damage, which can help in expert interpretation. In our opinion, in doubtful cases, it is an interesting indication to increase the possible defensibility of the case.
RecommendationsIt is evident that IONM provides reliable and useful information on the state of neurological structures in the perioperative context of spinal surgery. Therefore, although there is no general obligation to use it, it could be recommended in some cases.
In turn, such a recommendation could have different degrees. For example, in a canal stenosis with myelopathy and intubation difficulties, it may be much more advisable to use IONM than in a simple herniated cervical disc.
Likewise, the recommendation is greater if other surgical assistance systems are not going to be used, such as microsurgery, for example.
Consequently, a protocol for use could be based on 3 criteria:
- 1.
Type of intervention to be performed.
- 2.
Characteristics of the case.
- 3.
Use of other intraoperative assistance systems.
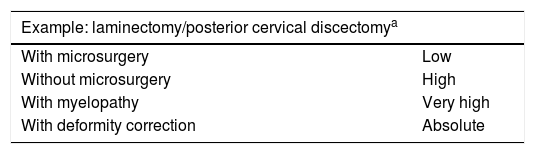
A possible example of a protocol, that can be extended to other diseases, is shown in Table 3.
Proposed guidelines for the use of IONM in spinal surgery.
| Example: laminectomy/posterior cervical discectomya | |
|---|---|
| With microsurgery | Low |
| Without microsurgery | High |
| With myelopathy | Very high |
| With deformity correction | Absolute |
It would be desirable for this system to be adopted by the scientific societies or, failing that, by each centre, thus offering a standard of care (general or institutional) for the ad hoc assessment of the praxis of care in IONM.
ConclusionsIONM is an effective and useful system for detecting neurological damage associated with spinal surgery. However, there is no evidence that it can reduce the neurological damage associated with surgery. According to current knowledge it does not constitute a standard of care and, therefore, if not used it does not amount to a breach of the lex artis. There may, however, be cases in which its use is more advisable than in others, depending on the disease, the characteristics of the case and the use or otherwise of other surgical aids. It also constitutes an element of evidence to detect time and type of neurological damage that influences medical-legal defensibility. The adoption is desirable of protocols for the use of IONM either by the scientific societies or by the hospital centres themselves, likely to adopt them as the lex artis ad hoc legal criterion in this matter.
Please cite this article as: Martínez Quiñones JV, Aso Escario J, Fernández Sánchez V, Marín Zaldivar C, Aso Vizán A, Consolini F, et al. Monitorización neurofisiológica intraoperatoria en cirugía raquimedular. Aspectos clínicos y médico-legales. Rev Esp Med Legal. 2020;46:20–27.