The neural mechanisms underlying neurodegenerative disorders in the elderly remain elusive, despite extensive neuroimaging research in recent decades. Amnestic type mild cognitive impairment (aMCI) and late-life major depressive disorder (MDD) are two such conditions characterized by intersecting cognitive and affective symptomatology, and they are at a higher risk for Alzheimer's disease.
Materials and methodsThis study analyzed the neural underpinnings of cognitive and depressive symptoms in a cohort comprising 12 aMCI subjects, 24 late-life MDD patients, and 26 healthy controls (HCs). Participants underwent a detailed neuropsychological assessment and completed a visual attentional oddball task during functional magnetic resonance imaging (fMRI), with evaluations at baseline and at 2-year follow-up.
ResultsInitial findings showed that aMCI subjects had reduced dACC activation during oddball (target) stimulus detection, a pattern that persisted in longitudinal analyses and correlated with cognitive functioning measures. For HCs, subsequent dACC activation was linked to depression scores. Furthermore, in the affective-cognitive altered groups, later dACC activation correlated with oddball and memory performance.
ConclusionsThese findings enhance our comprehension of the neurobiological basis of cognitive and depressive disturbances in aging, indicating that dACC activation in response to a visual attentional oddball task could serve as a neural marker for assessing cognitive impairment and depression in conditions predisposing to Alzheimer's disease.
In an increasingly aging population, growing attention is being paid to the mild end of the cognitive spectrum spanning normal aging to Alzheimer's disease (AD). The term, mild cognitive impairment (MCI), has been used to describe this intermediate state in severity between normal or non-pathological aging and dementia.1 Due to their primary cognitive deficits in memory, individuals with amnestic type MCI (aMCI) could be considered as an early manifestation of AD.2 However, it is well known that neurocognitive disorders have manifestations other than purely cognitive deficits, such as behavioral and emotional manifestations resembling depression,3 illustrating the challenges in evaluating and treating patients with these conditions.
The longitudinal comorbidity between depression and cognitive impairment has been well established.4,5 However, the neural correlates underlying the association between depressive symptoms and cognitive dysfunction remain poorly understood. In terms of their association with AD, individuals diagnosed with aMCI exhibit a higher likelihood of progressing to AD compared to those in a non-aMCI group,6 with an annual AD conversion rate ranging between 10 and 15%.7 Additionally, major depressive disorder (MDD) doubles the risk of transitioning to AD.8 Nevertheless, it is uncertain whether depression solely acts as a risk factor for cognitive decline, serves as a psychological reaction to cognitive impairment, or can be considered a prodromal symptom of dementia.9
In recent years, neuroimaging studies have investigated MDD10,11 and MCI12,13 association with AD separately and mainly focused on macrostructural abnormalities. To address this, a recent meta-analysis by Zacková5 of 48 voxel-based morphometry (VBM) studies of individuals with MDD, MCI, and age-matched controls showed that MDD and MCI patients shared volumetric reductions in the insula, superior temporal gyrus, amygdala, hippocampus, and thalamus. Although valuable, these studies do not inform about the functional correlates of MDD and MCI leaving unanswered whether and how anatomical deficits are related to the functional alterations.
To the best of our knowledge, only a limited number of studies have investigated the neural correlates of the co-occurrence of MCI and MDD at the brain's activation level.14–18 However, these investigations have frequently involved heterogeneous samples of MDD, encompassing patients with early onset, acute, and treatment-resistant and remitted episodes, as well as varied MCI samples, incorporating subjects with both amnestic and non-amnestic presentations. Consequently, the literature remains inconclusive.
In this study, we aimed to investigate brain alterations during a visual oddball task in subjects with aMCI and in patients with late-life MDD, who were compared with healthy controls (HCs). Based on the executive attention framework,19 this approach is expected to provide insights into the cognitive and attentional processes associated with these conditions. We also aimed to evaluate longitudinally these neuroimaging findings over 2 years, as well as their association with clinical and neuropsychological data. Notably, this is the first longitudinal assessment conducted in these affective-cognitive altered groups (ACAGs, i.e., aMCI and MDD), enabling the monitoring of disorder progression over time and the potential identification of time-framed changes in underlying neural activity associated with cognitive decline in aging. We hypothesized that subjects with aMCI and patients with late-life MDD would show impairment in the oddball task and this would likely relate to measures of integrity in different neuropsychological domains and disorder severity. We also anticipated that such alterations would discriminate between the ACAGs and worsen over time.
MethodsParticipantsThe study included three groups of participants: 26 HCs, 12 subjects with aMCI, and 24 patients with a primary diagnosis of late-life MDD. Participants from the ACAGs were consecutively recruited from the Neurology and Psychiatry Departments of Bellvitge University Hospital (Barcelona, Spain), and HCs were recruited via advertisements from the same University Hospital catchment area. All participants underwent the Spanish version of the Mini-International Neuropsychiatric Interview (MINI) to assess the presence of a psychiatric disorder. Late-life MDD diagnoses were established by two experienced psychiatrists (MU and VS) according to DSM-IV-TR criteria, and aMCI diagnoses were established by two experienced neurologists (JG and RR) according to Petersen criteria,1 based on a syndromic categorical cognitive staging approach rather than on the use of biomarkers.20
The study participants were subject to several exclusion criteria, including the following: (1) individuals aged below 60 or above 76 years, (2) individuals with a past or current diagnosis of other major psychiatric disorders, including substance abuse or dependence (excluding nicotine), (3) individuals with an intellectual disability or neurodevelopmental disorders, (4) individuals with neurological disorders, (5) individuals with a Hachinski Ischemic Score greater than 5, to exclude those with a high likelihood of cognitive deficits derived from vascular causes, (6) individuals exhibiting dementia based on DSM-IV-TR criteria and/or a Clinical Dementia Rating (CDR) score greater than 1, (7) individuals with severe medical conditions, (8) individuals who had undergone electroconvulsive therapy within the past year, (9) individuals with conditions that hindered neuropsychological assessment or magnetic resonance imaging (MRI) examinations, and (10) individuals with anatomical abnormalities detected in the MRI scan.
Written informed consent was obtained from all participants before taking part in the study. The study procedures were carried out following the Declaration of Helsinki and received the approval of the Clinical Research Ethics Committee of Bellvitge University Hospital (reference PR156/15, February 17th, 2016).
Clinical and neuropsychological assessmentAll participants underwent a comprehensive neuropsychological battery by an experienced neuropsychologist, at both baseline and the 2-year follow-up, including the Spanish version of the Mini-Mental State Examination (MMSE), and several tests to evaluate the following domains: attention, executive functions, memory, language and psychomotor speed (see Table S1 for the detailed list of the neuropsychological battery used). Furthermore, to assess depression severity but not for diagnostic purposes, all participants completed the validated Spanish versions of the Geriatric Depression Scale (GDS) and the Hamilton Depression Rating Scale 17-item version (HDRS17).
Imaging data acquisitionBoth baseline and 2-year follow-up data were acquired on the same 3.0T clinical MRI scanner (Ingenia, Philips Healthcare, Best, The Netherlands), equipped with a 32-channel head coil at the Imaging Diagnostic Institute (IDI) in Barcelona, Spain. All participants performed a visual oddball task inside the scanner (see below), during which we acquired an echo planar imaging (EPI) sequence sensitive to fluctuations in the Blood Oxygenation Dependent Level (BOLD) contrast with the following parameters: 435 volumes (excluding the four initial dummy volumes), 40 axial interleaved slices perpendicular to the floor of the fourth ventricle, over a matrix of 80mm×80mm, repetition time (TR)=2000ms, echo time (TE)=25ms, flip angle (FA)=90°, 3mm isometric voxel size, field of view (FOV)=24cm. This acquisition lasted for 14min 42s. The scanning also included a whole-brain T1 weighted three-dimensional inversion-recovery prepared spoiled gradient echo sequence (TR=10.46ms, TE=4.79ms, FA=8°, 0.75mm isometric voxel size, FOV=24cm, 233 axial slices over a matrix of 320mm×318mm) that lasted for 5min 04s.
fMRI visual oddball taskWe used a well-validated two-stimulus oddball paradigm adapted from Murphy.21 A full description of the task can be found elsewhere.22 It consisted of the presentation of a big purple circle (standard stimuli) and a small purple circle (target/oddball stimuli). The participant's task was to indicate the oddball stimuli by speedily pressing a button with their right index finger in an MRI-compatible fiber optic response box (Lumina 3G Controller, Cedrus Corporation). All stimuli were presented for 100ms in the center of a dark gray background matched for luminance. Oddball stimuli (target) represented 20% of the trials and were pseudorandomly distributed throughout the task.
fMRI preprocessing and analysisFor modeling and despiking motion artifacts, functional series sequences were revised using the Brain-Wavelet toolbox v2.0.23 Afterward, functional and anatomical images were preprocessed using CONN functional connectivity toolbox release 21.a. A thorough description of the preprocessing pipeline including realignment with correction of susceptibility distortion interactions, slice timing correction, outlier detection, direct segmentation, MNI-space normalization, smoothing, and denoising can be found in the Supplementary Material.
First-level (within-subject) maps for oddball stimuli vs. standard stimuli were estimated using SPM12 software, in baseline and follow-up acquisitions separately. Using a summary statistics approach, the resulting contrast images were used in subsequent group-level random effects analyses. Between-group comparisons (ACAGs vs. HCs or aMCI vs. MDD vs. HCs) and longitudinal differences were examined in whole-brain voxel-wise activations. Significance thresholding from all derived differences in activation was set to satisfy a family-wise error (FWE) rate correction of pFWE<0.05.
To explore the association between significant brain-derived between-group differences from the visual oddball task and clinical and neuropsychological measures, peak activation eigenvalues from regions displaying significant differences were extracted.
Statistical analyses of non-imaging dataStatistical analyses of non-imaging data were conducted with IBM SPSS Statistics v. 23. Differences were considered statistically significant if p<0.05. One-way ANOVA was employed for the global comparison of study variables between the groups (i.e., demographic, clinical, and neuropsychological data), and repeated measures ANOVA to explore group×time interactions. All analyses were controlled for age and sex.
A principal component analysis (PCA) was conducted on the 20 clinical and neuropsychological variables, with orthogonal rotation (varimax). The Kaiser–Meyer–Olkin (KMO) measure verified the sampling adequacy for the analysis: KMO=0.85, and all KMO values for individual tests were greater than 0.50. An initial analysis was run to obtain eigenvalues for each component in the data. Component loadings of an absolute value above 0.30 were considered. Furthermore, linear associations between brain-derived eigenvalues and PCA results were assessed using Pearson's parametric correlations (r).
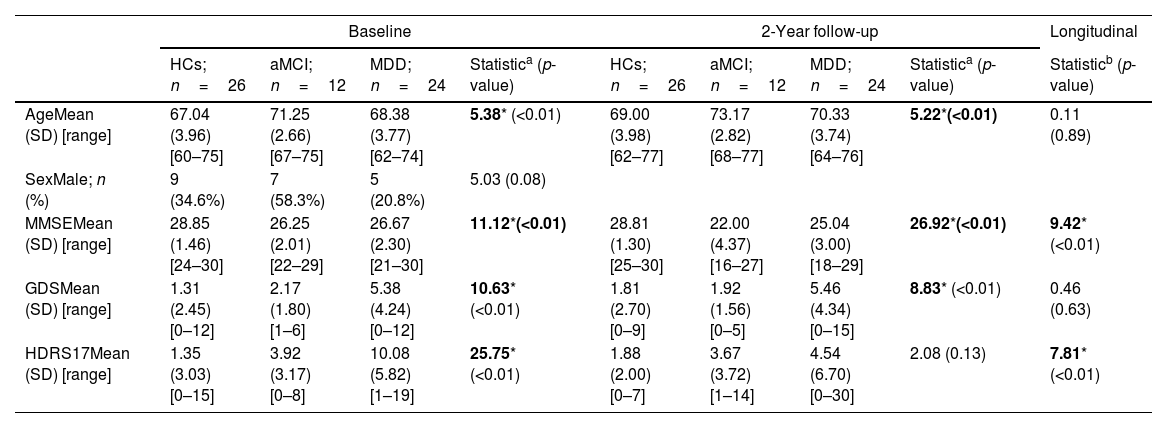
ResultsDemographic, clinical, and neuropsychological characterizationTable 1 summarizes demographic and clinical measures in the three groups and in both evaluation times (baseline and 2-year follow-up). HCs endorsed higher scores on the MMSE, in both evaluations, compared to the ACAGs. Table S2 summarizes demographic and clinical measures in HCs and ACAGs. The three groups showed a decrease in the MMSE scores over time, showing a significant group-by-time interaction in the repeated measures ANOVA (F=9.42; p=<0.01). As would be expected, patients with late-life MDD endorsed higher scores of depression severity on the GDS and the HDRS17 in both evaluations compared to HCs and subjects with aMCI, but only HDRS17 scores showed a significant group-by-time interaction (F=7.81; p=<0.01). Likewise, there were significant differences in age between groups, which were controlled for in subsequent analyses, as mentioned above.
Sample description.
| Baseline | 2-Year follow-up | Longitudinal | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HCs; n=26 | aMCI; n=12 | MDD; n=24 | Statistica (p-value) | HCs; n=26 | aMCI; n=12 | MDD; n=24 | Statistica (p-value) | Statisticb (p-value) | |
| AgeMean (SD) [range] | 67.04 (3.96) [60–75] | 71.25 (2.66) [67–75] | 68.38 (3.77) [62–74] | 5.38* (<0.01) | 69.00 (3.98) [62–77] | 73.17 (2.82) [68–77] | 70.33 (3.74) [64–76] | 5.22*(<0.01) | 0.11 (0.89) |
| SexMale; n (%) | 9 (34.6%) | 7 (58.3%) | 5 (20.8%) | 5.03 (0.08) | |||||
| MMSEMean (SD) [range] | 28.85 (1.46) [24–30] | 26.25 (2.01) [22–29] | 26.67 (2.30) [21–30] | 11.12*(<0.01) | 28.81 (1.30) [25–30] | 22.00 (4.37) [16–27] | 25.04 (3.00) [18–29] | 26.92*(<0.01) | 9.42* (<0.01) |
| GDSMean (SD) [range] | 1.31 (2.45) [0–12] | 2.17 (1.80) [1–6] | 5.38 (4.24) [0–12] | 10.63* (<0.01) | 1.81 (2.70) [0–9] | 1.92 (1.56) [0–5] | 5.46 (4.34) [0–15] | 8.83* (<0.01) | 0.46 (0.63) |
| HDRS17Mean (SD) [range] | 1.35 (3.03) [0–15] | 3.92 (3.17) [0–8] | 10.08 (5.82) [1–19] | 25.75* (<0.01) | 1.88 (2.00) [0–7] | 3.67 (3.72) [1–14] | 4.54 (6.70) [0–30] | 2.08 (0.13) | 7.81* (<0.01) |
Abbreviations: aMCI, amnestic type mild cognitive impairment subjects; GDS, Geriatric Depression Scale; HCs, healthy controls; HDRS17, Hamilton Depression Rating Scale; MDD, late-life major depressive disorder patients; MMSE, Mini-Mental State Examination.
Online Supplementary Table S3 presents the detailed across-group comparisons in neuropsychological testing and oddball performance, where both aMCI and MDD performed worse than HCs. Five components had eigenvalues over Kaiser's criterion of 1 and explained 76.9% of the variance. Table S4 shows the component loadings after rotation. The tests that cluster on the same factor suggest that factor 1 represents language and inhibitory control, factor 2 represents verbal memory, factor 3 represents working memory, factor 4 represents verbal fluency, and factor 5 represents mental control.
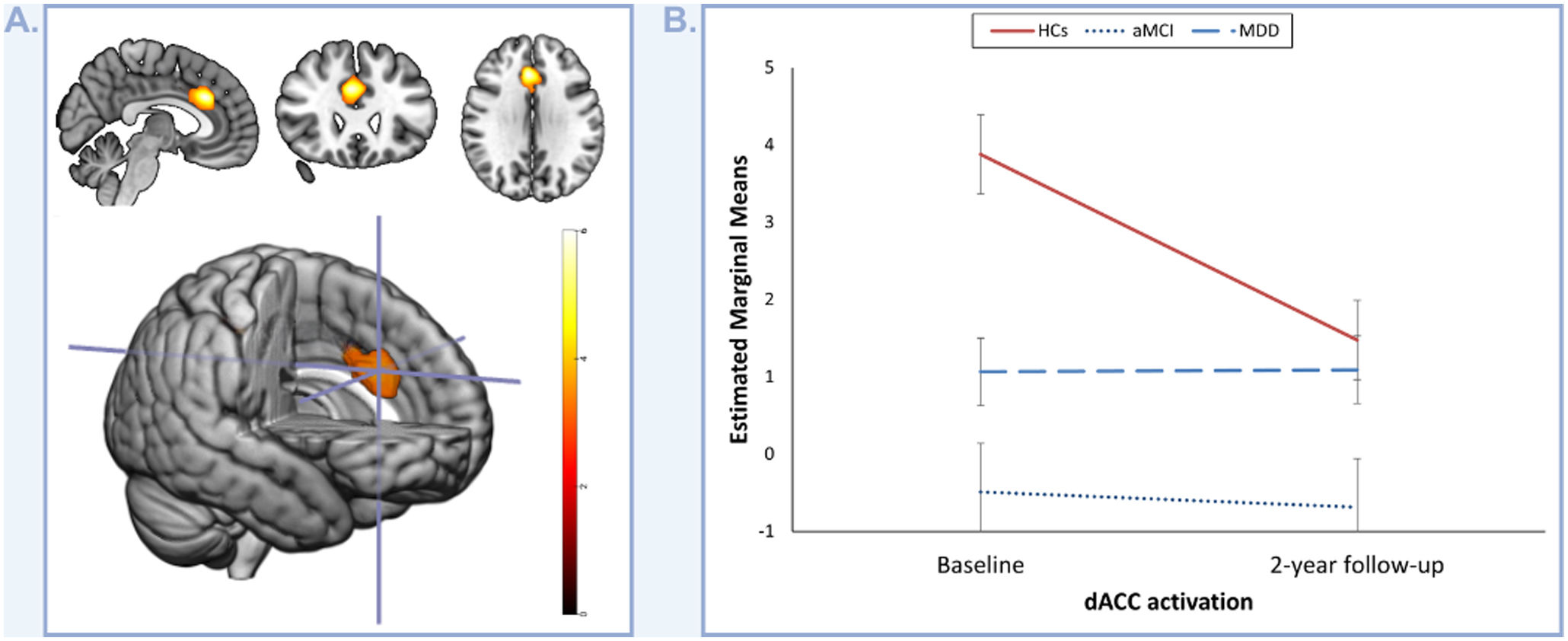
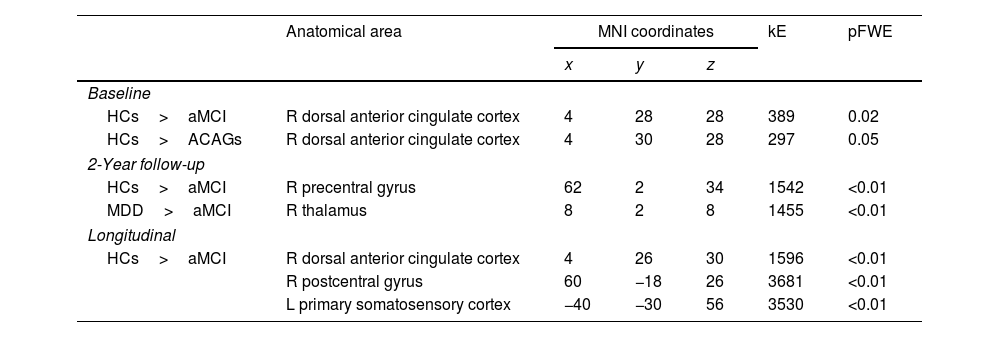
fMRI task-related activationsGroup comparisons at baseline found significantly decreased activation in the dorsal anterior cingulate cortex (dACC) in subjects with aMCI in comparison to HCs (pFWE=0.02; Table 2) during oddball stimuli detection. This decreased activation in the dACC was also observed when comparing the ACAGs (combined aMCI and MDD groups) to the HCs (pFWE=0.05; Table 2) but not in the MDD group individually. Remarkably, even though this finding was not observed in the 2-year follow-up analyses (see Table 2), the longitudinal analysis showed a significantly decreased activation in the dACC in subjects with aMCI in comparison to HCs (pFWE=<0.01; Table 2; Fig. 1A).
Group comparisons of fMRI oddball task activations for the oddball > standard contrast.
| Anatomical area | MNI coordinates | kE | pFWE | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Baseline | ||||||
| HCs>aMCI | R dorsal anterior cingulate cortex | 4 | 28 | 28 | 389 | 0.02 |
| HCs>ACAGs | R dorsal anterior cingulate cortex | 4 | 30 | 28 | 297 | 0.05 |
| 2-Year follow-up | ||||||
| HCs>aMCI | R precentral gyrus | 62 | 2 | 34 | 1542 | <0.01 |
| MDD>aMCI | R thalamus | 8 | 2 | 8 | 1455 | <0.01 |
| Longitudinal | ||||||
| HCs>aMCI | R dorsal anterior cingulate cortex | 4 | 26 | 30 | 1596 | <0.01 |
| R postcentral gyrus | 60 | −18 | 26 | 3681 | <0.01 | |
| L primary somatosensory cortex | −40 | −30 | 56 | 3530 | <0.01 | |
Abbreviations: ACAGs, affective-cognitive altered groups; aMCI, amnestic type mild cognitive impairment; FWE, family-wise error; HCs, healthy controls; kE, cluster extent; MDD, late-life major depressive disorder; L, left; MNI, Montreal Neurological Institute; R, right.
Longitudinal group differences in the dorsal anterior cingulate cortex (dACC) activations. (A) Depicts the identified longitudinal decrease in dACC activation in subjects with amnestic type mild cognitive impairment (aMCI) compared to healthy controls (HCs) during the oddball stimuli>standard stimuli contrast. The color bar represents t-values. (B) Depicts the evolution of dACC activation in healthy controls (HCs), subjects with amnestic type mild cognitive impairment (aMCI), and patients with late-life major depressive disorder (MDD) over the two-year follow-up period.
Interestingly, dACC activation in HCs decreased between baseline and 2-year follow-up (F=9.72; p=<0.01; Fig. 1B), but seems to remain stable throughout the 2-year period in the ACAGs.
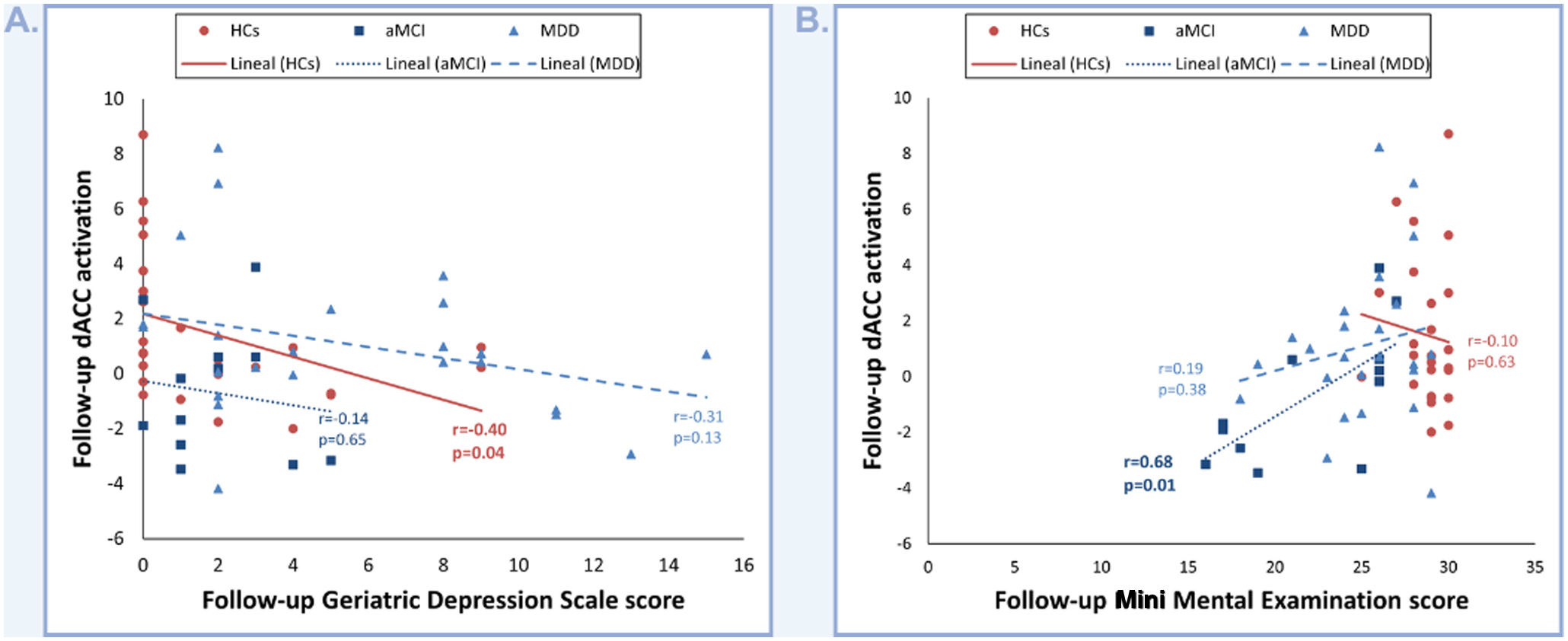
Correlations between dACC activation and clinical, neuropsychological and oddball performance dataCorrelation analysis revealed that decreases in follow-up dACC activity in HCs significantly correlate with increases in follow-up GDS scores (r=−0.40; p=0.04; Fig. 2A). Conversely, follow-up dACC activity in aMCI positively correlates with follow-up MMSE scores (r=0.68; p=0.01; Fig. 2B).
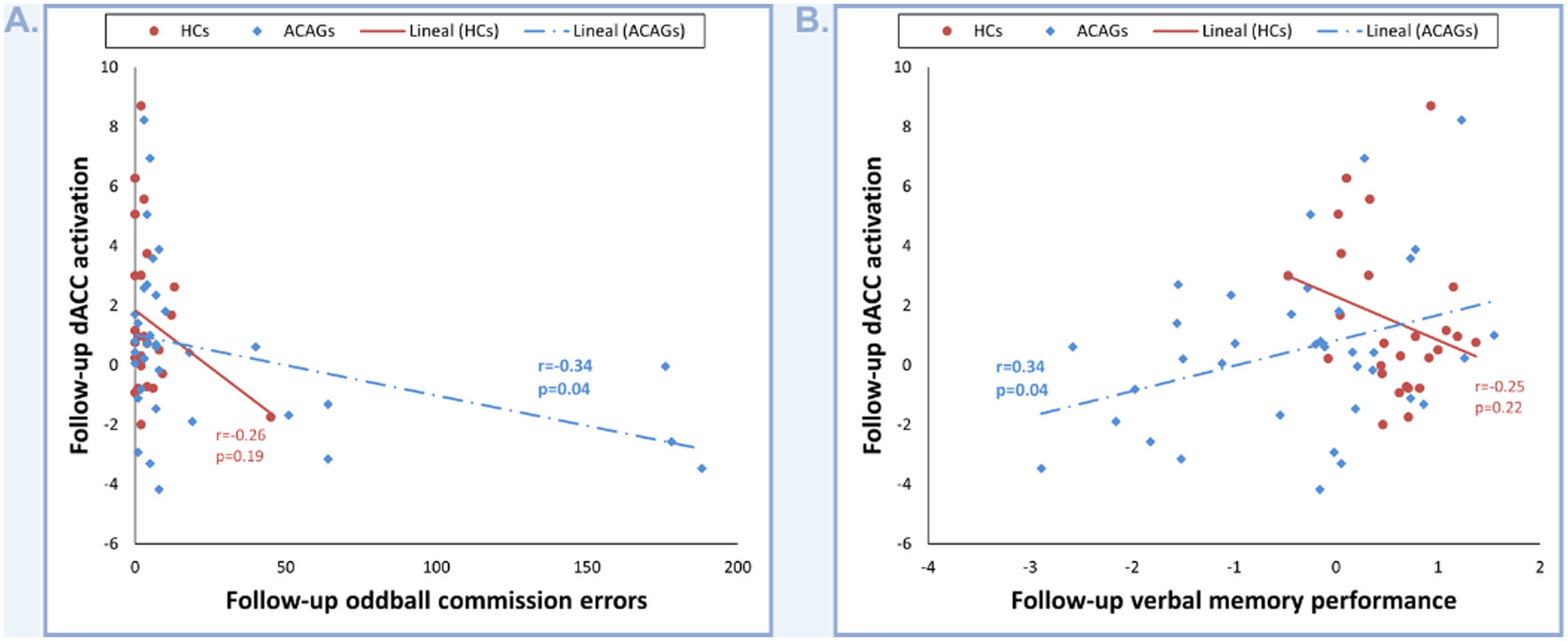
Regarding neuropsychological and oddball scores, when compared individually (HCs, subjects with aMCI, and patients with MDD), we didn’t find any significant correlation between dACC activation and these data. However, when compared as a single group, the combined aMCI and MDD groups (ACAGs) showed a significant positive correlation between dACC activation at follow-up and oddball commission errors (r=−0.34; p=0.04; Fig. 3A), as well as with follow-up verbal memory performance, represented by factor 2 (r=0.34; p=0.04; Fig. 3B).
DiscussionOur study compared task-related brain activations between HCs and two groups of subjects with brain conditions conferring an increased risk for AD. Notably, at baseline, individuals with aMCI displayed a reduction in dACC activation compared to HCs, a discrepancy persisting over a 2-year follow-up. Furthermore, this diminished dACC activation characterized the group of affective-cognitive altered individuals as a whole, when compared to HCs. Significantly, this diminishment correlated with clinical severity, offering pivotal insights into the neural substrates of aMCI and late-life MDD. These findings posit dACC activation during a visual oddball task as a promising biomarker for gauging cognitive impairment and depression symptoms within these patient cohorts.
As a pivotal node within the Salience Network (SN), the dACC assumes a critical role in discerning the most biologically and cognitively pertinent stimuli to dynamically guide attention and behavior.24 Our investigation unveils heightened dACC activation in HCs compared to ACAGs during the visual oddball task, implying a more robust engagement of the SN in HCs. This heightened activation suggests a superior and more effective utilization of attention and working memory resources in HCs to identify relevant yet infrequent stimuli, consistent with prior research findings.25,26 The observed structural degradation in AD patients has fostered the ‘disconnection hypothesis’ of AD pathology, postulating disruptions in crucial brain networks.27 Moreover, late-life MDD and aMCI have been conceptualized as disconnection syndromes, characterized by impaired brain network integrity vital for multifaceted cognitive and behavioral functions.16,28 Conversely, the decrement in dACC activation noted in HCs over the two-year period, juxtaposed with the relatively stable pattern in ACAGs, hints at an accelerated aging trajectory within the dACC network among our ACAGs, suggesting a distinct aging effect on dACC activation. This phenomenon necessitates further scrutiny to unravel its underlying mechanisms. In this regard, it is recommended to focus on earlier stages within the Alzheimer's disease continuum, such as Subjective Cognitive Decline, which could provide insights into early functional changes before the onset of cognitive and depressive symptoms.29,30
The dACC is pivotal in navigating cognitive, homeostatic, motivational, and affective systems,31,32 and its dysfunction has been thoroughly established as a contributing factor in various psychiatric conditions, including MDD.33 Our research further elucidates this relationship, uncovering a significant inverse correlation between dACC activity at follow-up and the depression scores in HCs. This finding indicates a possible influence of the dACC on mood regulation. From a broader network perspective, the regulation of emotions in HCs appears to be supported by increased activity in the dACC, along with stronger functional connectivity between the dACC and key regions such as the dorsolateral prefrontal cortex and the amygdala.34 Importantly, this enhanced network activity is also observed in older adults who exhibit more pronounced dACC-centered network engagement during tasks involving emotional facial recognition and the reappraisal of emotional content.32 This suggests a greater reliance on this neural network for emotion regulation with advancing age.32 Additionally, a reduction in functional connectivity between the dACC and specific areas of the brain, namely the right occipital lobe and the right lingual gyrus, has been identified as significant in AD patients suffering from depression.31 While our study does not encompass AD patients, it is remarkable to note that the neural regions compromised in MDD show considerable similarity to those affected in the early stages of AD by amyloid-beta (Aβ) and tau pathologies. Moreover, depressive symptoms in individuals who do not meet the full criteria for MDD might originate from neurofibrillary damage to monoaminergic neurons in areas like the dorsal raphe and locus coeruleus (LC), which are among the first to be impacted in AD.35 Reflecting on our previous findings, we observed that alterations in connectivity between the LC and the ACC were inversely related to the severity of depression in MDD patients.22 These observations regarding mood alterations in later life could have significant clinical implications, potentially serving as indicators of impending cognitive decline and emphasizing the need for early intervention.
Individuals with aMCI also displayed a positive association between dACC activation during follow-up and their MMSE scores. This suggests a compensatory mechanism at work, where increased dACC recruitment may help sustain or reach a level of cognitive functioning that is comparatively normal. This aligns with various research findings that have reported augmented activation in the ACC among subjects with aMCI, interpreting such hyperactivity as an attempt to offset diminished supervisory capabilities of the SN over key neural networks like the default-mode and central executive networks.36 Further adding to our understanding of the dACC's role, we found significant correlations between its activation in follow-up assessments and oddball and verbal memory performance in the ACAGs. This underscores the dACC's contribution to attention and memory processes within these conditions,37 and aligns with previous neuroimaging evidence of ACC activation anomalies in patients with MDD both at rest and during cognitive tasks.38,39 Our verbal memory evaluation integrates various aspects of learning and recall abilities, as gauged by the Wechsler Memory Scale – Third Edition (WMS-III) and the recognition tasks from the Neuropsychological Battery of the Cognitive Assessment Exercise (NBACE). The WMS-II is particularly focused on the ability to memorize a list of words through repetition and to retrieve this information over longer periods, thereby providing insight into episodic memory capabilities—a cognitive domain notably impaired in both aMCI and MDD.40,41 Intriguingly, research by Lin et al.42 has indicated that older adults with superior cognitive abilities exhibit higher intrinsic connectivity in the ACC compared to their age-matched counterparts. This finding suggests that the ACC's enhanced connectivity may play a role in cognitive reserve mechanisms, potentially contributing to the maintenance of cognitive function despite the presence of neuropathology.
Regarding the study's limitations, it is important to note that while all participants underwent thorough clinical and neuropsychological assessments, the inherent nature of our naturalistic and sequential recruitment approach resulted in the age-related aMCI group being both older and smaller in size compared to the other groups. This discrepancy may have compromised our ability to identify significant variances. Additionally, even though the disparity in sex composition of our groups was not statistically significant, this imbalance, combined with a relatively small sample size, may have led to the overlooking of significant sex-related differences. Although we adjusted for sex and age in our analyses, these adjustments may not fully mitigate their impact. Future research with larger and more demographically balanced samples is needed to validate our findings and ensure their generalizability. Furthermore, our research did not uncover a direct linkage between dACC activation and performance on the oddball task. This finding implies that the dACC's involvement in cognitive operations might be more generalized rather than being strictly tied to specific tasks. Therefore, it is essential to approach these results with a degree of caution. Specifically, these outcomes should not be interpreted as concrete evidence of a direct association between dACC alterations and the execution of the oddball stimuli detection. Rather, they ought to be viewed in the context of a broader understanding of cognitive processes and attentional dysfunctions.
ConclusionsIn summary, our research offers valuable contributions to the understanding of common neurobiological foundations that aMCI and late-life MDD may share. The observed reduction in dACC activation during target stimuli detection during the visual oddball task, across both ACAGs relative to HCs, hints at the possibility of a shared neural biomarker characterizing these conditions. This insight advances our comprehension of the neurobiological intricacies associated with these disorders in later life, potentially guiding future diagnostic and therapeutic strategies.
Role of the funding sourceThis study was supported by the Agency for Management of University and Research Grants of the Catalan Government (2021SGR01017), the Department of Health of the Generalitat de Catalunya (PERIS grant SLT002/16/249), the Carlos III Health Institute, Spain (Grant PIE14/00034, PI19/01040 and INT21/00055), FEDERER Funds/European Regional Development Fund (ERDF) (A way to build Europe) and CIBERSAM. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interestIdC served as a consultant for Worldwide Clinical Trials. The other authors report no biomedical financial interests or potential conflicts of interest regarding this work.
The authors are grateful to all the study participants and their families, and to the staff and technicians of Bellvitge University Hospital and Duran I Reynalds Hospital who helped to recruit the sample for this study. We thank the CERCA Programme/Generalitat de Catalunya for institutional support. The Institute of Neurosciences of the University of Barcelona is a María de Maeztu Unit of Excellence CEX2021-001159-M of the Ministry of Science and Innovation of Spain.