Brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) are neurotrophins that play critical roles in brain neuronal function. Previous studies have established the association between BDNF and NGF signaling and severe mental disorders, but changes in BDNF plasma levels and electroconvulsive therapy (ECT) response are controversial. The aim of his study was to explore the acute effects of a single session of ECT on these neurotrophins signaling.
Plasma levels of BDNF and NGF and their tyrosine kinase-type receptors expression in peripheral blood mononuclear cells (PBMCs) were determined before and two hours after a single ECT session in 30 subjects with a severe mental disorder.
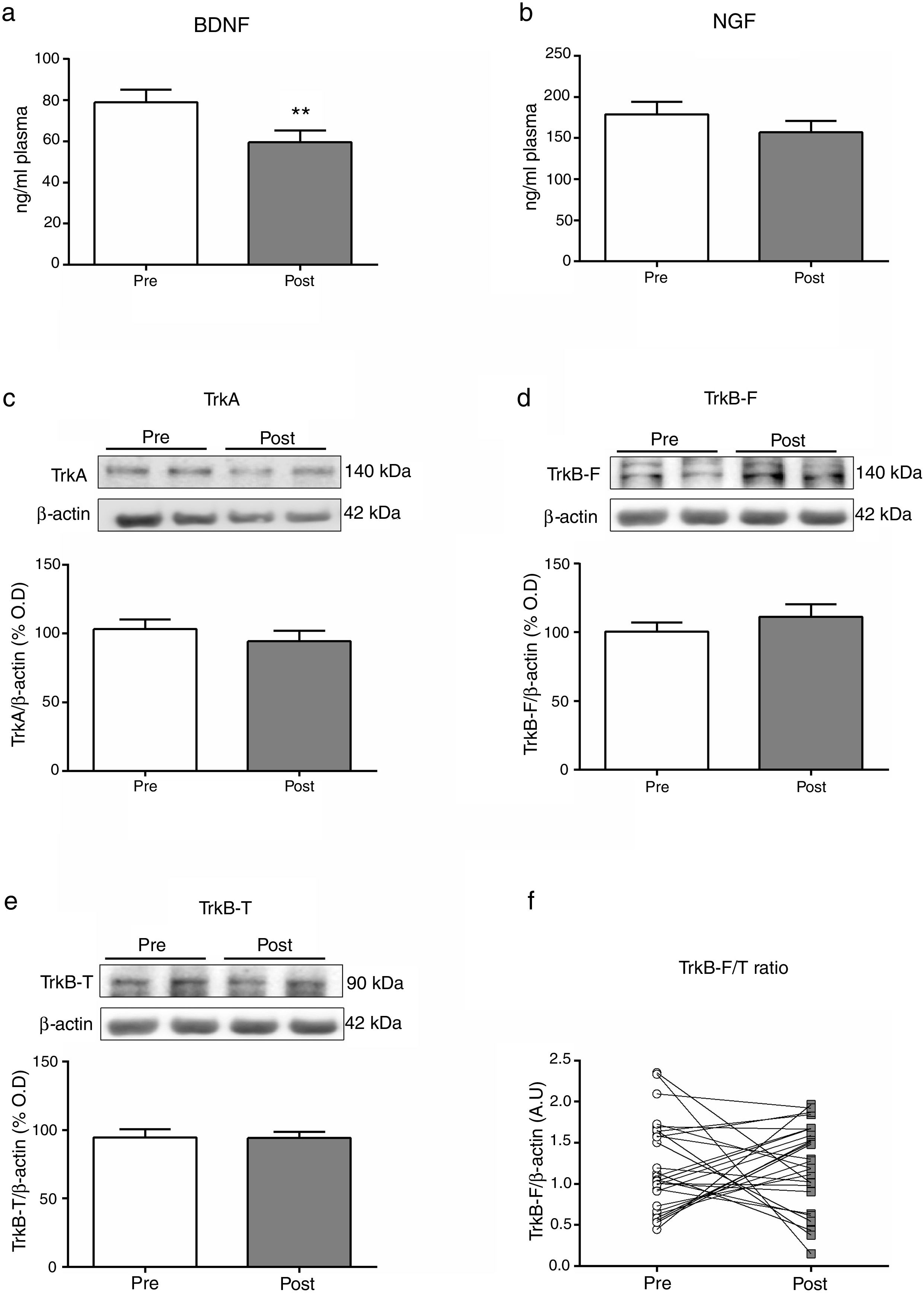
Two hours after an ECT session we found a statistically significant decrease of BDNF plasma levels (p=0.007). We did not find significant acute effects on NGF plasma levels or receptors expression in PBMCs. We found a significant inverse correlation between the time of convulsion and BDNF plasma levels decrease (r=−0.041, p=0.024).
We have identified a decrease in BDNF plasma levels after 2h of a single ECT session. These results indicate the interest for future research in the role of neurotrophins in the response and safety of ECT.
Electroconvulsive therapy (ECT) is recognized as one of the most effective therapies for certain severe mental disorders resistant to other treatments or in clinical scenarios that need a rapid response.1–6 Despite its efficacy, it is necessary to expand the available knowledge about its mechanisms of action and to identify useful biological marked linked either to clinical response or to the appearance of side effects.
ECT affect a wide range of molecules, including neurotransmitters, inflammatory pathways and neurotrophic factors.7,8 Brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) are neurotrophins that play critical roles in neurodevelopment and a wide range of adult neuronal functions.9,10 Both BDNF and NGF are activated by their own tyrosine kinase-type receptors, named TrkB and TrkA respectively.11 In the case of TrkB, two types of receptors have been described: an active full-length form of the receptor (TrkB-F) and a truncated form (TrkB-T). The last one lacks kinase activity and inhibits the TrkB-F function by competing in binding to BDNF.12 For its part, TrkA mediates on multiple effects of NGF, including neuronal differentiation and programmed death inhibition.11
Several studies and meta-analyses have established the association between BDNF and NGF signaling and severe mental disorders, including major depressive disorder (MDD), bipolar disorder (BD) and schizophrenia (SZ).13–16 A meta-analysis conducted by Molendijk et al. concluded that serum BDNF levels were reduced in patients suffering from major depressive disorder (MDD) and in patients receiving a course of antidepressants.17
Despite a growing number of studies, findings on changes in BDNF levels and the response to ECT are controversial.18 A recent meta-analysis of 22 studies of BDNF blood levels after ECT in patients with major depressive disorder concluded that BDNF levels may increase after ECT, being a candidate to be used as an indicator of treatment response after one or more weeks of ECT.19 However, some studies reported that serum or plasma BDNF levels increase at different time points after ECT,20–29 while other research groups did not find any influence of ECT on BDNF levels.30–35 Recently, Sorri and collaborators reported a decrease of BDNF levels during the fifth ECT session, between the baseline and the 2-h samples, with no association between serum or plasma BDNF levels and remission.10 Finally, baseline serum BDNF levels and the BDNF Val66Met polymorphism did not show any clinical utility in predicting ECT response in treatment-resistant MDD patients.36 Taken together, these results indicate a need for further research of the effects of ECT on the neurotrophins signaling and of the predictive value of BDNF plasma determination in patients who will receive this treatment.
Considering this background, the main objective of the present study is to explore the acute effects of a single session of ECT on the neurotrophins signaling in a selected group of patients with a severe mental disorder. Besides, we pretend to analyze if there are different effects considering clinical features of the studied sample and ECT parameters.
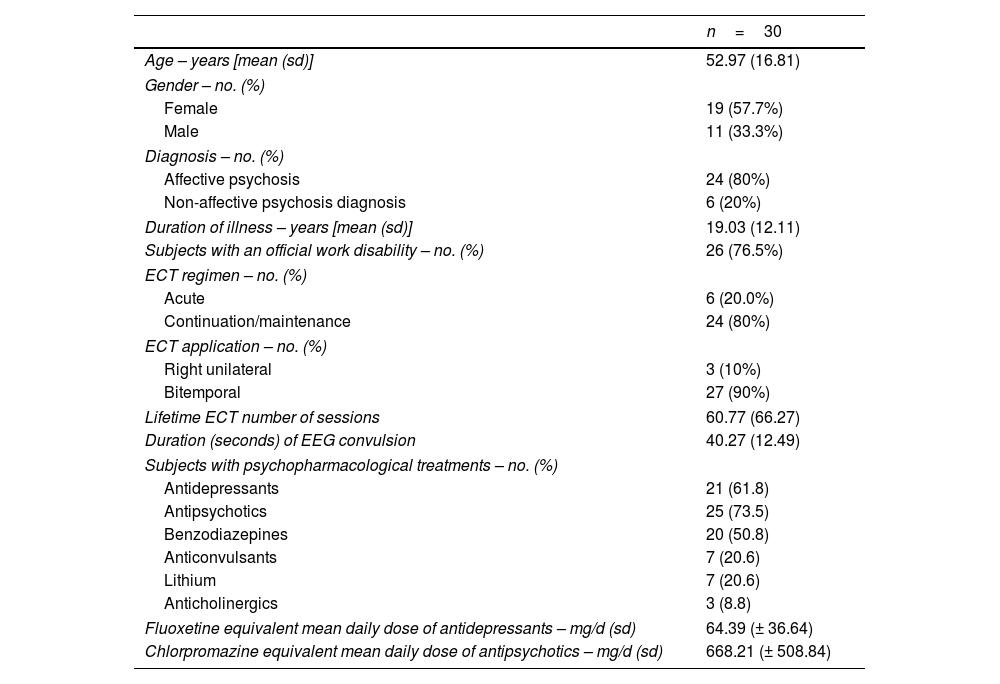
Subjects and methodsSubjectsWe recruited 30 subjects who attended an ECT session in the Hospital Clínic de Barcelona following the usual practice in this center, with the following inclusion criteria: (1) meet DSM-5 criteria for a severe mental disorder (Major Depressive Disorder, Bipolar Disorder, Schizophrenia or Schizoaffective Disorder); (2) age between 18 and 70 years; and (3) be able to sign the informed consent. Participants could be in any ECT regimen (acute or continuation/maintenance). The exclusion criteria were: (1) being pregnant; (2) be in treatment with anti-inflammatory drugs (including steroids), antioxidants, antibiotics or immunological therapies; (3) present fever (>38°) or leukocytosis (>10,000) in the previous 24h; (4) have received a vaccine in the previous 4 weeks; and (5) present a history of neurological diseases, a history of traumatic brain injury with loss of consciousness, mental retardation or generalized developmental disorders.
All the subjects who met the inclusion/exclusion criteria during the period of recruitment were discussed in a specific ECT committee and were informed about the study by members of the research group, obtaining the informed consent from all participants.8 Together with the medical records, sociodemographic and clinical data of the patients were collected in an interview prior to the ECT session. The study had been approved by the Ethics Committee of the Hospital Clínic de Barcelona (record CB/2017/0369).
A MECTA SPECTRUM 5000Q® device (MECTA Corp, Lake Oswego, USA) was used for all the ECT stimuli administration. Electroencephalographic (EEG) and motor seizure manifestations were monitored to ensure that an adequate ictal response occurred (>20s) or to detect prolonged seizure activity. Patients were continuously monitored (non-invasive arterial blood pressure, EKG and heart rate and pulse oximetry) using a PHILIPS MP-20 Anesthesia monitor (PHILIPS®, Boeblingen, Germany). Succinylcholine (30–120mg), atropine (0–1mg), and sodium tiopenthal (75–450mg) were used for general anesthesia. Ventilation was assisted with face mask at high oxygen concentration before and after the stimulus and mild hyperventilation was maintained for at least one minute before the electrical stimuli. The characteristics of the electrical stimulus were automatically recorded by the MECTA EMR® software connected to the stimulator. Patients could be in any ECT regimen (acute: first 6–12 ECT sessions; continuation: first six months of treatment after acute regimen; maintenance: after first six months).
Demographic characteristics of the patients (age, gender), diagnosis, duration of the illness, information regarding work disability, ECT regimen (acute, continuation or maintenance), electrode placement, EEG time of convulsion and total number of lifetime ECT sessions were recorded. Prescribed psychopharmacological treatment was also recorded. The prescribed daily doses of antipsychotics were converted to an estimated equivalent amount of chlorpromazine following the international consensus,37 while the prescribed daily doses of antidepressants were converted to an estimated equivalent amount of fluoxetine following published guidelines.38
Sample collection and biochemical measurementsVenous blood samples (10mL) were collected between 8:30h and 9:30h (Pre-ECT), after overnight fasting, and two hours after the stimulation (Post-ECT).8 We decided to sample at two hours post-ECT in order to be able to compare our results to previous comparable studies that had taken this same measure,10 and to ensure that both measures (pre and post ECT session) were being collected under fasting conditions.8 Samples were maintained at 4°C until preparation after approximately 1h. Blood tubes were centrifuged (641g×10min). The resultant plasma samples were collected and stored at −80°C. The rest of the sample was 1:2 diluted in culture medium (RPMI 1640, LifeTech) and a gradient with Ficoll-Paque (GE Healthcare) was used to isolate mononuclear cells by centrifugation (800g×40min, room temperature [RT]). Peripheral blood mononuclear cells (PBMC) layer was aspired and resuspended in RPMI and centrifuged (1116g×10min, RT). The supernatant was removed, and the mononuclear cell-enriched pellet was stored at −80°C. Once the whole sample recruitment was finished in Hospital Clinic de Barcelona, all samples were sent to Universidad Complutense de Madrid for subsequent ELISA and Western Blot determinations.
Plasma levels of BDNF and NGF were determined using enzymatic assays (ELH-BDNF-1, RayBiotech®; ab99986, abcam), according to the manufacturer's instructions, and measured using a Synergy 2 multi-mode reader (BioTek®). The sensitivity of the assay for BDNF was 80pg/mL and <14pg/ml for NGF; for both kits the manufacturer's intra- and interassay coefficients of variation (CV) were respectively <10% and <12%, while own lab's CVs were <7% and <8%.
Protein levels of TrkA, TrkB-F, and TrkB-T receptors in PBMCs were quantified by Western Blot analysis. In brief, 15μg of cytosolic extracts were loaded onto electrophoresis gels.39 Protein samples were separated and transferred onto nitrocellulose membrane (Transfer Pack, Biorad®). After blocking, membranes were incubated with specific antibodies: (1) TrkA, rabbit polyclonal antibody dilution of 1:750 in BSA 1% (sc118, SCB); (2) TrkB-F, rabbit polyclonal antibody dilution of 1:750 in BSA 1% (sc12, SCB); (3) TrkB-T, rabbit polyclonal antibody dilution of 1:1000 in BSA 1% (ab1987, abcam); (4) β-actin mouse monoclonal in a dilution 1:10,000 (A5441, Sigma, Spain). Proteins were recognized by the respective horseradish peroxidase-linked secondary antibodies and visualized using an Odyssey® Fc System (Li-COR Biosciences®) and quantified by densitometry (NIH ImageJ® software). Values were normalized to the loading control (β-actin, the blots are shown in Fig. 1c–e). All western blots were performed at least three times in separate assays.
Mean differences (SD; univariate analysis) on biomarkers at baseline (Pre) and two hours after (Post) a single electroconvulsive session. BDNF and NGF plasma levels (a, b); Protein expression of TrkA, TrkB-F and TrkB-T in PBMC (c-e); Ratio of TrkB-F/TrkB-T expression (f). BDNF: Brain-derived neurotrophic factor; NGF: Nerve growth factor; PBMC: Peripheral blood mononuclear cells; TrkA: Tyrosine kinase-type A receptor; TrkB-F: Full-length form of the tyrosine kinase-type B receptor; TrkB-T: Truncated-length form of the tyrosine kinase-type B receptor.
Given the counterbalancing effect of TrkB-T and TrkB-F, we decided to choose the ratio of TrkB-F to TrkB-T expression (hereafter F/T ratio) as our index variable for describing BDNF receptor expression.
Statistical analysisDifferences in biomarkers expression before and after the ECT session were assessed using a paired t-test on those variables (TrkA, TrkBF, TrkBT and F/T ratio) which distribution met the assumption of normality in the Kolmogorov–Smirnov (with Lillierfors correction) test. For BDNF and NGF plasma levels analysis, a nonparametric Wilcoxon Signed Rank test was used.
Several sub-analyses were performed to explore whether the changes in the expression of biomarkers were associated with some of the sociodemographic, clinical or treatment characteristics of ECT. To limit the number of post hoc analyses, we only explored the changes in those biomarkers with significant changes in the activity. Thus, a mixed between-within subjects analysis of variance was conducted to assess the impact categorical variables (gender, diagnosis, ECT regimen, electrodes placement, prescribed treatment) on these biomarkers expression. The relationship between the biomarkers changes and continuous variables were investigated using Pearson correlation coefficient. Preliminary analyses were performed to ensure no violation of the assumptions of normality, linearity and homoscedasticity. The strength of these correlations were evaluated according to Cohen's guidelines (small r=0.10–0.29; medium r=0.30–0.49; large r=0.50–1).40 Being these exploratory analysis, corrections were not made for multiple comparisons.41
A value of p<0.05 was taken to be statistically significant in all analysis. Data were managed and analyzed with the IBM SPSS Statistics v.23.
ResultsTable 1 shows general demographic, clinical and ECT parameters data of the 30 participants included in the study. No incidences were recorded during the ECT procedures in these patients. The graphic representation of the main results is summarized in Fig. 1.
Demographic, clinical characteristics and electroconvulsive therapy parameters.
| n=30 | |
|---|---|
| Age – years [mean (sd)] | 52.97 (16.81) |
| Gender – no. (%) | |
| Female | 19 (57.7%) |
| Male | 11 (33.3%) |
| Diagnosis – no. (%) | |
| Affective psychosis | 24 (80%) |
| Non-affective psychosis diagnosis | 6 (20%) |
| Duration of illness – years [mean (sd)] | 19.03 (12.11) |
| Subjects with an official work disability – no. (%) | 26 (76.5%) |
| ECT regimen – no. (%) | |
| Acute | 6 (20.0%) |
| Continuation/maintenance | 24 (80%) |
| ECT application – no. (%) | |
| Right unilateral | 3 (10%) |
| Bitemporal | 27 (90%) |
| Lifetime ECT number of sessions | 60.77 (66.27) |
| Duration (seconds) of EEG convulsion | 40.27 (12.49) |
| Subjects with psychopharmacological treatments – no. (%) | |
| Antidepressants | 21 (61.8) |
| Antipsychotics | 25 (73.5) |
| Benzodiazepines | 20 (50.8) |
| Anticonvulsants | 7 (20.6) |
| Lithium | 7 (20.6) |
| Anticholinergics | 3 (8.8) |
| Fluoxetine equivalent mean daily dose of antidepressants – mg/d (sd) | 64.39 (± 36.64) |
| Chlorpromazine equivalent mean daily dose of antipsychotics – mg/d (sd) | 668.21 (± 508.84) |
In ECT regimen: “Acute” refers to the first 6–12 ECT sessions, “Continuation” for the following sessions during the first six months and “Maintenance” for the sessions received after this first six-month period. ECT: electroconvulsive therapy; EEG: electroencephalographic.
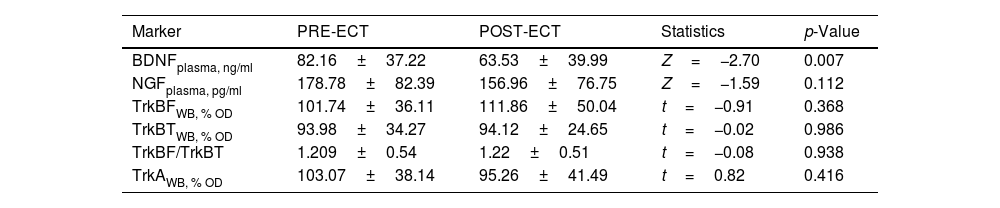
Table 2 summarizes the changes in neurotrophins plasma levels and in the expression of the receptors in PBMCs before and two 2h after the ECT session, when there was a significant decreased of the BDNF plasma levels (from 82.16±6.79 to 63.53±6.75; Z=−2.7, p=0.007).
Mean differences (± SD) in neurotrophins plasma levels and expression of receptors in PBMCs between pre-ECT and post-ECT session (2hours after stimulus) in 30 patients with severe mental disorders.
| Marker | PRE-ECT | POST-ECT | Statistics | p-Value |
|---|---|---|---|---|
| BDNFplasma, ng/ml | 82.16±37.22 | 63.53±39.99 | Z=−2.70 | 0.007 |
| NGFplasma, pg/ml | 178.78±82.39 | 156.96±76.75 | Z=−1.59 | 0.112 |
| TrkBFWB, % OD | 101.74±36.11 | 111.86±50.04 | t=−0.91 | 0.368 |
| TrkBTWB, % OD | 93.98±34.27 | 94.12±24.65 | t=−0.02 | 0.986 |
| TrkBF/TrkBT | 1.209±0.54 | 1.22±0.51 | t=−0.08 | 0.938 |
| TrkAWB, % OD | 103.07±38.14 | 95.26±41.49 | t=0.82 | 0.416 |
Data expressed as mean±standard deviation. BDNF: Brain-derived neurotrophic factor; NGF: Nerve growth factor; OD: Optical density; Plasma: plasma levels; TrkA: Tyrosine kinase-type A receptor; TrkBF: Full-length form of the tyrosine kinase-type B receptor; TrkBT: Truncated-length form of the tyrosine kinase-type B receptor; WB: Western Blot. See Methods section for detail.
There were no baseline (pre-stimulation) differences between genders, diagnosis (affective disorders vs. schizophrenia related disorders) or ECT regimen (acute vs. continuation/maintenance). We carried out a series of exploratory sub-analyses to detect whether the significant changes in BDNF plasmatic levels were associated with certain sociodemographic, clinical or ECT treatment characteristics. We found a significant inverse correlation for the EEG time of convulsion and BDNF plasma levels at the endpoint (p=0.024), with a medium strength of the relationship (r=−0.041).
We conducted group of analyses to explore if the observed changes in BDNF plasma levels after the ECT session were modified by concurrent pharmacological treatment studied. A mixed between-within subjects analysis of variance was conducted to assess the impact of concurrent psychopharmacologic treatment on BDNF levels changes after the ECT session. We did not find significant differences in BDNF levels changes in patients with or without prescriptions of antipsychotic, antidepressant, benzodiazepines, anticonvulsants, lithium or anticholinergics, meaning that changes of BDNF levels were not related to being or not under those treatments. We neither found significant correlation between BDNF plasma levels changes and antipsychotic (p=0.17) or antidepressant (p=0.76) doses.
DiscussionThe results of this study point to the acute effects that a single ECT session produces on the neurotrophins pathway. Specifically, two hours after an ECT session we found a statistically significant decrease of the levels of BDNF in plasma (Fig. 1a). We also found a significant inverse correlation for the EEG duration of convulsion and BDNF plasma levels decrease (r=−0.041, p=0.024). We did not find a significant acute effect over NGF plasma levels or receptors expression in PBMCs (Fig. 1b–e).
These results agree with previous reports from Sorri and collaborators,10 who recently reported a decrease of BDNF levels between the baseline and the 2-h samples during the fifth ECT session. Stelzhammer and collaborators also reported decreased serum BDNF levels after 4 weeks of acute ECT.42 By contrast, some studies have reported plasma BDNF levels increases at different time points after ECT.20–28 A recent meta-analysis of 22 studies, revealed no significant changes in the serum BDNF levels between patients who responded and those that did not respond to ECT, but reported a significant increase in the plasma BDNF levels after ECT treatment in the first week and month in patients with MDD.21 Other research groups did not report any influence of ECT on BDNF plasma levels.30–35
In our opinion, a major difference between these studies and ours is the timing of blood sampling.10,42 In some of studies mentioned above BDNF plasma levels were measured during a course of ECT, while in others blood samples were taken in a variable period of one day to one month after finishing the whole ECT course (generally 9–12 sessions). Besides, previous studies have shown that BDNF levels stored in plasma and serum might differ significantly, which could explain in part the controversial findings so far.19
Overall, these apparently controversial results are consistent with the hypothesis that BDNF undergoes differential regulation after different time periods following ECT.42 In that vein, a gene profiling study found increased BDNF expression 2h after both acute and chronic ECT, with decreased levels after 6h.43 The mechanisms of action of ECT and psychotropic drugs may be different, so their effects on specific biomarkers of therapeutic response may be different too.
Some limitations should be considered when analyzing these results. First, the relatively small sample size, which could be limiting the statistical power. Secondly, we studied the effects of a single ECT session after two hours, while it would very informative to have re-tested the patients at different moment after the ECT session (i.e. 1h, 3h, 24h, 48h, or even one week later) and at the end of the whole acute treatment (generally 9–12 sessions). Thirdly, there was heterogeneity of the subjects included in the study in clinical variables such as diagnostics, psychopharmacological treatment or ECT regimen. Fourthly, we do not know the effects of anesthesia, the apnea time or of the tonic-clonic seizures on the pathway studied. Finally, we did not have a group of healthy controls paired with the study sample with which to compare the expression of biomarkers at baseline.
Despite these limitations, we believe that our study has identified significant acute effects of ECT treatment over BDNF plasma levels in a group of patients with severe mental disorders, which deserve more attention in future research. Future studies could identify the optimal time point to predict the levels of BDNF in plasma, serum or even directly from the brain, comparing favorable responders, partial responders, and non-responders for plasma and serum BDNF levels across different timepoints during and after ECT treatment.21 Besides, large genetic consortium such as the International Consortium on the Genetics of Electroconvulsive Therapy and Severe Depressive Disorders (Gen-ECT-ic) may be able to determine the role of the different BDNF genetic polymorphisms in ECT response.44
In conclusion, and regarding to implications for clinical practice, in this study we have identified a decrease of BDNF plasma levels after 2h of an ECT session. Nevertheless, predictive value of BDNF for effects of ECT remains uncertain. The results indicate that this is an area of interest for future research, in which the role of neurothrophins in the response and safety of ECT should be clarified.
Authors’ contributorsMBi conducted the literature review, recruited the participants, applied the ECT session, collected data, conducted the main statistical analysis, wrote the first draft of the manuscript and handled subsequent drafts after receiving coauthors feedback. KSMD & CF performed all biochemical determinations in plasma and in cells and prepared sub-cellular samples, assisted with the analysis and wrote the first draft of the manuscript. AM collected the biological samples and separated plasma and PBMC. EM recruited the participants, collected the blood samples and applied the ECT session. The rest of coauthors participated in the ECT procedures, collected data and commented on drafts. All of the authors contributed to the final version of the paper.
FundingThis study was supported by Instituto de Salud Carlos III, Fondo Europeo de Desarrollo Regional, Unión Europea, “Una manera de hacer Europa”; MINECO SAF2016/75500-R; Centro de Investigación Biomédica en Red de salud Mental, CIBERSAM, and by Generalitat de Catalunya and Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement (2014SGR441 and 2014SGR398).
Conflicts of interestDr. Bernardo has been a consultant for, received grant/research support and honoraria from, and been on the speakers/advisory board of ABBiotics, Adamed, Angelini, Casen Recordati, Eli Lilly, Janssen-Cilag, Lundbeck, Otsuka, Takeda, Somatics and has obtained research funding from the Ministry of Education, Culture and Sport, the Spanish Ministry of Economy, Industry and Competitiveness (CIBERSAM), by the Government of Catalonia, Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement (2017SGR1355), Foundation European Group for Research In Schizophrenia (EGRIS), and the 7th Framework Program of the European Union. Dr. Bioque has been a consultant for, received grant/research support and honoraria from, and been on the speakers/advisory board of has received honoraria from talks and/or consultancy of Adamed, Ferrer, Janssen-Cilag, Lundbeck, Otsuka, Pfizer and Sanofi. Dr. Valentí has received research grants from Eli Lilly & Company and has served as a speaker for Abbott, Bristol-Myers Squibb, GlaxoSmithKline, Janssen-Cilag, and Lundbeck. The rest of authors report no competing interests for this study.
Authors want to acknowledge Sonia Hernández and all the members of the neuroanesthesia section at the Hospital Clínic de Barcelona.