We compared effective connectivity from the locus coeruleus (LC) during the resting-state in patients with late-life Major Depressive Disorder (MDD), individuals with amnestic Mild Cognitive Impairment (aMCI), and Healthy Controls (HCs).
Participants23 patients with late-life MDD, 22 patients with aMCI, and 28 HCs.
Material and methodsParticipants were assessed in two time-points, 2 years apart. They underwent a resting-state functional magnetic resonance imaging and a high-resolution anatomical acquisition, as well as clinical assessments. Functional imaging data were analyzed with dynamic causal modeling, and parametric empirical Bayes model was used to map effective connectivity between 7 distinct nodes: 4 from the locus coeruleus and 3 regions displaying gray matter decreases during the two-year follow-up period.
ResultsLongitudinal analysis of structural data identified three clusters of larger over-time gray matter volume reduction in patients (MDD+aMCI vs. HCs): the right precuneus, and the visual association and parahippocampal cortices. aMCI patients showed decreased effective connectivity from the left rostral to caudal portions of the LC, while connectivity from the left rostral LC to the parahippocampal cortex increased. In MDD, there was a decline in effective connectivity across LC caudal seeds, and increased connectivity from the left rostral to the left caudal LC seed over time. Connectivity alterations with cortical regions involved cross-hemisphere increases and same-hemisphere decreases.
ConclusionsOur discoveries provide insight into the dynamic changes in effective connectivity in individuals with late-life MDD and aMCI, also shedding light on the mechanisms potentially contributing to the onset of neurodegenerative disorders.
The locus coeruleus (LC), an elongated structure situated within the dorsolateral pontine tegmentum, serves as the primary source of norepinephrine (NE) within the brain. Notably, the LC has garnered considerable attention in research owing to its pivotal role in the preclinical phases of Alzheimer's disease (AD).1 This significance arises from the identification of abnormal intraneuronal tau, a hallmark of AD pathology, in the LC even preceding observable neuronal loss, and potentially initiating as early as young adulthood.
Recent investigations have unveiled a distinct pattern of neuronal loss in AD, particularly affecting the rostral and medial–dorsal projecting neurons within the LC. This loss profoundly impacts disease severity by reducing NE availability in critical areas like the forebrain and hippocampus, known for their involvement in attention, memory, and arousal.2
Given its close association with the aging process, AD predominantly affects individuals over 65 years, with its prevalence doubling approximately every five years, culminating in a time-dependent exponential rise.3 Mild cognitive impairment (MCI), a common condition among older adults, entails compromised cognitive function and poses a substantial risk for progression to dementia, especially the amnestic subtype (aMCI) characterized by memory dysfunction. Insightfully, the LC emerges as a key player in this scenario as neurofibrillary tangles, and their precursors, manifest during normal aging and escalate in visibility during the transition from MCI to early AD.4
The intricate interplay between mild cognitive impairment and late-life Major Depressive Disorder (MDD) remains an enigma, with both conditions frequently co-occurring in the older persons.5 Notably, depression has been implicated as a risk factor for the MCI-to-dementia trajectory.6 Furthermore, compelling evidence substantiates the linkage between late-life depression and heightened susceptibility for major neurocognitive disorders (MND), particularly AD.7 Remarkably, patients with MDD demonstrate LC neuronal loss, exacerbating the deficiency of NE input to the cerebral cortex. This degenerative process, albeit gradual, triggers compensatory mechanisms including axonal sprouting in LC noradrenergic projections to the hippocampus and the prefrontal cortex (PFC).8,9 Notably, neuroimaging techniques present a promising avenue for non-invasive evaluation of the LC and its connectivity as a biomarker of noradrenergic dysfunction.
In this study, we explored the 2-year evolution of resting-state effective connectivity (EC) from the LC, using spectral Dynamic causal modeling, among patients with major late-life depressive disorder or amnestic mild cognitive impairment, in comparison with healthy controls. Our investigation encompasses seven distinct regions, including four LC nodes stratified into caudal and rostral divisions, connected with three brain regions that in our sample exhibited differential volumetric changes across time points. We hypothesize that functional compensation mechanisms can occur alongside EC deficits associated with the early stages of Alzheimer's disease (AD).
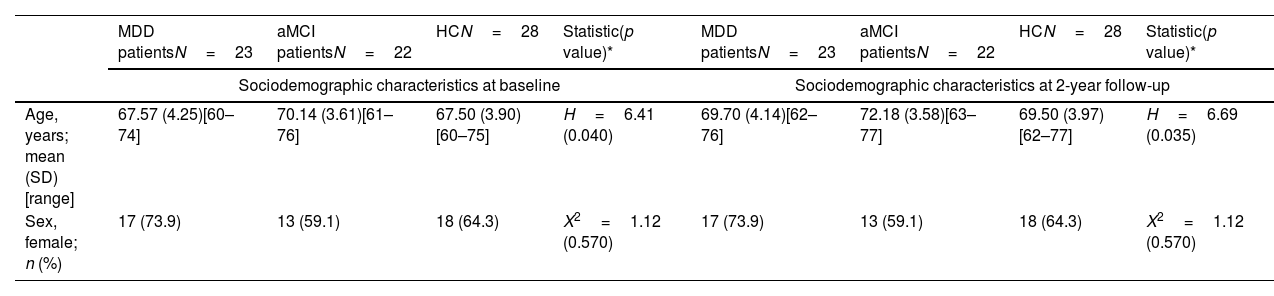
MethodsParticipantsThe study sample comprised 73 individuals. These participants were categorized into three distinct groups: 23 patients diagnosed with late-life Major Depressive Disorder (MDD), 22 individuals with amnestic Mild Cognitive Impairment (aMCI), and 28 Healthy Controls (HCs) (Table 1). MDD subjects were recruited consecutively from the Psychiatry Department of Bellvitge University Hospital in Barcelona, Spain. Inclusion criteria specified that major depression must be the primary diagnosis, and the onset of the first depressive episode should occur after 40 years of age, following the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR)10 criteria.
Sociodemographic and clinical characteristics of the study sample.
| MDD patientsN=23 | aMCI patientsN=22 | HCN=28 | Statistic(p value)* | MDD patientsN=23 | aMCI patientsN=22 | HCN=28 | Statistic(p value)* | |
|---|---|---|---|---|---|---|---|---|
| Sociodemographic characteristics at baseline | Sociodemographic characteristics at 2-year follow-up | |||||||
| Age, years; mean (SD) [range] | 67.57 (4.25)[60–74] | 70.14 (3.61)[61–76] | 67.50 (3.90)[60–75] | H=6.41 (0.040) | 69.70 (4.14)[62–76] | 72.18 (3.58)[63–77] | 69.50 (3.97)[62–77] | H=6.69 (0.035) |
| Sex, female; n (%) | 17 (73.9) | 13 (59.1) | 18 (64.3) | X2=1.12 (0.570) | 17 (73.9) | 13 (59.1) | 18 (64.3) | X2=1.12 (0.570) |
| Clinical characteristics at baseline | Clinical characteristics at 2-year follow-up | |||||||
|---|---|---|---|---|---|---|---|---|
| HDRS; mean (SD) [range] | 10.35 (5.83)[1–19] | 6.45 (5.34)[0–19] | 1.29 (2.93)[0–15] | H=35.18 (<0.001) | 3.96 (4.65)[0–16] | 4.64 (6.54)[0–30] | 1.86 (1.94)[0–7] | H=5.38 (0.068) |
| GDS; mean (SD) [range] | 5.57 (4.29)[0–12] | 2.95 (2.26)[0–9] | 1.32 (2.43)[0–12] | H=20.81 (<0.001) | 5.57 (4.28)[0–14] | 3.14 (3.41)[0–15] | 1.89 (2.57)[0–9] | H=12.89 (0.002) |
| MMSE; mean (SD) [range]** | 26.96 (2.18)[21–30] | 25.64 (2.50)[20–29] | 28.86 (1.41)[24–30] | H=27.27 (<0.001) | 25.39 (2.84)[19–29] | 22.09 (4.51)[13–27] | 28.79 (1.26)[25–30] | H=43.27 (<0.001) |
| Long term memory***; mean (SD) [range] | 6.39 (1.64)[4–10] | 2.05 (1.29)[0–4] | 8.39 (1.75)[6–12] | H=53.24 (<0.001) | 5.78 (2.70)[0–11] | 2.09 (2.45)[0–7] | 8.07 (1.76)[3–11] | H=38.19 (<0.001) |
aMCI; amnestic Mild Cognitive Impairment, GDS; Geriatric Depression Scale, HC; healthy controls, HDRS; Hamilton Depression Rating Scale, MDD; late-life Major Depression Disorder, MMSE; Mini-Mental State Examination, SD; standard deviation.
Statistic value corresponds to Kruskal–Wallis H test for continuous variables and Chi-square test for categorical variables.
Conversely, individuals with aMCI were consecutively recruited from the Department of Neurology of the same hospital. Diagnosis adhered to Petersen11 criteria, which included self-reported memory complaints corroborated by informants and objectively established long-term memory impairments (i.e., scores falling 1.5 standard deviations below age- and education-adjusted normative values in the delayed recall test from the Wechsler Memory Scale III12 (WMS-III)), along with Clinical Dementia Rating13 (CDR) scores of 0.5.
All participants underwent comprehensive evaluations, including the administration of the Mini-International Neuropsychiatric Interview (MINI) and the Mini-Mental State Examination (MMSE) as a cognitive screening measure. Depression severity in both groups was assessed using the Spanish brief version of the Geriatric Depression Scale (GDS) and the Hamilton Depression Rating Scale (HDRS), although not for diagnostic purposes. Importantly, aMCI participants had no history or present comorbidities with MDD, whereas seven out of the 23 MDD patients concurrently exhibited aMCI diagnoses.
Exclusion criteria comprised: (1) ages below 60 or above 76 years, (2) history or current diagnoses of other major psychiatric disorders, including substance abuse or dependence (excluding nicotine), (3) intellectual disabilities or neurodevelopmental disorders, (4) neurological disorders, (5) Hachinski Ischemic Score exceeding 5 to exclude those at high risk for vascular-derived cognitive deficits, (6) presence of major neurocognitive disorders (DSM-5 criteria), which corresponds to dementia as per DSM-IV-TR criteria and/or Clinical Dementia Rating (CDR) score>1, (7) significant medical conditions, (8) electroconvulsive therapy within the preceding year, (9) conditions impeding neuropsychological assessments or MRI procedures (e.g., blindness, deafness, claustrophobia, presence of pacemakers or cochlear implants), and (10) gross abnormalities in the MRI scan.
The study received approval from The Clinical Research Ethics Committee (CEIC) of Bellvitge University Hospital (reference PR156/15, February 17th, 2016), and adherence to the ethical standards outlined in the 1975 Declaration of Helsinki and its subsequent amendments (revised in 2000) was ensured. All participants provided written informed consent before participating in the study.
Neuroimaging data acquisition and preprocessingAll participants underwent identical imaging sessions at two time-points: baseline and a two-year follow-up. In each session, a functional magnetic resonance sequence during the resting-state and a whole-brain T1-weighted anatomical sequence were acquired. See the Supplementary material for details on image acquisition and preprocessing.
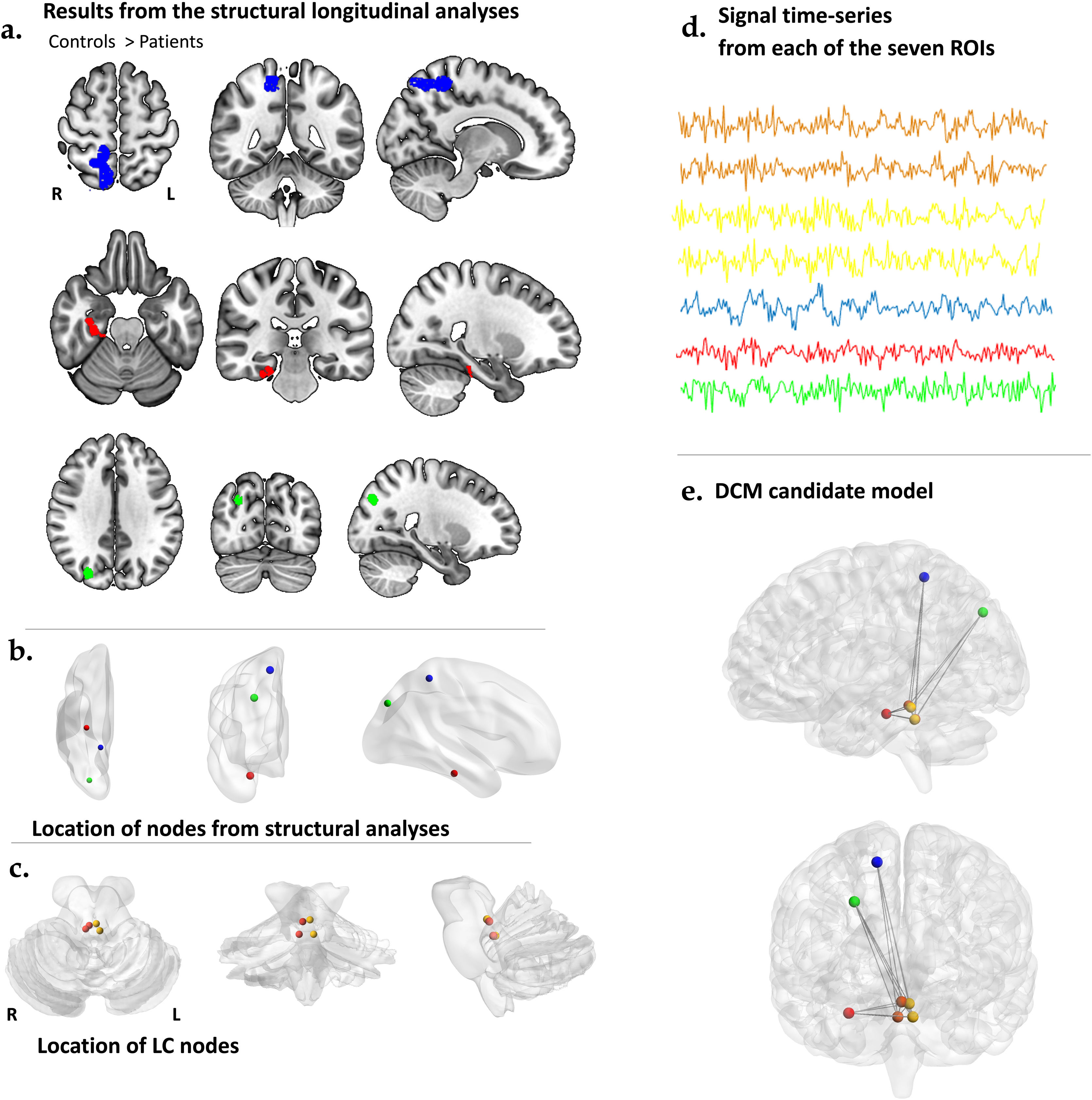
ROI selection and time series extractionOur functional analyses encompassed seven distinct nodes, as depicted in Fig. 1.
Effective connectivity analysis pipeline. (a) Regions that exhibited significant between-group differences in volume, where controls had greater values than patients, as revealed in the longitudinal structural data analyses. (b) These nodes, serving as the ROIs (region of interest) or seeds of interest, were selected from the peak coordinates of the structural analyses described in ‘a.’ These nodes include the posterior hippocampus (PHC), in red, the posterior cingulate cortex (PCUN), in light green, and the ventral anterior cingulate (VAC), in blue, all from the right hemisphere. (c) The LC ROIs were selected from two different divisions of each hemisphere nuclei: the right rostral and caudal divisions are depicted in orange, while the left rostral and caudal divisions are depicted in yellow. (d) Time series data extracted from each of the ROIs mentioned in ‘b.’ and ‘c.’ were utilized to perform spectral dynamic causal modeling. (e) In our model, we assumed intrinsic connectivity between the different divisions of the LC and the brain regions that exhibited differential volume decreases between patients and controls over time.
We first performed group comparisons of longitudinal volumetric changes between patients and HCs (i.e., MDD+aMCI vs. HCs) using an exploratory whole-brain and voxel-wise two-sample t-test comparison. See the Supplementary material for details on the preprocessing of structural data. Age and gender were included as confounding covariates in this analysis. Areas of increased and decreased GM volume were identified with a voxel-level peak detection threshold of p=0.001, uncorrected for multiple comparisons.
We then selected the three regions displaying longitudinal volume decrease according to such longitudinal analysis of structural data. These were the visual association cortex (VAC) (MNI: 27, −80, 36); the precuneus (PCUN) (MNI: 15, −45, 57); and the parahippocampal cortex (PHC) (MNI: 30, −23, −23); all within the right hemisphere (see results below).
Next, we selected four coordinates of the LC, as outlined in a published atlas,14 corresponding to the bilateral locations of the rostral and caudal divisions of the nucleus. Specifically, our regions were labeled as: the right rostral LC (RroLC) (MNI 2, −35, −17); the right caudal LC (RcaLC) (MNI 4, −39, −25); the left rostral LC (LroLC) (MNI −2, −36, −18); and the left caudal LC (LcaLC) (MNI −4, −38, −25).
The BOLD fMRI time series corresponding to the aforementioned Regions of Interest (ROIs) were obtained from the pre-processed data using the residuals of a General Linear Model (GLM) that included twenty-four head motion parameters15 and global white matter (WM) and cerebrospinal fluid (CSF) signals as nuisance regressors. Therefore, we selected the MNI coordinates of each ROI as the center of an 8-mm sphere to compute the subject-specific principal eigenvariate, correcting for such confounds.
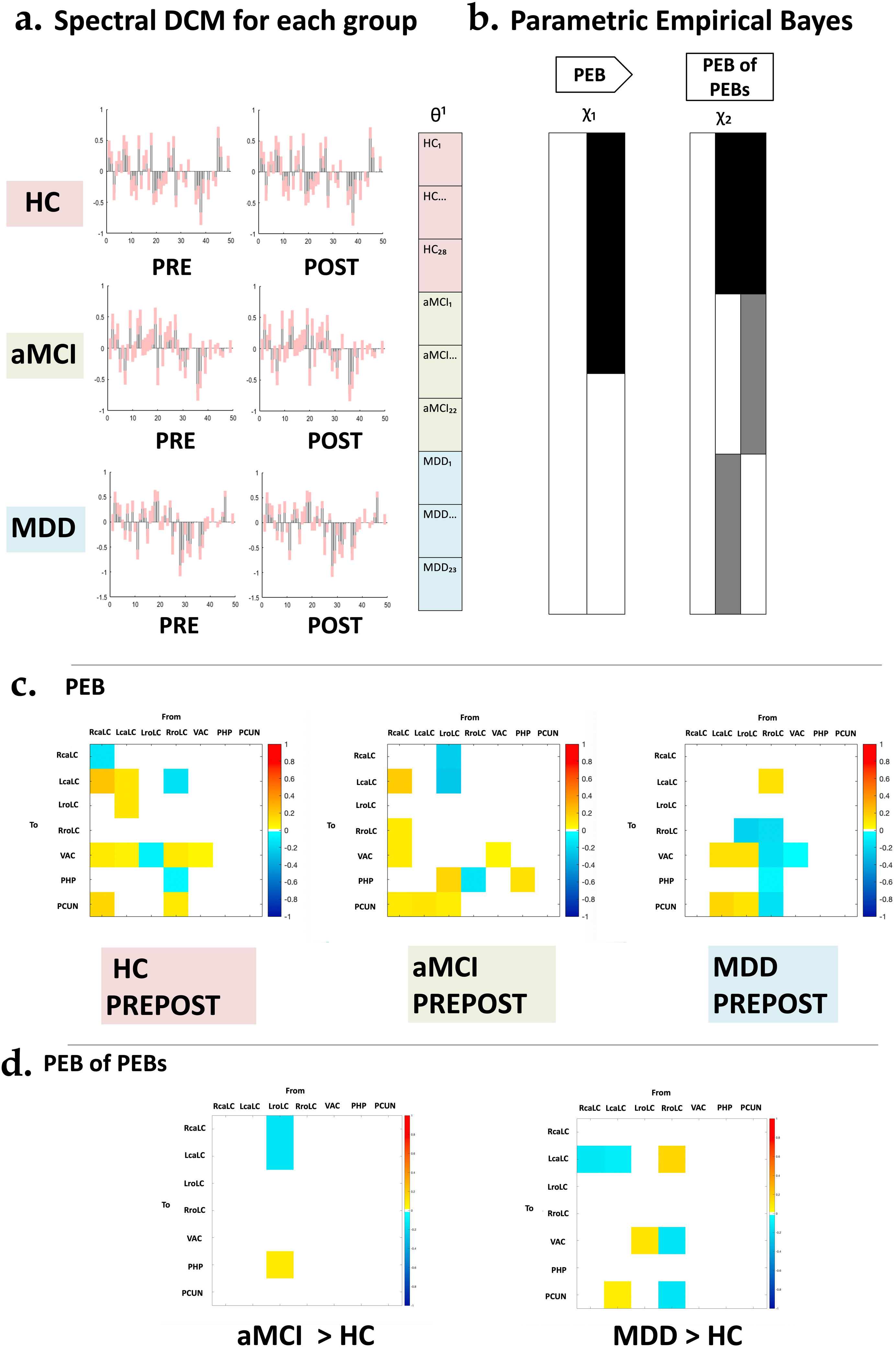
Neural network modeling: spectral dynamic causal modeling (spDCM)Spectral dynamic causal modeling (spDCM) analyses were conducted using functions from DCM12.5 revision 7497, implemented within SPM12. spDCM serves as a model-based analytical framework, facilitating the inference of intrinsic causal connections among specific regions through cross-spectral density. Unlike task-based approaches, spDCM computes endogenous coupling among regions without requiring explicit experimental input, making it computationally efficient and sensitive to group differences. Our primary focus for spDCM analyses was to investigate alterations in effective connectivity originating from the LC modulated over time within the MDD and aMCI groups. Fig. 2 presents a visual summary of the spDCM analysis outcomes. Further description of these analyses can be found in the Supplementary material.
Design of the parametric empirical Bayes (PEB) analysis. (a) Presentation of the Spectral Dynamic Causal Modeling (spDCM) results encompassing 31 estimated parameters. (b) These results were then analyzed using parametric empirical Bayes (PEB) analysis to explore group-level effects over time. The analysis employs a general linear model of connectivity parameters, illustrated by the design matrix χ2, which represents time (PRE-POST), and χ2, accounting for group effects, baseline (PRE), and two-year follow-up (POST). (c) Display the estimated parameters for PEB, focusing on time effects. (d) Reveals the estimated parameters for PEB of PEBs, emphasizing group effects. aMCI; amnestic Mild Cognitive Impairment, HC; healthy controls, MDD; late-life Major Depression Disorder.
The sample consisted of 73 individuals between 60 and 76 years: 23 patients with late-life MDD (17 females, mean age±standard deviation (SD): 69±4.1 years), 22 individuals with aMCI (13 females, mean age±SD: 73±3.5 years), and 28 healthy controls (18 females, mean age±SD: 69±3.9 years); see Table 1. The clinical outcomes are also presented in Table 1. Over the 2-year follow-up period, both patient groups exhibited significant decreases in the MMSE scores. Additionally, and as expected, the MDD group displayed notably higher scores both in the HDRS and the GDS assessments at baseline. Furthermore, it is noteworthy that HDRS scores exhibited a decrease during the follow-up period, unlike GDS scores. This trend suggests that quantitative (non-dichotomous) scoring systems may possess greater sensitivity to longitudinal symptom changes compared to GDS.
Structural data analysesThe longitudinal whole-brain voxel-based GM analyses comparing MDD+aMCI vs. HCs revealed different regions of GM volume reduction. Specifically, patients displayed GM volume decreases over time in the following right hemisphere regions in comparison to HCs: the precuneus (PCUN), the occipital lobe, encompassing visual motor and visual association cortex (VAC), and the parahippocampal cortex (PHC). Detailed results of this analysis can be found in Fig. 1a.
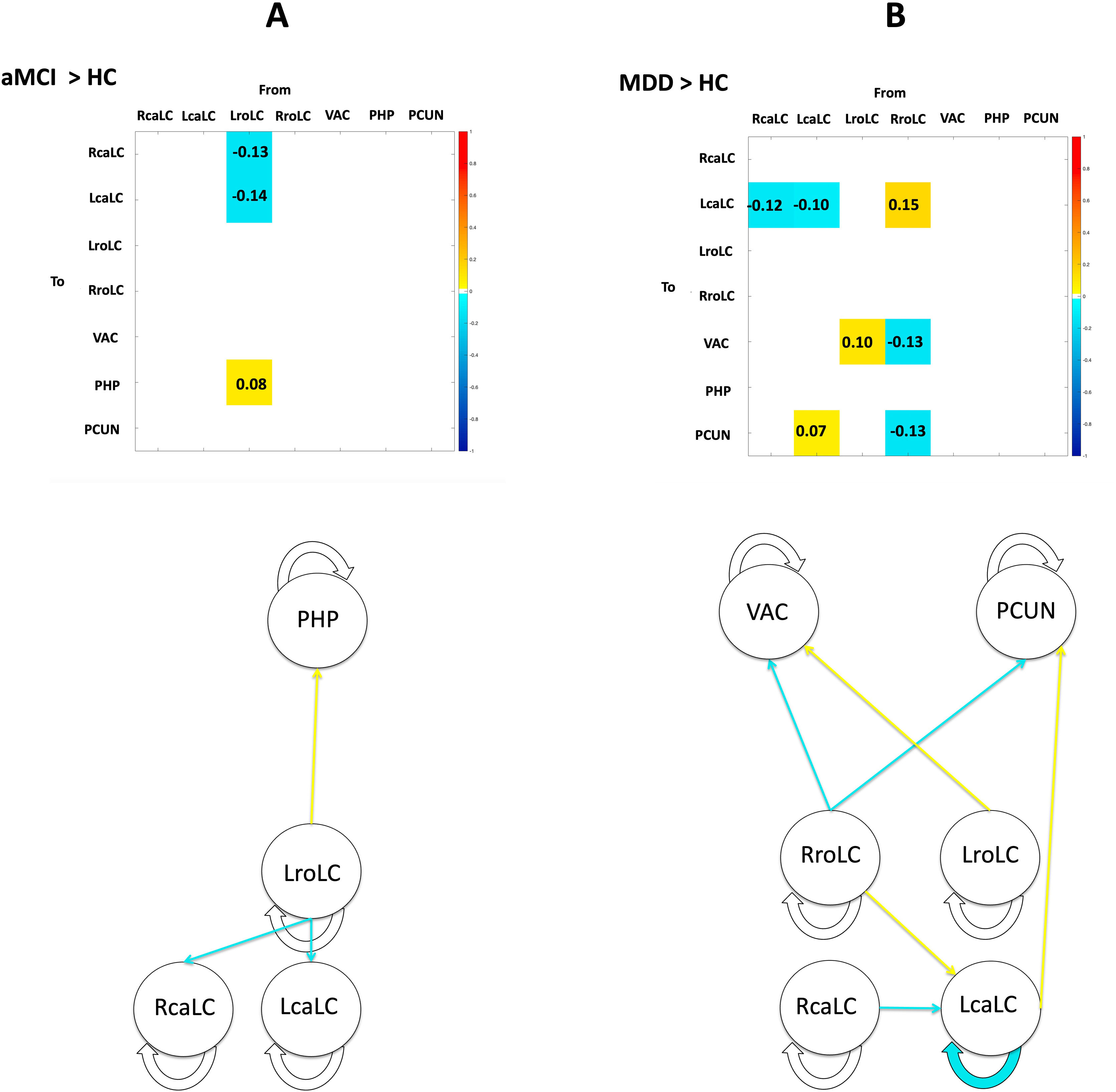
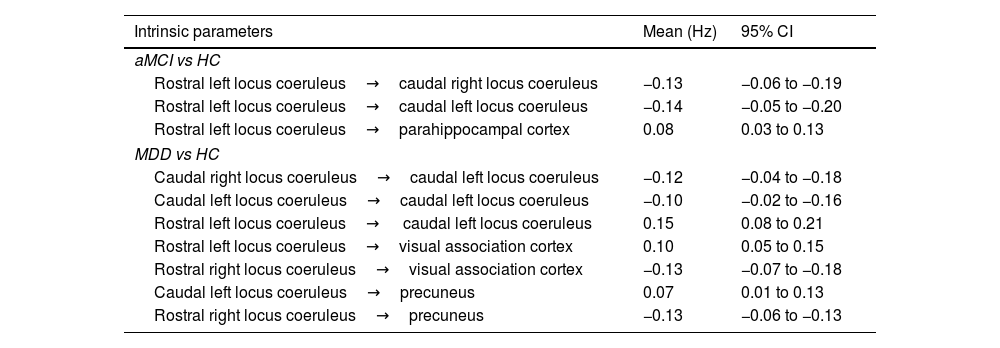
Spectral dynamic causal modeling and parametric empirical BayesThrough the application of parametric empirical Bayes (PEB), we estimated extrinsic connectivity along with associated uncertainties, the influence of time, and the differences between control and patient groups (Table 2). Our model focused on connections from the LC to regions displaying significant patient-control differences in volume changes over time. Employing Bayesian Model Reduction (BMR), we streamlined the model by excluding connections lacking substantial evidence, and subsequently applied Bayesian Model Averaging (BMA) to compute a weighted mean of parameters derived from BMR. Fig. 3 illustrates the BMA outcome, depicting group-level estimates of network connection strengths, their uncertainties, and posterior probabilities for surviving a 95% threshold.
DCM parameter estimates of intrinsic connections in second level PEB.
| Intrinsic parameters | Mean (Hz) | 95% CI |
|---|---|---|
| aMCI vs HC | ||
| Rostral left locus coeruleus→caudal right locus coeruleus | −0.13 | −0.06 to −0.19 |
| Rostral left locus coeruleus→caudal left locus coeruleus | −0.14 | −0.05 to −0.20 |
| Rostral left locus coeruleus→parahippocampal cortex | 0.08 | 0.03 to 0.13 |
| MDD vs HC | ||
| Caudal right locus coeruleus→caudal left locus coeruleus | −0.12 | −0.04 to −0.18 |
| Caudal left locus coeruleus→caudal left locus coeruleus | −0.10 | −0.02 to −0.16 |
| Rostral left locus coeruleus→ caudal left locus coeruleus | 0.15 | 0.08 to 0.21 |
| Rostral left locus coeruleus→visual association cortex | 0.10 | 0.05 to 0.15 |
| Rostral right locus coeruleus→visual association cortex | −0.13 | −0.07 to −0.18 |
| Caudal left locus coeruleus→precuneus | 0.07 | 0.01 to 0.13 |
| Rostral right locus coeruleus→precuneus | −0.13 | −0.06 to −0.13 |
CI; confidence interval, Hz; hertz, aMCI; amnestic Mild Cognitive Impairment, MDD; late-life Major Depression Disorder, HC; healthy controls.
Summary of the effective connectivity findings from the hierarchical parametric empirical Bayes analysis. In Panels A and B, tables illustrate the estimation of time X group interactions. Figures provide a simplified visualization of the parameters with a posterior probability exceeding 0.95. In both cases, over time decreases in effective connectivity are colored in blue, while increases are colored in yellow. LcaLC; the left caudal locus coeruleus, RcaLC; the right caudal locus coeruleus, LroLC; the left rostral locus coeruleus, RroLC; the right rostral locus coeruleus, PCUN; precuneus, PHC; parahippocampal cortex, VAC; visual association cortex, aMCI; amnestic Mild Cognitive Impairment, HC; healthy controls, MDD; late-life Major Depression Disorder.
Fig. 3A presents the comparison of patients with aMCI and healthy controls. Patients with aMCI present a decreased connectivity within the LC over time, which extends from the left rostral region to the bilateral caudal region. There is also an increase in effective connectivity over time from the left rostral LC to the right parahippocampal cortex.
Fig. 3B shows the difference between subjects with MDD and healthy subjects, in which we observed a decline in effective connectivity from the bilateral caudal seeds to the left caudal seed, as well as an increased connectivity from the right rostral to the left caudal LC seed over time. The analysis of the connectivity from the LC to cortical regions reveals increases in cross-hemisphere connections and decreases in same-hemisphere connections, originating predominantly from the rostral LC and extending to the visual association cortex and the precuneus of the right hemisphere.
DiscussionThis study employed spDCM to examine changes in resting-state effective connectivity over a two-year follow-up period in patients with aMCI or MDD, conditions associated with an elevated risk of developing AD. In comparison to a HC group, our findings revealed distinct effective connectivity patterns. In patients with aMCI, we observed a progressive decrease in LC connectivity over time, spanning from the left rostral to the bilateral caudal regions of the LC. Simultaneously, there was an increasing effective connectivity over time, specifically from the left LC to the right parahippocampal cortex within the same group. For individuals with MDD, we noted a decline in effective connectivity between LC caudal seeds when compared to the control group. However, there was an increased connectivity over time from the left rostral to the left caudal LC seed. Alterations in connectivity patterns with cortical regions were primarily marked by increases in cross-hemisphere connections and decreases in same-hemisphere connections, originating predominantly from the rostral LC and extending to the visual association cortex and the precuneus of the right hemisphere.
Longitudinal effective connectivity changes in aMCIThe observed effective connectivity reductions from the left rostral to bilateral caudal portions of the LC in aMCI individuals align with recent findings in the field. These results are consistent with a study by Galgani16 which conducted a 2.5-year follow-up in individuals with aMCI or AD. This study identified a substantial volume reduction in the left LC, particularly in its rostral subregion, at the outset of the study. Notably, this reduction was most pronounced in subjects with MCI who subsequently transitioned to MND/dementia, mirroring the volume reduction observed in the AD group. Our results also corroborate post-mortem evidence in humans, which suggests a progressive degeneration of the LC across various stages of AD, including the preclinical stage. They are also in line with age-dependent changes in LC functional connectivity, as reported by Song.17 These changes could be attributed to morphological alterations affecting neuromelanin-containing neurons in the rostral part of the nucleus. Such alterations may lead to a reduction in cell size and potentially result in cell loss, which could, in turn, be associated with changes in their projections.18 Another possible explanation lies in the accumulation of misfolded proteins in the aging brain, which may or may not lead to future neurodegeneration.19 The LC has been identified as an early site for tau deposition, and the accumulation of pathological proteins heightens the risk of neurodegenerative diseases such as AD. Histological studies have reported that the distribution of pathological proteins, particularly tau neurofibrillary tangles, is not uniform across the LC, with a preference for affecting the rostral–dorsal portion, irrespective of Braak stages, which is considered as a gradient of neuronal loss and dysfunction.20 Early morphological changes in tau-containing LC neurons include gradual dendritic atrophy and alterations in axonal morphology, indicative of impaired axonal transport. These changes may contribute to an early loss of connectivity in the rostral section of the LC during the aging process.
In contrast to the reductions in intra-LC connectivity, we observed a notable increase in effective connectivity over time, specifically from the left rostral LC to the right PHP cortex. One of the essential functions of the LC NE system is facilitating memory retrieval by activating hippocampal networks.21 The observed surge in effective connectivity between the left LC and the right PHP region could be interpreted as compelling evidence of an adaptive functional reorganization. This adaptive reconfiguration of connectivity, directing its focus toward episodic encoding processes in aMCI patients, may serve as a compensatory mechanism to counteract early memory alterations in the neurodegenerative process. Indeed, such compensatory activities, including heightened metabolism in surviving neurons or the reorganization of functional networks, have been documented in the initial stages of Alzheimer's disease (AD).22 Interestingly, in HCs resting-state functional connectivity between the LC and the PHC has been found to correlate with memory function, in particular with better encoding abilities.23 Furthermore, a recent study by Tang et al. (2023)24 suggests a decrease in structural covariance between the left rostral LC and the PHP in aMCI, a finding that is notably correlated with MMSE scores. Collectively, these findings lend support to Robertson's noradrenergic reserve theory.25 However, it is important to acknowledge that further research is imperative to uncover the underlying reasons for the pronounced left lateralization of LC connectivity findings in aMCI. Notably, similar effects have been observed in structural MRI studies, even in cognitively intact subjects.26,27 For instance, in a study focused on visualizing age-related differences in the LC using structural MRI, Liu et al., 201928 also reported left lateralized findings.
Longitudinal effective connectivity changes in late-life MDDThe role of the LC in depression has been firmly established by prior research, encompassing investigations into the depletion of noradrenergic neurons18 and alterations in both intra- and extra-neuronal signaling pathways.29 More specifically, the LC's significance in depression predominantly lies in its association with the development of anxiety, thus contributing to stress-induced depression.30 In our previous research with the same study sample, we discerned distinct LC alterations in patients with Major Depressive Disorder (MDD). Our initial fMRI exploration unveiled a reduction in functional connectivity of the LC during a visual oddball task.31 Subsequently, employing structural MRI, we revealed a marked decrease in LC structural integrity among patients with MDD.32
The dysregulation of the noradrenergic system observed in MDD holds relevance for the pathogenesis of this disorder. Previous studies have reported an increased density and sensitivity of central noradrenergic alpha-2 receptors in individuals with depression.33 It is noteworthy that the LC houses the highest density of alpha-2 receptors in the brain, and these receptors, among other functions, play a pivotal role in the autoregulation of NE secretion, governing approximately 80% of NE distribution through the LC. Notably, this region has been demonstrated to exhibit an elevated alpha-2 receptor binding capacity in MDD.34 The persistent dysregulation of the LC-NA system has been discussed in the context of chronic stress and it is associated with hormonal overstimulation.33
A potential explanation for this dysregulation lies in the observed decreases over time in the afferent connectivity within the caudal portions of the LC in our study. Particularly noteworthy is the reduced self-connection identified in the left caudal portion of the LC, which is believed to induce neural disinhibition. Self-connections exert inhibitory control on each region within the model, thereby regulating the excitatory and inhibitory connections between regions. This regulation is essential to prevent the development of a runaway positive feedback loop in the neurological model, as estimated by DCM. Consequently, the decrease in the self-connection parameter's magnitude may contribute to an increase in extrinsic connectivity within the left caudal LC.
The heightened external connectivity stemming from the left caudal LC can account for the observed rise in effective connectivity over time between this originating point and the contralateral precuneus. Numerous neuroimaging investigations have designated the precuneus as a pivotal hub in the human brain, attributed to its crucial role in supporting intricate cognitive functions and behaviors. The precuneus serves as a central component of the default mode network (DMN), which exhibits augmented activation during periods of rest and specific cognitive tasks, such as autobiographical memory recall.35 It also engages in distinct interactions within the broader network.36 Moreover, the precuneus demonstrates extensive connectivity, encompassing higher-order association regions, signifying its pivotal role in integrating both internally and externally driven information. In the context of DMN research, which is associated with introspection and internal attention, it has been observed that individuals with major depression exhibit heightened functional connectivity within this network.37 Importantly, this altered connectivity tends to normalize following treatment with antidepressants.38 Additionally, the left rostral LC also displayed heightened effective connectivity over time with the contralateral visual association cortex. Taken together, these cross-hemisphere connective increases resemble findings observed in the aMCI population and can therefore be interpreted in terms of potential compensatory mechanisms.
Longitudinal changes in intra- and inter-hemispheric effective connectivityExamining the evolving patterns of enhanced and diminished effective connectivity in both patient groups concerning intrahemispheric and interhemispheric networks presents an intriguing perspective. Over time, there is an observed decrease in intrahemispheric connectivity originating from the right portion of the LC, contrasted by an increase in interhemispheric connectivity between the left portion of the LC and cortical regions on the right hemisphere.
This rise in interhemispheric connectivity may be associated with compensatory mechanisms driven by neuronal plasticity. The brain's adaptive capacity to environmental changes enables compensation for declining cognitive functions, potentially mitigating the effects of AD symptoms over an extended period. Interhemispheric communication plays a pivotal role in cognitive and emotional processing. Coordinated interaction between both hemispheres becomes especially vital for executing complex tasks, particularly when task complexity or workload intensifies. During the initial stages of neurodegenerative processes, patients might sustain performance levels akin to those of controls by increasing interhemispheric communication, as observed in previous studies. For example, magnetoencephalography studies have shown greater interhemispheric gamma, beta, and alpha synchrony in individuals with MCI, performing similarly to controls in memory tasks.39
The escalating interhemispheric connections from the LC to the cortex in our patient cohorts as the disorder progresses align well with evidence suggesting that LC projection patterns exhibit a modular design. These projections indicate segregated output channels with the potential for diverse release and actions of NA on their projection fields.40 The distinct projection patterns of individual LC neurons might enable the differential modulation of various processes.41 This signals a paradigm shift in understanding LC operations and the influence of the LC-NA system on behavioral outcomes in both health and disease.
Strengths and limitationsThis study exhibits several strengths, notably the integration of hypothesis-driven and data-driven methods in defining regions of interest. Specifically, while we aimed a priori to assess effective connectivity from the LC, the cortical regions also included in DCM analyses were derived from longitudinal analyses of volume change. Moreover, the longitudinal design for effective connectivity analysis allowed us to track changes in connections, unveiling both reductions and increases. Notably, the identified increases may be indicative of compensatory neuronal mechanisms.
Likewise, the study has its share of limitations. Notably, the relatively small size of the LC presents a challenge for researchers in terms of determining the most suitable approach for processing image data. Spatial smoothing offers several advantages, such as enhancing the signal-to-noise ratio and mitigating anatomical and functional variations among subjects. However, the practice of spatial smoothing, which permits adjacent voxels to contribute to the estimated signal, can also elevate the risk that the modest LC activity area might be contaminated by noise stemming from the neighboring neural tissue. Consequently, the exact impact of spatial smoothing on the study's outcomes remains uncertain. To address this issue, high-field MRI acquisitions are warranted to bolster both resolution and signal acquisition quality. An additional limitation arises from the inclusion of individuals with concurrent aMCI diagnoses within our group of patients with MDD, potentially affecting the specificity of the findings related to individuals with MDD. While cognitive alterations in MDD are commonly observed clinically,42 future studies with larger and more robust samples should aim to explore the specificity of our findings. Furthermore, we exclusively present comparisons between patient populations and healthy controls, as the comprehensive examination of both disorders was beyond the scope of this manuscript. Nonetheless, it is worth noting that a comparative analysis between the two patient groups could offer valuable insights into the commonalities and distinctions between these conditions. Such an exploration could further enrich our understanding of the role played by the LC and noradrenergic neurotransmission in late-life pathology.
ConclusionsIn summary, our study utilizing spDCM uncovered distinctive resting-state effective connectivity patterns in patients with aMCI and MDD over a two-year follow-up. Our findings offer concrete insights into the evolving dynamics of neural connectivity in late-life MDD and aMCI, and underscore the intricacies of neural network adaptations. In addition to providing novel insights into the neurobiology of these late-life pathologies, with the potential to inform the development of biologically-informed remedies, our results also illuminate the underlying mechanisms that may contribute to the development of neurodegenerative disorders, including Alzheimer's disease.
FundingThis study was supported by the Agency for Management of University and Research Grants of the Catalan Government (2021SGR01017), the Carlos III Health Institute (Grants PIE14/00034, PI19/01040 and INT21/00055), the European Regional Development Fund (ERDF) “A way to build Europe” and CIBERSAM. The Institute of Neurosciences of the University of Barcelona is a María de Maeztu Unit of Excellence CEX2021-001159-M of the Ministry of Science and Innovation of Spain. We also thank CERCA Program/Generalitat de Catalunya for the institutional support.
Conflict of interestsThe sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript. The authors report no conflicts of interest with any product mentioned or concept discussed in this article.