About 30–50% of Primary Care (PC) users in Spain suffer mental health problems, mostly mild to moderate anxious and depressive symptoms, which account for 2% of Spain's total Gross domestic product and 50% of the costs associated to all mental disorders. Mobile health tools have demonstrated to cost-effectively reduce anxious and depressive symptoms while machine learning (ML) techniques have shown to accurately detect severe cases. The main aim of this project is to develop a comprehensive ML digital support platform (PRESTO) to cost-effectively screen, assess, triage, and provide personalized treatments for anxious and depressive symptoms in PC.
MethodsThe project will be carried out in 3 complementary phases: First, a ML predictive severity model will be built based on all the cases referred to the PC mental health support programme during the last 5 years in Catalonia. Simultaneously, a smartphone app to monitor and deliver psychological interventions for anxiety and depressive symptoms will be developed and tested in a clinical trial. Finally, the ML models and the app will be integrated in a comprehensive decision-support platform (PRESTO) which will triage and assign to each patient a specific intervention based on individual personal and clinical characteristics. The effectiveness of PRESTO to reduce waiting times in receiving mental healthcare will be tested in a stepped-wedge cluster randomized controlled trial in 5 PC centres.
DiscussionPRESTO will offer timely and personalized cost-effective mental health treatment to people with mild to moderate anxious and depressive symptoms. This will result in a reduction of the burden of mental health problems in PC and on society as a whole.
Trial registrationThe project and their clinical trials were registered in Clinical Trials.gov: NCT04559360 (September 2020).
In Spain, 30–50% of people attending Primary Care (PC) have mental health problems, the highest percentage being mild to moderate anxious and depressive symptoms.1 This group accounts for 2% of Spain's total gross domestic product (GDP) and more than 50% of the costs associated to mental disorders.2 About 5% of these cases will eventually receive specialized care in a referral process that can take up to 3 months given the significant number of referrals that must be assessed.3 The unresolved delay in receiving specialized mental health attention may lead to an increased and unnecessary risk of worsening of symptoms that affects the quality of life of patients and may even lead to irreparable consequences such as suicide.4,5 This situation produces a care demand that far exceeds the resources currently available at PC Centers (PCCs). The COVID-19 pandemic has and will increase even more the prevalence of mental health problems in PC,6,7 especially in healthcare workers,8,9 with potential severe mental health consequences.10–12
For instance, the European Study of the Epidemiology of Mental Disorders (ESEMeD) found that up to 30% of patients with depression reported not receiving any type of care in the previous year,13 and that Spain was among the countries offering one of the lowest levels in quality of care for patients with mental health problems in Europe.14 These data suggest that, in Spain, the therapeutic resources existing in PC to meet this growing demand are clearly insufficient: visits averaging 10min to screen, diagnose and treat mental health problems, non-existent psychological resources or mental health nurses, added to the lack of training of General Practitioners (GP) to approach mental health problems.15 It is therefore not surprising that these deficits have been reflected in a significant increase in the prescription of psychotropic drugs that can reach up to 1 in 2 service users, in many cases unnecessary and prescribed prematurely.16–18 These limitations may lead to unnecessary referrals to specialized care (i.e. people with mild to moderate mental health problems that could be attended in PC) and a lack of early detection of severe mental health problems (i.e. first psychotic episodes or suicidal behaviours).
Considering this complex and problematic challenge, several research groups and health systems have tried to tackle these deficits during the last 10 years. The Spanish National Health System (SNS) has promoted primary care mental health support programmes (PCMHSP) composed of mental health specialists, including psychiatrists, psychologists and nurses, who dedicate part of their workweek to PCCs.19 Recently, group-based cognitive-behavioural therapy (CBT) interventions have also been proposed for treating mental health problems in PC.20–22 Along the same lines, but exploring the potential of new communication technologies, other ongoing projects are evaluating the cost-effectiveness of hybrid (i.e., face-to-face plus online) CBT interventions for PC users suffering from depression.23
The effectiveness of mobile health (mHealth) platforms, including smartphone-based interventions through applications (apps) for treating anxious and depressive symptoms is well established.24–28 The apps allow a constant intervention that adapts to the needs, schedules, and status of the patient in a dynamic way from the progression of the patient and the active or passive data that may or may not be collected.29,30 Our group has demonstrated the feasibility of using apps to offer evidence-based psychological interventions to people suffering from severe mental disorders such as bipolar.31,32 Besides the effectiveness of mHealth, telemedicine, and hence telepsychiatry, have rapidly become compulsory due to social-distancing measures during the COVID-19 pandemic. The current change in paradigm is and will lead to the implementation of telepsychiatry and an increasing use of mHealth platforms in the upcoming years.33
In parallel to the mHealth platforms, another promising advance in the development of digital technology in healthcare is promoted by machine learning (ML) approaches in the context of artificial intelligence (AI). ML models, unlike traditional statistical models, offer two important advantages: (1) a higher predictive accuracy that can be reproduced and replicated as the models are trained, tested and validated with subsets of the total dataset, (2) interpretable models that can explain the causes of these predictions to improve them and increase the accuracy of the initial algorithm by back feeding it. Recently, the development and use of ML models has proven useful to identify severe cases of depression that would require specialized care among PC users.34 In addition, ML algorithms in PC are demonstrating use to help to personalize pharmacological treatments for depression.35–37
Considering this emerging evidence demonstrating the potential of mHealth and AI in PC, we hypothesize that, the use of a digital decision support platform combining ML models with a smartphone-based app for monitoring and delivering evidence-based psychological interventions for people with mild to moderate anxious and depressive symptoms will reduce waiting times and improve the quality of mental healthcare compared to treatment as usual in PCC in a cost-effective manner.
Methods and designAimsThis project aims at developing an integral platform of digital support for mental health problems in PC: PRimary carE digital Support ToOl in mental health (PRESTO), which will allow to improve the quality of care and reduce waiting times through the prioritization of the most severe cases to be assessed timely in specialized care, while it assigns mild to moderate cases to one of these interventions: (1) Watchful waiting and periodic follow-up by the GP, (2) Symptoms monitoring and a brief evidence-based psychological intervention through an app (PRESTOapp), or (3) A personalized psychopharmacological treatment. To achieve this objective, first, ML-based predictive severity models will be developed taking into account clinical variables from electronic health records (EHR) of all referrals from GPs to the Primary Care mental health support programme (PCMHSP) during the last 5 years from all PCCs in Catalonia, including a total population of 7.5 million users. Simultaneously, the effectiveness of an app for monitoring and delivering evidence-based psychological interventions for mild to moderate anxiety-depressive symptoms (PRESTOapp) will be developed and evaluated in a feasibility study and a randomized clinical trial. Finally, the ML models and the PRESTOapp will be integrated into a decision support platform (PRESTO) that will triage and personalize the interventions for each case in a supervised manner. Yet, the platform will be dynamically updated, and the triage algorithm will be automatically re-trained with the results of the evolution and response to the treatment of each case included to improve its accuracy.
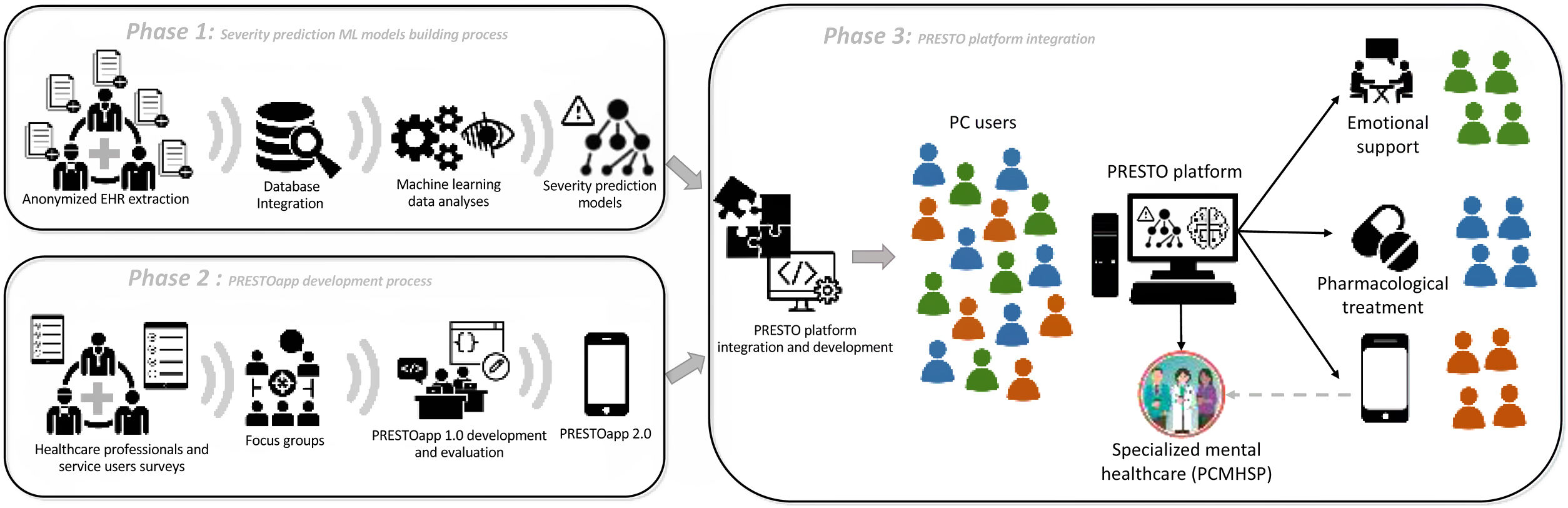
Study design and phasesThe PRESTO project will be structured in three phases, described in detail below, in order to finally integrate the different modules validated in each phase in the final objective of evaluating the PRESTO platform (Fig. 1). Phase 1 and 2 will be conducted simultaneously.
Phase 1: Building machine learning models that allow the categorization of the severity of people with mental health problems in PCThe regional shared EHR of all referrals from GPs to the PCMHSP during the last 5 years from all PCCs in Catalonia and a control sample of non-referred PC users will be independently retrieved and anonymized by the Data analytics programme for health research and innovation (PADRIS)38 from the Catalan Agency for Health Quality and Evaluation (AQuAS).39 Afterwards, the data will be analyzed in an aggregated manner.
Based on previous research of predictors of depression severity in patients from PC,40 the baseline data that will be prioritized in the initial model are the following variables: sex, age, education level, ethnicity, employment status, marital status, housing and living conditions, present or past medical and psychiatric co-morbidities, past-present drug use and pharmacological treatments, and recent life stressors. Additionally, any previous mental health care service provided such as emergency room or hospitalizations admissions registered in any public health care facility in Catalonia will be included to the model. Simultaneously the same variables from PC users who were not referred or required to the PCMHSP will be retrieved from public EHR to train the ML models.
Once all the data is retrieved and merged from the diverse sources aforementioned, the team (AS, JR) will use ML techniques to building, debugging, modelling, training and evaluating predictive variables to differentiate PC users that required specialized mental health care from those who didn’t, as well as predictive variables of severity. The severity (target feature) will be determined by the diagnosis by the PCMHSP of a severe mental disorder or not, time off work, the use of second- or third-line antidepressant treatments, prolonged follow-up, referral to the mental health centre, hospitalization in a psychiatric units or consultations in the psychiatric emergency departments.
Potential problems that can be assumed in predictable data cleansing and modelling include cases that left the monitoring by the PCMHSP, people who changed the basic health area sector, or cases that preferred to be monitored and treated in the private network. It will also be decided on the basis of the quality and granularity of the data available whether to proceed with a binary, categorical or cluster classification.
Phase 2: Development and evaluation of the feasibility, acceptability, usability, satisfaction and effectiveness of PRESTOappBased on the team's experience in the development and evaluation of mental health apps,31,32 the next steps will be followed:
a. Development of PRESTOapp 1.0: A User-centred development (UCD) approach will be adopted which has evidence of improved user retention and satisfaction with the end product.41,42
This phase will begin by conducting two online surveys: one directed to PC users who have been referred to PCMHSP about their interests and preferences on the use of a mental health app, and another survey directed to GPs and mental health professionals (psychiatrists, psychologists, specialized nurses, etc.) about the needs they think ought to be covered by a mental health app.
Subsequently, two focus groups, one with PC users/people who have been referred to PCMHSP and another with GPs and mental health professionals, with a minimum of 8 people each and a duration of 1.5hours approximately, will be performed in an online format. During the focus group, ideas, models, designs, characteristics and functionalities of the proposed mental health app, considering the results from the surveys, will be presented to find out the participants’ interest and opinions.
Based on the surveys and the data collected during the focus groups, the research team will outline the priority aspects of the development of the application as well as the contents of the evidence-based psychological interventions to be included.
The technical development of PRESTOapp will be carried out with the software development collaborating company through an iterative, collaborative, multidisciplinary process that preserves patient confidentiality at all times and will be monitored by the researchers as it will be displayed in real time at the collaborative working platform. This approach has been successfully implemented by the team in previous e-mental health projects.43
b. Evaluation of the feasibility of PRESTOapp 1.0: A minimum of 50 PC users will be recruited to participate in the feasibility study of PRESTOapp 1.0 app for 1 month. The inclusion criteria will take into account persons between 18 and 65 years of age who are referred to or are being followed up by the PCMHSP who are found to have mild-to-moderate depressive (PHQ-9: 5–14) and/or anxious (GAD-7: 5–14) symptoms,44,45 and have a compatible smartphone (i.e. Android or iPhone) and sufficient digital skills to download and use an app. The criteria for exclusion will be the previous or present diagnosis of a severe mental disorder, or a mild, moderate or severe risk of suicide. A baseline assessment will be conducted to collect sociodemographic (including occupation to identify health workers), medical and psychiatric history and evaluate depressive (PHQ-9) and anxious (GAD-7) symptoms. At a single follow-up visit after 1 month, depressive and anxious symptoms will be reassessed and acceptability will be explored with the Technology Acceptance Model (TAM) questionnaire,46 usability with the System usability scale (SUS),47 and satisfaction with the Health App Usability Questionnaire (MAUQ).48 Data on usage, retention and adherence with the app will be retrieved and analyzed directly from the servers.
c. Randomized controlled clinical trial with PRESTOapp 2.0: Based on participants’ feedback and suggestions from the feasibility study, PRESTOapp will be further improved to a 2.0 version. We will conduct a single-blind randomized controlled clinical trial offering their participation to all patients referred to our PCMHSP from our catchment area that includes 5 PCCs. The sample size calculation will contemplate as a primary outcome the reduction of depressive symptoms assessed by PHQ-9 taking into account two groups (PRESTOapp 2.0 vs. treatment as usual (TAU) in addition to previous results of effect sizes between 0.30 and 0.35 from similar studies.49,50 We have set for this purpose a power of 0.80 and a α of 0.05. Experience in similar studies indicates an expected 25–30% drop-out. Therefore, it was decided to add 15 more subjects per branch for preventive purposes and to ensure that at the end of the study there would be a sufficient sample to guarantee the strength of the data. Hence, 76 participants per branch will be recruited. Considering similar inclusion and exclusion criteria as the previous feasibility study, the intervention group will be asked to use the app for a period of 2 months. The control group will receive the usual follow-up and treatment during the same time by the PCMHSP team. Participants from both groups will be asked to participate in an initial evaluation and a follow-up in 2 months, unless the app indicates or symptoms that require urgent care arise. Both interviews will include the collection of sociodemographic and clinical variables alongside standardized scales PHQ-9, GAD-7, Holmes and Rahe Stress Scale.51
Statistical analyses will be conducted using specific R packages. The analyses of all the subjects included will be considered until the dropout or end of the study. The main variable is the change in symptoms measured by PHQ-9, GAD-7 during the 2 months, controlled by Holmes and Rahe Stress Scale. We will use a mixed effects linear model with random interception for each participant. The differences in the primary measures will be analyzed first in an unadjusted manner and then adjusted for sociodemographic and clinical factors. An analysis by intention to treat (ITT) with last observation carried forward (LOCF) will also be employed. A threshold of statistical significance p≤0.05 (two-tailed) will be set.
Among the limitations of this study, it is worth mentioning that researcher and patients blinding will not be possible for logistical and ethical reasons, nor is there a placebo in the case of the control group. The main reason for this design is that the methodological effects of providing a placebo app to the control group are unclear. Assumable risks are the placebo and Hawthorne effect in the intervention group, natural progression and regression to the mean. Digital literacy will likely be widely variable among the included sample. This may hinder the use of the app in some of the included subjects. To minimize bias in measuring engagement, inclusion criteria in phase 2 takes into account having sufficient digital skills to download and use an app. Moreover, the design of the app's user-interface will be clean, user-friendly, and include preliminary instructions of use.
Health workers are one of the most affected populations regarding mental health due to the COVID-19 pandemic. Beside from anxiety and depression symptoms, they may suffer from posttraumatic stress disorder (PTSD) symptoms.9 Moreover, their medical literacy is usually higher than the general population. To avoid selection bias, health workers included in the study will be identified and a sensitivity analysis performed. It is worth noting that a version of the PRESTOapp adapted to specific situations and particularities of health workers – in particular on the detection and management of PTSD symptoms and the medical language used – is under current development in a parallel project.
Phase 3: Integration and evaluation of the PRESTO platforma. Calculation of predictive models of response to each assigned intervention based on ML: development and integration in the PRESTO platform: From the ML predictive models built in phase 1 and the data collected on the evolution of patients with continuous monitoring through the app in phase 2, the PRESTO platform will be integrated and developed in a web interface.
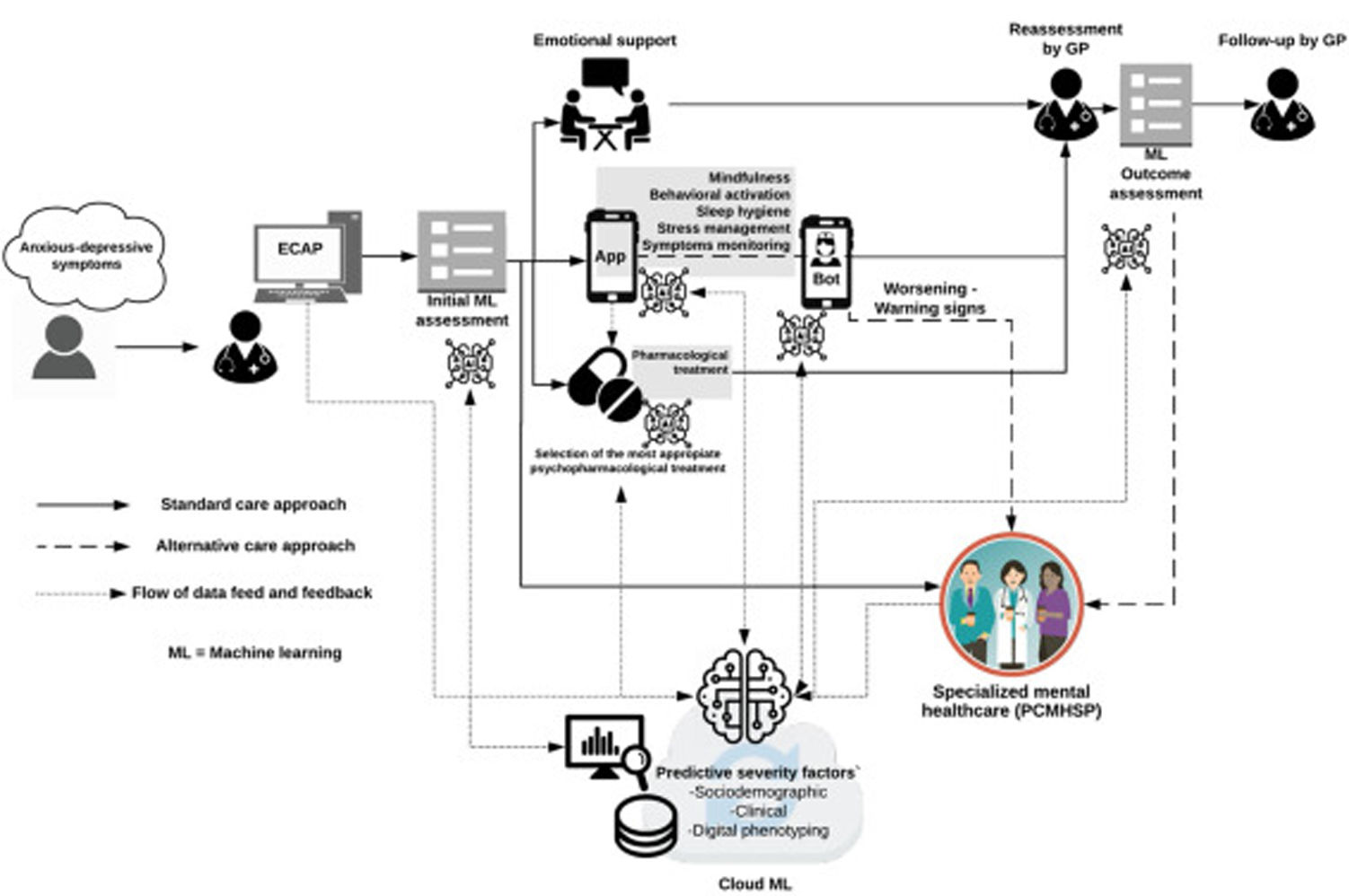
The main aim of this phase is to test the PRESTO platform, a dynamic triage system for all patients who are referred to PCMHSP by GPs that will be assigned, in a supervised manner, the most appropriate and personalized approach to each case. However, beyond the friendly web environment that will host the platform, the background algorithms will progressively be fed to increase with outcomes and follow-up data derived from face-to-face visits as well as those collected by the PRESTOapp. This will allow a two-way loop flow of information that with a machine learning approach will “learn in a supervised manner” and will allow to predict with increasing precision the evolutionary course of the patients and the best type of the approach that will be required (Fig. 2).
Final PRESTO platform framework and dataflow prototype. The picture shows a diagram of the final PRESTO platform prototype 1.56 alongside their dataflow channels allowing to created loop of self-learning data from data collecting during the follow-up and treatment assigned to each specific case.
b. Stepped-wedge cluster randomized trial to assess effectiveness in reducing waiting lists: The investigation of PRESTO effectiveness in reducing waiting times will be carried out by means of a pragmatic design study without distorting or interrupting the health care of service users as well as minimizing its risks. The design will therefore be of a Stepped-wedge cluster randomized trial, in which, for predetermined periods of 2 months, the platform will be implemented randomly to clusters of referrals to PCMHSP of each of the 5 PCCs from our catchment area. The rest of the time the current care method will be used as a control. All users will be informed of the system being used through the PRESTO platform and they must accept and sign the informed consent for study participation. Otherwise, they be provided with the current healthcare approach. The main variable for assessing efficiency will be the reduction in waiting time of a first contact with the PCMHSP of GP referrals, which will be calculated for each PCC.
Regarding statistical analyses, the waiting times will be compared with other centres and also with the average of each period of the historical records of the last 5 years. In order to minimize selection and unbalance bias, individual and cluster characteristics will be compared among groups beforehand and appropriate corrections will be conducted. A generalized mixed-effects model will be adopted. This model will make it possible to determine the efficiency of the platform by being able to adjust it by age, sex, seasonal patterns, as well as the characteristics and care burden of each PCC (population covered, socioeconomic situation, number of individuals per GP). A randomized slope model for treatment will also be considered given the variability of intervention by each centre following the recommendations of Davey et al.52 As a sensitivity analysis, the use of a mixed-effects linear Gaussian model for the different variances within each cluster will be adopted. Calculations will be made using specific R packages. In order to provide an extensive use of the PRESTO platform among GPs, and avoid a restricted use due to limited digital literacy, the user-interface will be clean, user-friendly and intuitive.
Ethical considerationsThe PRESTO project has been approved by the Clinical Research Ethics Committees (CEIC) of the Hospital Clinic of Barcelona (HCB/2020/0735). All the data will be collected by the project researchers, and stored in encrypted and secure servers following the guidelines and standards of the 2018 European General Data Protection Regulation (GDPR). The project protocols as described in this manuscript were registered at Clinicaltrials.gov (NCT04559360) in September 2020.
Project status and preliminary resultsAt the time of writing this protocol, the process of anonymized data extraction from EHR are already underway with the collaboration of the PADRIS programme from the AQuAS agency, as planned in Phase 1. In parallel, we have finished the exploratory surveys about the attitudes, uses and preferences of a digital mental health platform from both PC users and health professionals.
The participation in PC users survey was offered at the centres waiting rooms with cards and posters including a QR code, while the invitation to complete the health professionals survey was distributed by internal mailing list containing a link. The link to the surveys were accessible from June to November 2020. Both surveys were composed of 47 sequential, multi-choice and non-mandatory questions.
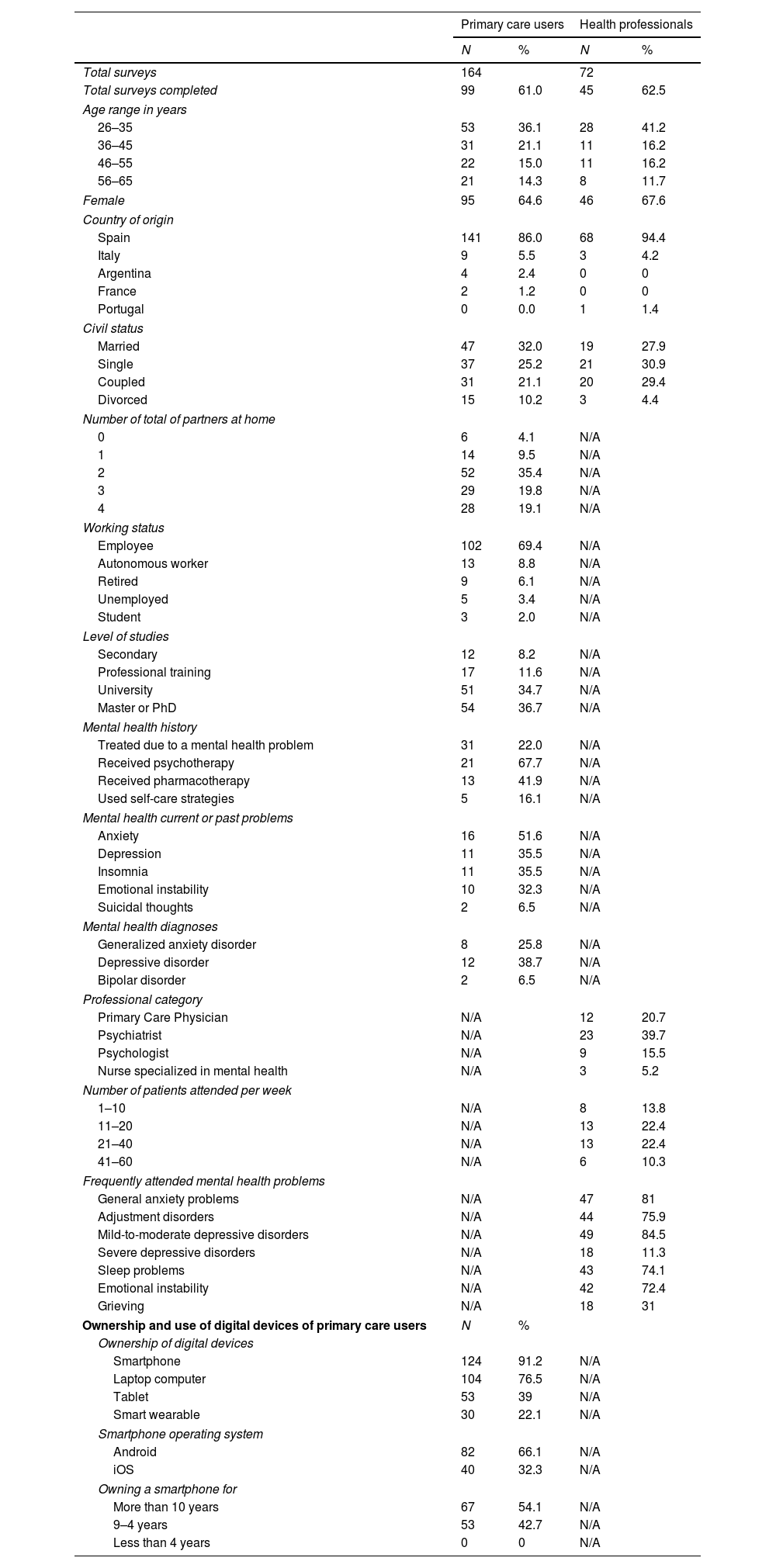
One hundred and sixty-two PC users and 72 health professionals took part in the survey. The sociodemographic and clinical characteristics of the respondents are described in Table 1. About 1 in 5 (22%) of PC users had previously been treated for mental health problems, most of them due to “anxiety” (51.6%), “depression” and “sleep problems” (35.5% both).
Sociodemographic and clinical characteristics.
| Primary care users | Health professionals | |||
|---|---|---|---|---|
| N | % | N | % | |
| Total surveys | 164 | 72 | ||
| Total surveys completed | 99 | 61.0 | 45 | 62.5 |
| Age range in years | ||||
| 26–35 | 53 | 36.1 | 28 | 41.2 |
| 36–45 | 31 | 21.1 | 11 | 16.2 |
| 46–55 | 22 | 15.0 | 11 | 16.2 |
| 56–65 | 21 | 14.3 | 8 | 11.7 |
| Female | 95 | 64.6 | 46 | 67.6 |
| Country of origin | ||||
| Spain | 141 | 86.0 | 68 | 94.4 |
| Italy | 9 | 5.5 | 3 | 4.2 |
| Argentina | 4 | 2.4 | 0 | 0 |
| France | 2 | 1.2 | 0 | 0 |
| Portugal | 0 | 0.0 | 1 | 1.4 |
| Civil status | ||||
| Married | 47 | 32.0 | 19 | 27.9 |
| Single | 37 | 25.2 | 21 | 30.9 |
| Coupled | 31 | 21.1 | 20 | 29.4 |
| Divorced | 15 | 10.2 | 3 | 4.4 |
| Number of total of partners at home | ||||
| 0 | 6 | 4.1 | N/A | |
| 1 | 14 | 9.5 | N/A | |
| 2 | 52 | 35.4 | N/A | |
| 3 | 29 | 19.8 | N/A | |
| 4 | 28 | 19.1 | N/A | |
| Working status | ||||
| Employee | 102 | 69.4 | N/A | |
| Autonomous worker | 13 | 8.8 | N/A | |
| Retired | 9 | 6.1 | N/A | |
| Unemployed | 5 | 3.4 | N/A | |
| Student | 3 | 2.0 | N/A | |
| Level of studies | ||||
| Secondary | 12 | 8.2 | N/A | |
| Professional training | 17 | 11.6 | N/A | |
| University | 51 | 34.7 | N/A | |
| Master or PhD | 54 | 36.7 | N/A | |
| Mental health history | ||||
| Treated due to a mental health problem | 31 | 22.0 | N/A | |
| Received psychotherapy | 21 | 67.7 | N/A | |
| Received pharmacotherapy | 13 | 41.9 | N/A | |
| Used self-care strategies | 5 | 16.1 | N/A | |
| Mental health current or past problems | ||||
| Anxiety | 16 | 51.6 | N/A | |
| Depression | 11 | 35.5 | N/A | |
| Insomnia | 11 | 35.5 | N/A | |
| Emotional instability | 10 | 32.3 | N/A | |
| Suicidal thoughts | 2 | 6.5 | N/A | |
| Mental health diagnoses | ||||
| Generalized anxiety disorder | 8 | 25.8 | N/A | |
| Depressive disorder | 12 | 38.7 | N/A | |
| Bipolar disorder | 2 | 6.5 | N/A | |
| Professional category | ||||
| Primary Care Physician | N/A | 12 | 20.7 | |
| Psychiatrist | N/A | 23 | 39.7 | |
| Psychologist | N/A | 9 | 15.5 | |
| Nurse specialized in mental health | N/A | 3 | 5.2 | |
| Number of patients attended per week | ||||
| 1–10 | N/A | 8 | 13.8 | |
| 11–20 | N/A | 13 | 22.4 | |
| 21–40 | N/A | 13 | 22.4 | |
| 41–60 | N/A | 6 | 10.3 | |
| Frequently attended mental health problems | ||||
| General anxiety problems | N/A | 47 | 81 | |
| Adjustment disorders | N/A | 44 | 75.9 | |
| Mild-to-moderate depressive disorders | N/A | 49 | 84.5 | |
| Severe depressive disorders | N/A | 18 | 11.3 | |
| Sleep problems | N/A | 43 | 74.1 | |
| Emotional instability | N/A | 42 | 72.4 | |
| Grieving | N/A | 18 | 31 | |
| Ownership and use of digital devices of primary care users | N | % | ||
| Ownership of digital devices | ||||
| Smartphone | 124 | 91.2 | N/A | |
| Laptop computer | 104 | 76.5 | N/A | |
| Tablet | 53 | 39 | N/A | |
| Smart wearable | 30 | 22.1 | N/A | |
| Smartphone operating system | ||||
| Android | 82 | 66.1 | N/A | |
| iOS | 40 | 32.3 | N/A | |
| Owning a smartphone for | ||||
| More than 10 years | 67 | 54.1 | N/A | |
| 9–4 years | 53 | 42.7 | N/A | |
| Less than 4 years | 0 | 0 | N/A | |
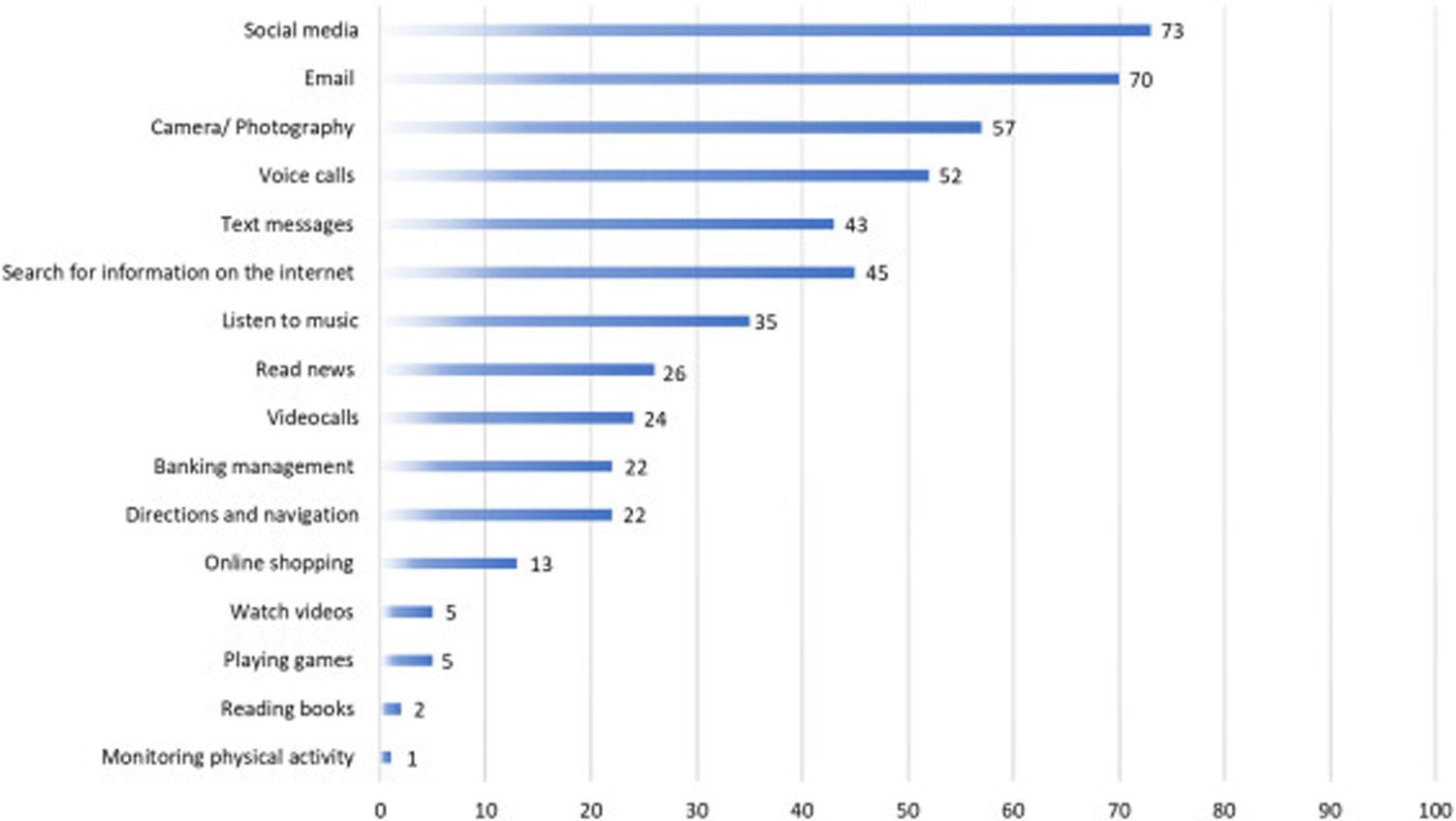
The ownership and use of digital devices of PC users are reported in Table 1, most of the them reported owning a smartphone (91.2%) with an Android operating system (66.1%), and 54.1% reported having been using a smartphone for more than 10 years. Fig. 3 details the most common uses of their smartphone informed by the participants.
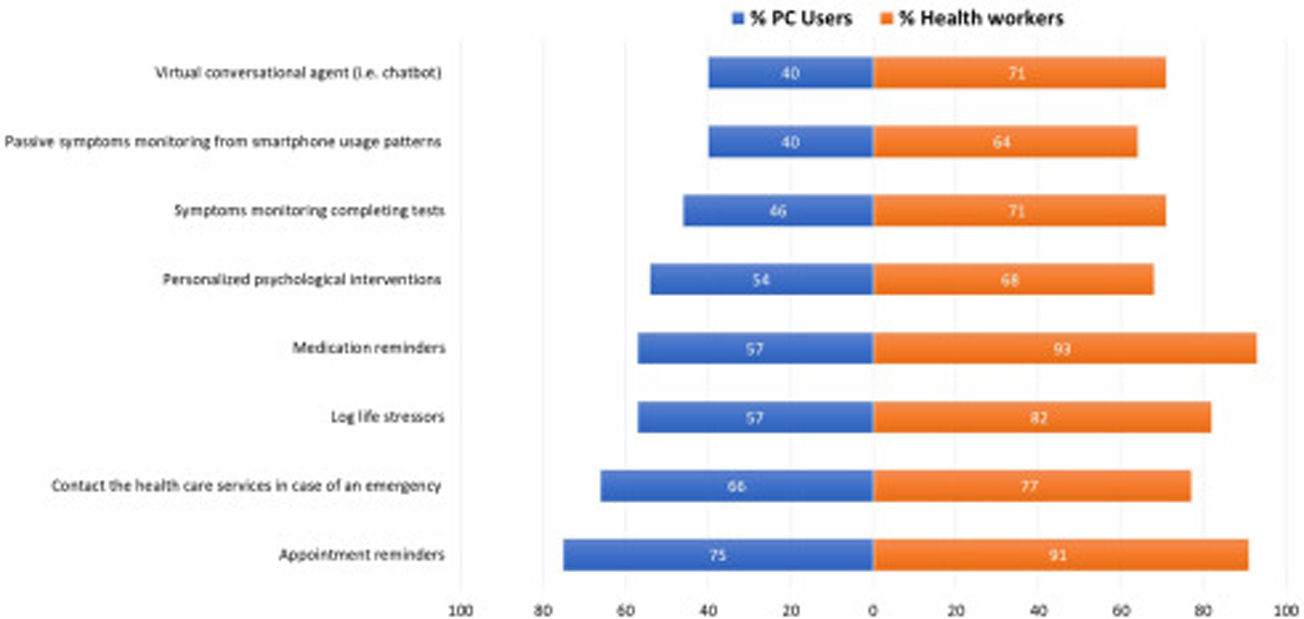
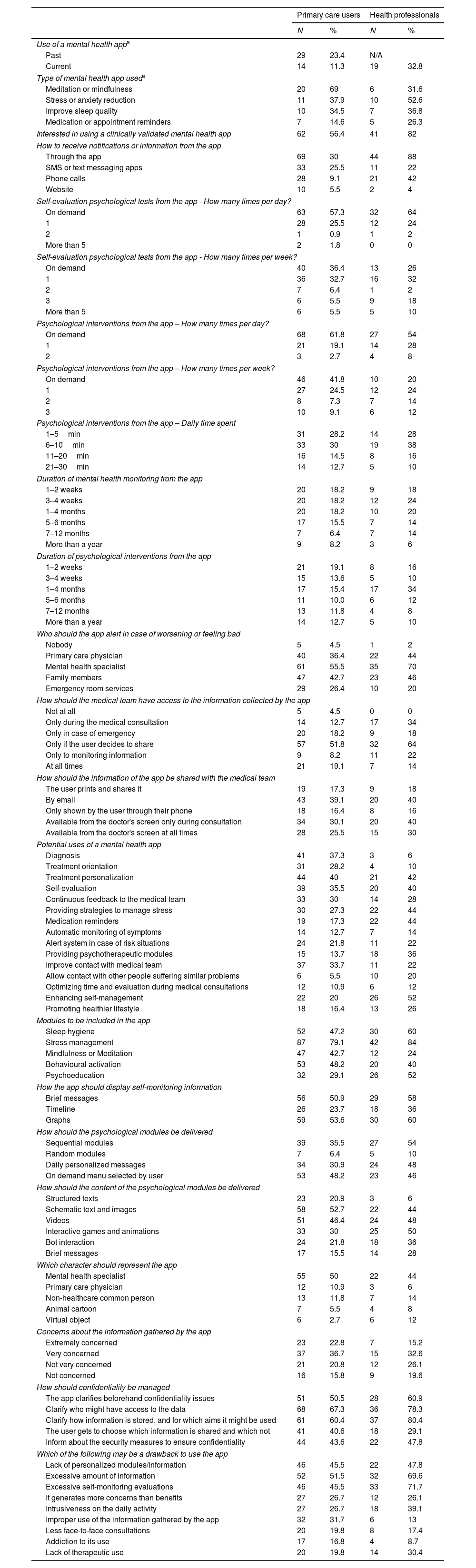
The use of smartphone applications (app) for mental health reasons and the potential use of a clinically validated mental health app are detailed in Table 2. Almost one-quarter of PC users (23.4%) reported having used a mental health app and an important percentage of health professionals (32.8%) found out that their patients used or were already using mental health apps. The most common type of mental health apps used were for meditation/mindfulness, to reduce stress and improve sleep quality. As a result, more than half of PC users (56.4%) and 82% of health professionals were interested on having available a clinically-validated mental health app for use or to prescribe to their patients with the purposes and features detailed in Fig. 4.
Mental health apps: use and interests.
| Primary care users | Health professionals | |||
|---|---|---|---|---|
| N | % | N | % | |
| Use of a mental health appa | ||||
| Past | 29 | 23.4 | N/A | |
| Current | 14 | 11.3 | 19 | 32.8 |
| Type of mental health app useda | ||||
| Meditation or mindfulness | 20 | 69 | 6 | 31.6 |
| Stress or anxiety reduction | 11 | 37.9 | 10 | 52.6 |
| Improve sleep quality | 10 | 34.5 | 7 | 36.8 |
| Medication or appointment reminders | 7 | 14.6 | 5 | 26.3 |
| Interested in using a clinically validated mental health app | 62 | 56.4 | 41 | 82 |
| How to receive notifications or information from the app | ||||
| Through the app | 69 | 30 | 44 | 88 |
| SMS or text messaging apps | 33 | 25.5 | 11 | 22 |
| Phone calls | 28 | 9.1 | 21 | 42 |
| Website | 10 | 5.5 | 2 | 4 |
| Self-evaluation psychological tests from the app - How many times per day? | ||||
| On demand | 63 | 57.3 | 32 | 64 |
| 1 | 28 | 25.5 | 12 | 24 |
| 2 | 1 | 0.9 | 1 | 2 |
| More than 5 | 2 | 1.8 | 0 | 0 |
| Self-evaluation psychological tests from the app - How many times per week? | ||||
| On demand | 40 | 36.4 | 13 | 26 |
| 1 | 36 | 32.7 | 16 | 32 |
| 2 | 7 | 6.4 | 1 | 2 |
| 3 | 6 | 5.5 | 9 | 18 |
| More than 5 | 6 | 5.5 | 5 | 10 |
| Psychological interventions from the app – How many times per day? | ||||
| On demand | 68 | 61.8 | 27 | 54 |
| 1 | 21 | 19.1 | 14 | 28 |
| 2 | 3 | 2.7 | 4 | 8 |
| Psychological interventions from the app – How many times per week? | ||||
| On demand | 46 | 41.8 | 10 | 20 |
| 1 | 27 | 24.5 | 12 | 24 |
| 2 | 8 | 7.3 | 7 | 14 |
| 3 | 10 | 9.1 | 6 | 12 |
| Psychological interventions from the app – Daily time spent | ||||
| 1–5min | 31 | 28.2 | 14 | 28 |
| 6–10min | 33 | 30 | 19 | 38 |
| 11–20min | 16 | 14.5 | 8 | 16 |
| 21–30min | 14 | 12.7 | 5 | 10 |
| Duration of mental health monitoring from the app | ||||
| 1–2 weeks | 20 | 18.2 | 9 | 18 |
| 3–4 weeks | 20 | 18.2 | 12 | 24 |
| 1–4 months | 20 | 18.2 | 10 | 20 |
| 5–6 months | 17 | 15.5 | 7 | 14 |
| 7–12 months | 7 | 6.4 | 7 | 14 |
| More than a year | 9 | 8.2 | 3 | 6 |
| Duration of psychological interventions from the app | ||||
| 1–2 weeks | 21 | 19.1 | 8 | 16 |
| 3–4 weeks | 15 | 13.6 | 5 | 10 |
| 1–4 months | 17 | 15.4 | 17 | 34 |
| 5–6 months | 11 | 10.0 | 6 | 12 |
| 7–12 months | 13 | 11.8 | 4 | 8 |
| More than a year | 14 | 12.7 | 5 | 10 |
| Who should the app alert in case of worsening or feeling bad | ||||
| Nobody | 5 | 4.5 | 1 | 2 |
| Primary care physician | 40 | 36.4 | 22 | 44 |
| Mental health specialist | 61 | 55.5 | 35 | 70 |
| Family members | 47 | 42.7 | 23 | 46 |
| Emergency room services | 29 | 26.4 | 10 | 20 |
| How should the medical team have access to the information collected by the app | ||||
| Not at all | 5 | 4.5 | 0 | 0 |
| Only during the medical consultation | 14 | 12.7 | 17 | 34 |
| Only in case of emergency | 20 | 18.2 | 9 | 18 |
| Only if the user decides to share | 57 | 51.8 | 32 | 64 |
| Only to monitoring information | 9 | 8.2 | 11 | 22 |
| At all times | 21 | 19.1 | 7 | 14 |
| How should the information of the app be shared with the medical team | ||||
| The user prints and shares it | 19 | 17.3 | 9 | 18 |
| By email | 43 | 39.1 | 20 | 40 |
| Only shown by the user through their phone | 18 | 16.4 | 8 | 16 |
| Available from the doctor's screen only during consultation | 34 | 30.1 | 20 | 40 |
| Available from the doctor's screen at all times | 28 | 25.5 | 15 | 30 |
| Potential uses of a mental health app | ||||
| Diagnosis | 41 | 37.3 | 3 | 6 |
| Treatment orientation | 31 | 28.2 | 4 | 10 |
| Treatment personalization | 44 | 40 | 21 | 42 |
| Self-evaluation | 39 | 35.5 | 20 | 40 |
| Continuous feedback to the medical team | 33 | 30 | 14 | 28 |
| Providing strategies to manage stress | 30 | 27.3 | 22 | 44 |
| Medication reminders | 19 | 17.3 | 22 | 44 |
| Automatic monitoring of symptoms | 14 | 12.7 | 7 | 14 |
| Alert system in case of risk situations | 24 | 21.8 | 11 | 22 |
| Providing psychotherapeutic modules | 15 | 13.7 | 18 | 36 |
| Improve contact with medical team | 37 | 33.7 | 11 | 22 |
| Allow contact with other people suffering similar problems | 6 | 5.5 | 10 | 20 |
| Optimizing time and evaluation during medical consultations | 12 | 10.9 | 6 | 12 |
| Enhancing self-management | 22 | 20 | 26 | 52 |
| Promoting healthier lifestyle | 18 | 16.4 | 13 | 26 |
| Modules to be included in the app | ||||
| Sleep hygiene | 52 | 47.2 | 30 | 60 |
| Stress management | 87 | 79.1 | 42 | 84 |
| Mindfulness or Meditation | 47 | 42.7 | 12 | 24 |
| Behavioural activation | 53 | 48.2 | 20 | 40 |
| Psychoeducation | 32 | 29.1 | 26 | 52 |
| How the app should display self-monitoring information | ||||
| Brief messages | 56 | 50.9 | 29 | 58 |
| Timeline | 26 | 23.7 | 18 | 36 |
| Graphs | 59 | 53.6 | 30 | 60 |
| How should the psychological modules be delivered | ||||
| Sequential modules | 39 | 35.5 | 27 | 54 |
| Random modules | 7 | 6.4 | 5 | 10 |
| Daily personalized messages | 34 | 30.9 | 24 | 48 |
| On demand menu selected by user | 53 | 48.2 | 23 | 46 |
| How should the content of the psychological modules be delivered | ||||
| Structured texts | 23 | 20.9 | 3 | 6 |
| Schematic text and images | 58 | 52.7 | 22 | 44 |
| Videos | 51 | 46.4 | 24 | 48 |
| Interactive games and animations | 33 | 30 | 25 | 50 |
| Bot interaction | 24 | 21.8 | 18 | 36 |
| Brief messages | 17 | 15.5 | 14 | 28 |
| Which character should represent the app | ||||
| Mental health specialist | 55 | 50 | 22 | 44 |
| Primary care physician | 12 | 10.9 | 3 | 6 |
| Non-healthcare common person | 13 | 11.8 | 7 | 14 |
| Animal cartoon | 7 | 5.5 | 4 | 8 |
| Virtual object | 6 | 2.7 | 6 | 12 |
| Concerns about the information gathered by the app | ||||
| Extremely concerned | 23 | 22.8 | 7 | 15.2 |
| Very concerned | 37 | 36.7 | 15 | 32.6 |
| Not very concerned | 21 | 20.8 | 12 | 26.1 |
| Not concerned | 16 | 15.8 | 9 | 19.6 |
| How should confidentiality be managed | ||||
| The app clarifies beforehand confidentiality issues | 51 | 50.5 | 28 | 60.9 |
| Clarify who might have access to the data | 68 | 67.3 | 36 | 78.3 |
| Clarify how information is stored, and for which aims it might be used | 61 | 60.4 | 37 | 80.4 |
| The user gets to choose which information is shared and which not | 41 | 40.6 | 18 | 29.1 |
| Inform about the security measures to ensure confidentiality | 44 | 43.6 | 22 | 47.8 |
| Which of the following may be a drawback to use the app | ||||
| Lack of personalized modules/information | 46 | 45.5 | 22 | 47.8 |
| Excessive amount of information | 52 | 51.5 | 32 | 69.6 |
| Excessive self-monitoring evaluations | 46 | 45.5 | 33 | 71.7 |
| It generates more concerns than benefits | 27 | 26.7 | 12 | 26.1 |
| Intrusiveness on the daily activity | 27 | 26.7 | 18 | 39.1 |
| Improper use of the information gathered by the app | 32 | 31.7 | 6 | 13 |
| Less face-to-face consultations | 20 | 19.8 | 8 | 17.4 |
| Addiction to its use | 17 | 16.8 | 4 | 8.7 |
| Lack of therapeutic use | 20 | 19.8 | 14 | 30.4 |
Overall, PC users were predominantly interested in symptoms monitoring (46%) and receiving psychological contents (54%) through a smartphone app preferably only when they needed (on-demand) (57.3%) during 1 to 10min per day (58.2%), 1 to 3 times per week (81%), over a period from 1 to 6 months (58.2%). Most of the participants preferred to share with the health care team the information collected by the app only when they decided it (51.8%), by e-mail (39.1%) and alert the mental health specialist if worsening (55.5%). Most of the respondents (59.5%) were extremely or very worried about the confidentiality and safety of the potential data collected by the app, stating that they would feel safer about it if the app clarifies beforehand who might have access to their data (68%), where and how it is stored, and for which aims it might be used (60%). The main drawback identified to use the app was an excessive amount of information (51.5%).
Health professionals that responded the survey, mostly psychiatrists and GPs, regularly cared for people suffering from depression and anxiety problems (more than 80% of patient's attended). The preferences of a clinically-validated mental health app were very similar to those from PC users (Table 2). The greatest discrepancies between them were that health professionals were more interested in apps including psychoeducation modules (52 vs 29%) whereas PC users showed more interest in meditation/mindfulness modules (43 vs 24%). Also, compared to PC users, only few health professionals considered that an app would be useful for diagnostic (6 vs 37%) or treatment orientation purposes (10 vs 28%). Finally, health professionals considered that self-evaluations should be performed more times per week as compared to patients’ preferences.
Based on all the feedback received with both surveys, we have drafted the first mock-ups and prototypes of the potential mental health app to present and discuss at the focus groups which are scheduled to be held in February 2021 with a modified online approach due to the covid-19 crisis.
DiscussionTo our knowledge this is the first attempt to integrate these novel technologies—smartphone apps and real-time machine learning—in a single integral decision and therapeutic support platform to address mental health issues in PC. However, it must be noted that independent computerized decision tools have already been successfully tested at PC settings in other medical disciplines.53–58
In addition, in the context of mental health, statistical analyses using ML techniques have demonstrated to correctly predict treatment resistance in major depressive disorder and identify patients in PC who might need specialized care. However, these analyses and their findings were made a posteriori and not in real time as this project proposes. Considering this novel approach and in order to ensure its feasibility, safety and test its potential effectiveness an iterative sequential process will be undertook adopting a supervised approach to ML. This kind of supervised ML approach allows and enables an easier understanding of ML outputs, which makes it especially suitable to identify, modify and improve clinical algorithms thus preventing the black box problem.59
Moreover, the design of the study by means of a stepped-wedge cluster randomized controlled trial, will facilitate its pragmatic evaluation simultaneously with the usual healthcare. Furthermore if the main hypothesis of the effectiveness of this approach is confirmed, it would make its implementation feasible in other national or international centres by considering the characteristics of each particular sector and population facilitated by the ML approach.60
It is important to highlight that innovations in psychiatry have not been promoted only by stakeholders and healthcare institutions for cost-effectiveness purposes. Healthcare professionals are also willing to embrace these technologies to deliver psychological interventions, symptoms monitoring as well as other therapeutic purposes.61 Moreover, as has been emphasized by several recent studies62,63 and re-confirmed by our initial survey, there is a great interest and preferences of adopting mHealth tools by primary care service users and healthcare professionals to support their mental health. In fact, a relevant percentage of them are already using non-clinically or scientifically validated mHealth tools to cope with their mental health issues representing a potential danger to their health. One of the main reasons for this phenomenon is the currently scarce options of commercially available apps developed or tested by academic or healthcare institutions. We have previously proposed frameworks and methodologies to facilitate these technologies to reach end-users which will be fully adopted throughout this project.64
There are several potential limitations which have been mentioned in the methodology of each sub-study above. However, one of the most concerning issues developing mHealth tools and decision support platform is its long-term lack of use in real-world clinical practice either by the patients or clinicians. Despite this possibilities, as has also been mentioned above, we are going to dedicate a strict user-centred design approach, thus adapting the final PRESTO app and platform to the needs and requirements of end-users.65 If successfully validated and its effectiveness is confirmed, we have set out in advance a valorization, cooperation and transferability plan to the public health system.
Altogether, the PRESTO platform has the unique potential to transform the current dilemma of mental health in PC by optimizing waiting times and improving the flow of care for patients in real-time according to their personal and clinical characteristics, adapting cost-effective interventions for each case and thus alleviating existing deficits. This unique approach embraces and attempts to make a reality the long-awaited precision psychiatry which have been proposed for many years with theoretical models.66
Authors’ contributionsDH, MC, EV and GA designed the project, studies and drafted the manuscript. DH and GA coordinated the project development with the involved companies. MP, XS, and IM will participate in the development of the psychological contents of PRESTOapp. VV will advise and help with the coordination of PRESTOapp development. VR, AAS, ES, SM, MSC will participate in the recruitment. AY and RZ will advise on the pharmacological treatment module development. JB will advise and coordinate the data analysis with PADRIS-AQUAS. CVB and JFMC will perform the anonymized data extraction from PADRIS-AQUAS electronic health records. EV reviewed the manuscript and made several suggestions to improve it. JR, AS, and MF revised and suggested the statistical analyses. JR, AS, MF, and EV supervised, reviewed and improved the project and manuscript. All authors read and approved the final version of the manuscript.
Consent for publicationAll participants will be asked to provide written informed consent prior to their inclusion in the study.
Availability of data and materialsThe investigators are currently collecting data. This is an ongoing study. Hence, preliminary results have not yet been published. The datasets used and/or analyzed during the current study will be available from the corresponding author on reasonable request.
FundingThe PRESTO project has been funded by Fundació Clínic per a la Recerca Biomèdica through the Pons Bartan 2020 grant (PI046549).
Conflicts of interestDr. Anmella has received CME-related honoraria, or consulting fees from Janssen-Cilag, Lundbeck and Angelini with no financial or other relationship relevant to the subject of this article. Dr. Murru has received CME-related honoraria or consulting feed from Janssen-Cilag, with no financial or other relationship relevant to the subject of this article. Dr. Vieta has received research support from or served as consultant, adviser or speaker for AB-Biotics, Abbott, Allergan, Angelini, Dainippon Sumitomo Pharma, Ferrer, Gedeon Richter, Janssen, Lundbeck, Otsuka, Sage pharmaceuticals, Sanofi-Aventis, Shire, Sunovion, Takeda, and reports no financial or other relationship relevant to the subject of this article. Dr. Hidalgo-Mazzei received CME-related honoraria from Abbott, Angelini and Janssen-Cilag with no financial or other relationship relevant to the subject of this article. Dr. Zahn has received honoraria for talks at medical symposia sponsored by Lundbeck as well as Janssen. He has collaborated with EMIS PLC for this study and advises Depsee Ltd. He is affiliated with the D’Or Institute of Research and Education, Rio de Janeiro and advises the Scients Institute, Palo Alto. Prof. Young received honoraria for lectures and advisory boards for all major pharmaceutical companies with drugs used in affective and related disorders with no financial or other relationship relevant to the subject of this article.
Dr. Anmella's research is supported by a Pons Bartran 2020 grant (PI046549). Dr. Murru thanks the support of the Spanish Ministry of Science and Innovation (PI19/00672). Prof. Vieta and Radua thank the support of the Spanish Ministry of Science and Innovation (PI15/00283, PI18/00805, PI19/00394, CPII19/00009) integrated into the Plan Nacional de I+D+I and co-financed by the Instituto de Salud Carlos III (ISCIII)-Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional (FEDER); the ISCIII; the CIBER of Mental Health (CIBERSAM); the Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement (2017 SGR 1365), the CERCA Programme, and the Departament de Salut de la Generalitat de Catalunya for the PERIS grant SLT006/17/00357. Dr. Hidalgo-Mazzei's research is supported by a Juan Rodés JR18/00021 granted by the Instituto de Salud Carlos III (ISCIII). Prof. Young and Dr. Zahn's independent research is funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.