Clinical high risk for psychosis (CHR) states are associated with an increased risk of transition to psychosis. However, the predictive value of CHR screening interviews is dependent on pretest risk enrichment in referred patients. This poses a major obstacle to CHR outreach campaigns since they invariably lead to risk dilution through enhanced awareness. A potential compensatory strategy is to use estimates of individual pretest risk as a ‘gatekeeper’ for specialized assessment. We aimed to test a risk stratification model previously developed in London, UK (OASIS) and to train a new predictive model for the Swiss population.
MethodThe sample was composed of 513 individuals referred for CHR assessment from six Swiss early psychosis detection services. Sociodemographic variables available at referral were used as predictors whereas the outcome variable was transition to psychosis.
ResultsReplication of the risk stratification model developed in OASIS resulted in poor performance (Harrel's c=0.51). Retraining resulted in moderate discrimination (Harrel's c=0.67) which significantly differentiated between different risk groups. The lowest risk group had a cumulative transition incidence of 6.4% (CI: 0–23.1%) over two years.
ConclusionFailure to replicate the OASIS risk stratification model might reflect differences in the public health care systems and referral structures between Switzerland and London. Retraining resulted in a model with adequate discrimination performance. The developed model in combination with CHR assessment result, might be useful for identifying individuals with high pretest risk, who might benefit most from specialized intervention.
In 2019, the Federal Social Insurance Office (OFAS) in Switzerland reported that schizophrenia and other psychotic disorders account for 9.4 and 14.4%, respectively, of new disability pensions for mental disorders.1 These disorders affect social, academic and professional functioning, as well as motivation and interest.2 Impairments are present during the first episode of psychosis (FEP),3 but in the majority of cases, they already begin before the onset of overt psychotic symptoms.4
The field of prevention and early detection of psychosis highlighted that a main factor contributing to a poor prognosis is the delay between the onset of symptoms and first specialized treatment.5 This led to an increased focus on early detection and the clinical high risk (CHR) state of psychosis. CHR is generally defined by the presence of ultra high-risk (UHR) criteria.6,7 These capture symptoms similar to positive psychotic phenomenology which, however, do not reach the severity or duration required for a diagnosis of a FEP. UHR criteria are usually assessed with semi-structured interviews such as the Structured Interview for Prodromal Symptoms (SIPS)8 and the Comprehensive Assessment of at Risk Mental States (CAARMS)7 that identify three risk categories: Brief Limited Intermittent Symptoms (BLIPS); Attenuated Positive Symptoms (APS); and Genetic Risk with Functional Decline (GRFD). Another approach, mostly implemented in German-speaking countries, considers subtle, subjectively experienced cognitive and perceptual experiences (‘basic symptoms’) as a potential indicator of psychosis risk. These are generally assessed by the Schizophrenia Proneness Instrument, Adult (SPI-A)9 and Child & Youth (SPI-CY)10 versions.
Identifying CHR states is of major importance for introducing measures to prevent or delay psychosis onset, as well as to tackle attenuated symptoms and functional impairments that may cause substantial illness burden regardless of transition risk.11–13 Around 25% of patients screening positive for high risk in the above-mentioned specialized interviews (CHR+) develop psychosis within the next 3 years.14 However, the predictive value of CHR screening is dependent on pretest risk: Fusar-Poli et al. (2015)15 estimated that CHR interviews had an excellent predictive value (AUC=0.90; 95% CI: 0.87–0.93) in help-seeking patients referred to early intervention services (EIS), who present a higher-than-average pretest risk. In contrast, the computed predictive value of a positive screening in unselected primary care patients was negligible based on the low incidence of psychotic disorders in the general population.16 Thus, CHR screening instruments are dependent on substantial ‘risk enrichment’ to be clinically useful; the term ‘risk enrichment’ refers to assessment and recruitment strategies that aim to increase the proportion of patients with ‘true’ risk among patients receiving a screening.17 Accordingly, the European Psychiatric Association guidance on the early detection of CHR18 recommends that CHR assessments should be offered only in help-seeking individuals suffering from mental health problems or seeking clarification of their current risk based on a pre-existing (e.g., genetic) vulnerability. Failure to follow this recommendation may lead to increase of false-positive referrals in EIS services (i.e., dilution of risk19). This represents a major obstacle to outreach campaigns, negating their usefulness for creating awareness and promoting early detection.
Based on the above, a potential strategy to prevent risk dilution while keeping the advantages of increased awareness and outreach might be to use individualized pretest risk estimates as a ‘gatekeeper’ for specialized assessment referrals in patients with suspected CHR. According to this notion, patients with high individualized pretest risk would go on to receive specialized assessment. In contrast, patients with low pretest risk would not be referred due to the low predictive value of a positive screening in these cases, which would make screening uninformative and, therefore, unnecessary.
Pretest risk estimates can be computed based on non-clinical factors affecting the incidence of psychosis, which thus may be used for risk stratification.18,20–23 Fusar-Poli et al. (2016)24 developed such a model using sociodemographic variables such as age, gender, race/ethnicity, socioeconomic status and source of referral to stratify patients referred to Outreach and Support in South London CHR service (OASIS) according to their risk for a later diagnosis of schizophrenia spectrum disorder. In their sample, race/ethnicity and source of referral were significantly associated with pretest risk. The model showed adequate discrimination and was externally validated using data from different boroughs of the catchment area of the service. However, it has not yet been independently validated outside OASIS. Thus, its generalizability to different contexts remains uncertain.
The present study aimed to develop an individualized pretest risk stratification model for use in Swiss early psychosis services. PsyYoung,25 launched in 2019, is a Swiss transcantonal collaborative project that aims to improve pathways to care by two-fold action, based on the above considerations: (a) increase awareness of psychosis risk in potential referring professionals in the health and education sectors, while at the same time (b) implementing a stepped model of care that includes individualized pretest risk estimates to guide access to specialized CHR assessment and intervention, in order to avoid risk dilution and preserve the predictive value of CHR screening. In this context, the aim of the present study was to replicate the pretest risk stratification model developed by Fusar-Poli, Rutigliano24 or, failing that, to train a new model, in a population of Swiss patients referred for CHR screening.
MethodThe study followed the guideline for Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD26).
Six Swiss early detection for psychosis services contributed data to the present analysis: (i) Basel Project for the Early Detection of Psychoses (Früherkennung von Psychosen, FePsy) (until mid-2017) and (ii) Basel Early Treatment Service (BEATS) (from mid-2017 on) at the University Psychiatric Clinics Basel; (iii) Bern Early Recognition and Intervention Centre for Mental Crisis (FETZ Bern)27 at the University Psychiatric Services Bern; (iv) Outpatient program for young adults with emerging mental disorders (JADE) at the University Hospital of Geneva; (v) Medico-Pedagogical Office (OMP) at the Department of Public Instruction, Education and Youth of the Canton of Geneva, (vi) At Risk Mental State (ARMS) and Treatment and Early Intervention in Psychosis (TIPP)28 Service at the University Hospital of Lausanne (CHUV).
SampleWe included all patients presenting for CHR assessment between 2015 (the earliest time, for which data were available for all services) and 2021, with the exception of patients who were classified as having a FEP at baseline, and those who did not complete the assessment. Patients who transitioned to psychosis within less than 4 weeks from baseline were excluded, to preclude potential misclassification at baseline. The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethikkomission Nordwest-und Zentralschweiz (EKNZ) (protocol code 2020-02384 and date of approval: 8 February 2021), in the context of the project PsyYoung. According to the Swiss Federal Human Research Act, no ethics authorization or informed consent is required for projects that use anonymized datasets, as in this case.
In three services (BEATS Basel, ARMS/TIPP Lausanne, OMP), CHR status was determined based on the SIPS for assessment of UHR criteria, as well as the SPI-A or SPI-CY for assessment of basic symptoms in adults and adolescents, respectively. JADE used the CAARMS for UHR determination. FETZ Bern used both the CAARMS and the SIPS, as well as the SPI-A. At FePsy Basel, CHR status was determined based on the Basel Screening Instrument for Psychosis (BSIP).29 Transition to psychosis was determined based on criteria for a first psychotic episode as defined in the respective instrument (SIPS, CAARMS, BSIP) used at each site.
Statistical analysisAnalyses were performed in R version 3.6.0. The outcome of interest was time to transition, censored at the last follow-up appointment of the patient, or at six years for patients still in follow-up on 31 January 2022. Transition was defined according to criteria determined by the specific instrument in use at each site (SIPS/SOPS, CAARMS or BSIP); we did not define transition based on the criterion of an ICD-10 diagnosis of psychotic disorder, as this would encompass diagnoses of acute polymorphic psychotic disorders (used for coding of brief intermittent psychotic symptoms, although these fall into the CHR range according to screening instruments) and/or codes used to indicate suspected or excluded diagnoses for billing purposes. Cumulative transition incidence in the analyzed sample was estimated using the Kaplan–Meier failure function.
For replication of the pretest risk stratification model by Fusar-Poli et al.,24 we calculated a prognostic index (PI) using the regression coefficients provided in their original publication for significant predictors of pretest risk (i.e., race/ethnicity and referral source) and used it to calculate Harrel's c index and to predict transition to psychosis in an univariable Cox regression model.
To retrain the predictive model, we used the following predictors: sex, age (linear and quadratic effect), interaction of sex with linear and quadratic age effects, race/ethnicity, relationship status, and referral source. Race/ethnicity, relationship status and referral source were re-coded at all sites to correspond to the categories used by Fusar-Poli et al.,24 and cross-checked by the last author. All categorical predictors were split into dummy variables. For model development, we used the mlr3 package for R.30 We trained a Cox proportional hazards model using the Least Absolute Shrinkage and Selection Operator (LASSO) method, a penalized regression analysis method that helps control overfitting problems. We used nested cross-validation with 10 folds/10 repeats in the inner loop, and 5 folds/5 repeats in the outer loop. The inner loop was used to determine the optimal value of the hyperparameter lambda, while the outer loop was used to provide an unbiased performance evaluation. We were primarily interested in discrimination rather than calibration of the model, given that we intended it for use to guide allocation of clinical resources rather than for communicating transition risk to single patients. Therefore, we used Harrel's c-index to tune lambda (across a 10-step sequence from 10−5 to 10).
Subsequently, we calculated a prognostic score for each patient based on the regression coefficients estimated in the LASSO model, and used the 25th, 75th and 95th percentiles of these scores to stratify the patient sample into four risk groups (corresponding to low, moderately low, moderately high, and high risk), according to the recommendation by Machin et al., 2006.31 Three contrasts were defined to compare the average survival of higher vs. lower-level risk groups (low risk vs. all higher-risk groups, low and moderately low vs. high and moderately high-risk groups, and high risk vs. all lower-risk groups); p-values were adjusted for multiple comparisons using the Bonferroni procedure.
For a subsample of patients (n=290; 156 CHR, of which 49 experienced a transition to psychosis) who had signed a general consent agreeing to the use of their health care data for research purposes, residential addresses were available and could be used to calculate socioeconomic position (SEP) based on the Swiss neighborhood index of SEP32,33 as an additional predictor in the model. Details of this analysis are reported in the Supplement, section ‘Supplementary analyses’.
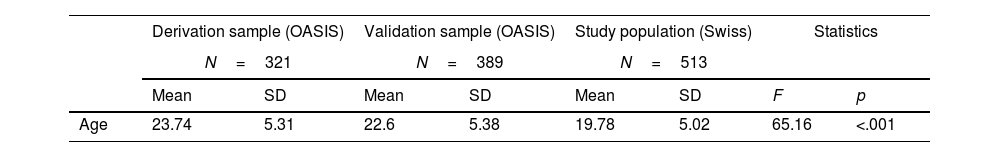
ResultsOf 561 referred patients meeting inclusion criteria, complete data were available for 513 patients, who constituted the study population. Sample characteristics and their comparison to the OASIS derivation and validation samples24 are presented in Table 1. Characteristics of the sample according to the canton of origin are presented in the Supplement (Table S1).
Characteristics of the current study population compared to the OASIS derivation and validation samples24 (Fusar-Poli et al., 2016).
| Derivation sample (OASIS) | Validation sample (OASIS) | Study population (Swiss) | Statistics | |||||
|---|---|---|---|---|---|---|---|---|
| N=321 | N=389 | N=513 | ||||||
| Mean | SD | Mean | SD | Mean | SD | F | p | |
| Age | 23.74 | 5.31 | 22.6 | 5.38 | 19.78 | 5.02 | 65.16 | <.001 |
| n | % | n | % | n | % | χ2 | p | |
|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||
| Male | 185 | 57.63 | 214 | 55.01 | 319 | 62.2 | 4.9 | .086 |
| Female | 136 | 42.37 | 175 | 44.99 | 194 | 37.8 | ||
| Transition to psychosis | ||||||||
| No | 280 | 87.23 | 349 | 89.72 | 448 | 87.33 | 1.49 | .475 |
| Yes | 41 | 12.77 | 40 | 10.28 | 65 | 12.67 | ||
| Ethnicity | ||||||||
| Black | 80 | 26.49 | 78 | 21.79 | 31 | 6.04 | 141.41 | <.001 |
| White | 143 | 47.35 | 186 | 51.96 | 414 | 80.70 | ||
| Asian | 9 | 2.98 | 21 | 5.87 | 17 | 3.31 | ||
| Caribbean | 17 | 5.63 | 15 | 4.19 | 3 | 0.58 | ||
| Mixed | 17 | 5.63 | 18 | 5.03 | 22 | 4.29 | ||
| Other | 36 | 11.92 | 40 | 11.17 | 26 | 5.07 | ||
| Marital status | ||||||||
| Married | 8 | 2.84 | 11 | 3.19 | 8 | 1.56 | 84.89 | <.001 |
| Divorced or separated | 11 | 3.9 | 8 | 2.32 | 4 | 0.78 | ||
| Single | 253 | 89.72 | 319 | 92.46 | 409 | 79.73 | ||
| In a relationship | 10 | 3.55 | 7 | 2.03 | 92 | 17.93 | ||
| Referral source | ||||||||
| Self | 29 | 9.03 | 37 | 9.51 | 26 | 5.07 | 325.42 | <.001 |
| Caregivers or relatives | 5 | 1.56 | 8 | 2.06 | 14 | 2.73 | ||
| Schools and colleges | 1 | 0.31 | 5 | 1.29 | 13 | 2.53 | ||
| Social services and supported accommodation | 4 | 1.25 | 7 | 1.8 | 3 | 0.58 | ||
| General medical practitioners | 119 | 37.07 | 124 | 31.88 | 38 | 7.41 | ||
| Community mental health services | 61 | 19 | 104 | 26.74 | 291 | 56.73 | ||
| Child and adolescent mental health services | 32 | 9.97 | 29 | 7.46 | 35 | 3.82 | ||
| Early intervention for psychosis services | 30 | 9.35 | 17 | 4.37 | 1 | 0.19 | ||
| Accident and emergency departments | 13 | 4.05 | 33 | 8.48 | 19 | 3.7 | ||
| Inpatient mental health services | 5 | 1.56 | 9 | 2.31 | 63 | 12.28 | ||
| Police and criminal justice system | 4 | 1.25 | 3 | 0.77 | 0 | 0 | ||
| Physical health services | 18 | 5.61 | 13 | 3.34 | 10 | 1.95 | ||
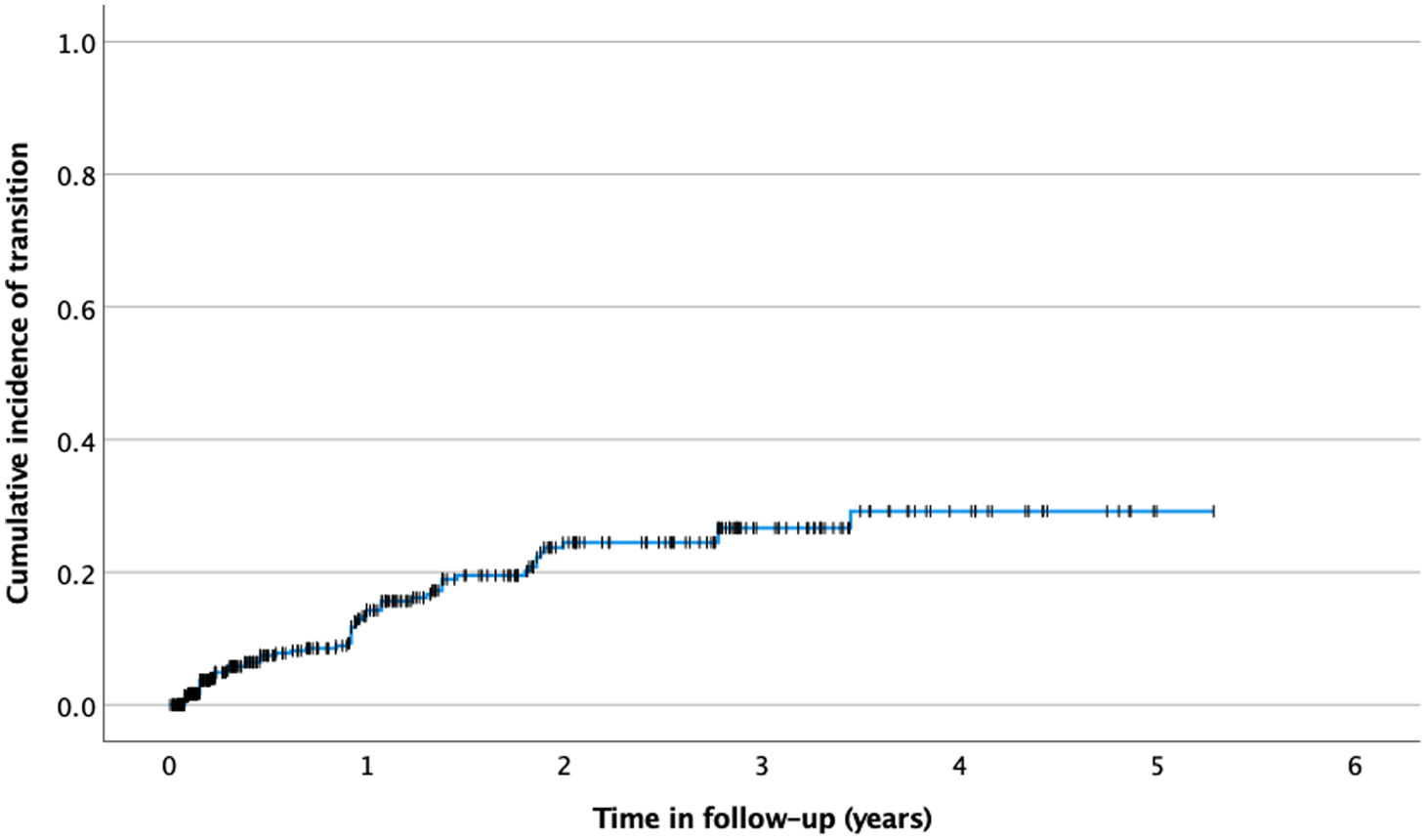
The mean follow-up time was 214.23 weeks (95% CI: 200.02–228.45 weeks). There were 65 transition events in total, of which 62 in patients diagnosed with CHR at baseline. The last transition was observed at 180 weeks; at that time, 29 patients were still in follow-up. The mean time to transition was 44.56 weeks (95% CI: 34.90–54.23 weeks). The estimated cumulative incidence of transition risk is depicted in Fig. 1 and was 14.3% (95% CI: 10.2–18.2%), 24.5% (95% CI: 18.4–30.1%) and 29.2% (95% CI: 20.9–36.7%) respectively at 1, 2 and 5 years.
Pretest risk predictionThe model using the PI derived from the equation provided by Fusar-Poli et al. (2016)24 performed no better than chance, with a Harrell's c of 0.51 (95% CI: 0.43–0.59), an integrated Brier score of 0.160 and a regression slope of −0.164 (95% CI: −0.452 to 0.124; p=0.264). Updating the model to optimize calibration (regression slope=1) did not improve model performance.
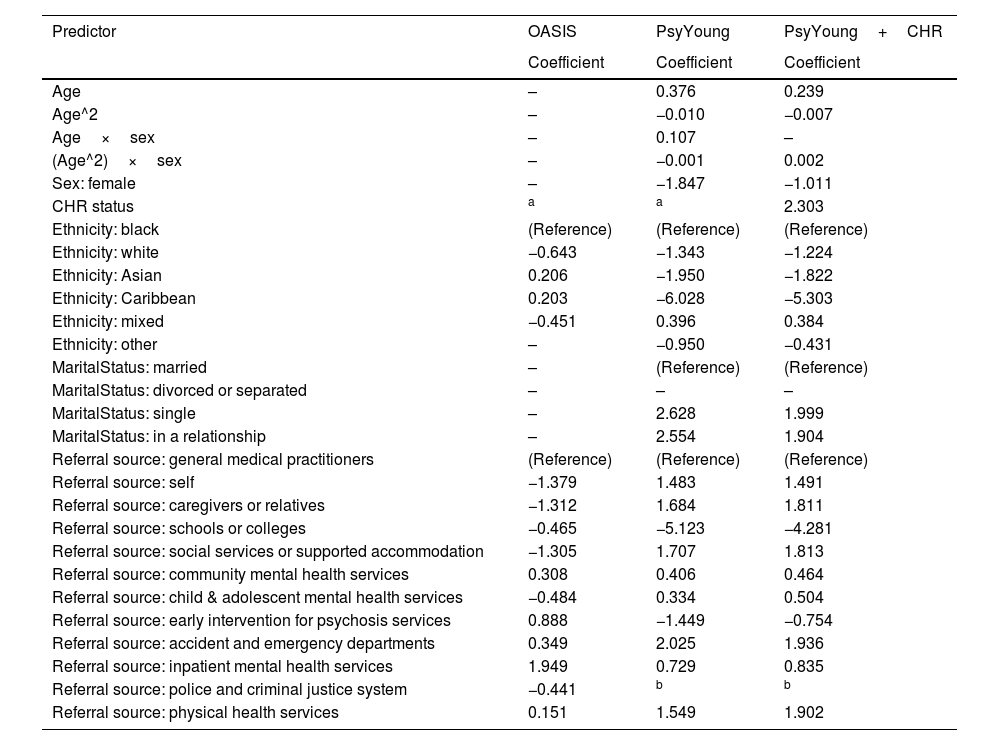
Retraining a LASSO Cox proportional hazards model resulted in a model with moderate discrimination (Harrell's c index=0.69), an integrated Brier score of 0.112, and a regression slope of 1.028 (aggregate values in hold-out samples). All predictor variables but one (marital status: divorced or separated) had a non-zero regression coefficient in the new model (see Table 2). Excluding all subjects with a follow-up time shorter than 4 weeks did not substantially change model performance (aggregate hold-out sample Harrel's c=0.69; see Supplement, section ‘Supplementary analyses’).
Predictor coefficients in the original OASIS (London) model,24 in the PsyYoung study model, and in the PsyYoung model with CHR as additional predictor.
| Predictor | OASIS | PsyYoung | PsyYoung+CHR |
|---|---|---|---|
| Coefficient | Coefficient | Coefficient | |
| Age | – | 0.376 | 0.239 |
| Age^2 | – | −0.010 | −0.007 |
| Age×sex | – | 0.107 | – |
| (Age^2)×sex | – | −0.001 | 0.002 |
| Sex: female | – | −1.847 | −1.011 |
| CHR status | a | a | 2.303 |
| Ethnicity: black | (Reference) | (Reference) | (Reference) |
| Ethnicity: white | −0.643 | −1.343 | −1.224 |
| Ethnicity: Asian | 0.206 | −1.950 | −1.822 |
| Ethnicity: Caribbean | 0.203 | −6.028 | −5.303 |
| Ethnicity: mixed | −0.451 | 0.396 | 0.384 |
| Ethnicity: other | – | −0.950 | −0.431 |
| MaritalStatus: married | – | (Reference) | (Reference) |
| MaritalStatus: divorced or separated | – | – | – |
| MaritalStatus: single | – | 2.628 | 1.999 |
| MaritalStatus: in a relationship | – | 2.554 | 1.904 |
| Referral source: general medical practitioners | (Reference) | (Reference) | (Reference) |
| Referral source: self | −1.379 | 1.483 | 1.491 |
| Referral source: caregivers or relatives | −1.312 | 1.684 | 1.811 |
| Referral source: schools or colleges | −0.465 | −5.123 | −4.281 |
| Referral source: social services or supported accommodation | −1.305 | 1.707 | 1.813 |
| Referral source: community mental health services | 0.308 | 0.406 | 0.464 |
| Referral source: child & adolescent mental health services | −0.484 | 0.334 | 0.504 |
| Referral source: early intervention for psychosis services | 0.888 | −1.449 | −0.754 |
| Referral source: accident and emergency departments | 0.349 | 2.025 | 1.936 |
| Referral source: inpatient mental health services | 1.949 | 0.729 | 0.835 |
| Referral source: police and criminal justice system | −0.441 | b | b |
| Referral source: physical health services | 0.151 | 1.549 | 1.902 |
Outcome is time to transition in a Cox regression model. Coefficients of predictors that did not contribute to a model are denoted with “–”.
In the subsample of patients with available SEP data, SEP had a non-zero contribution as a predictor of transition, while age and the interaction of its linear and quadratic effects with sex were not predictors. Discrimination performance did not improve (aggregate hold-out sample Harrel's c=0.66; see Supplement, section ‘Supplementary analyses’ and Tables S3 and S4).
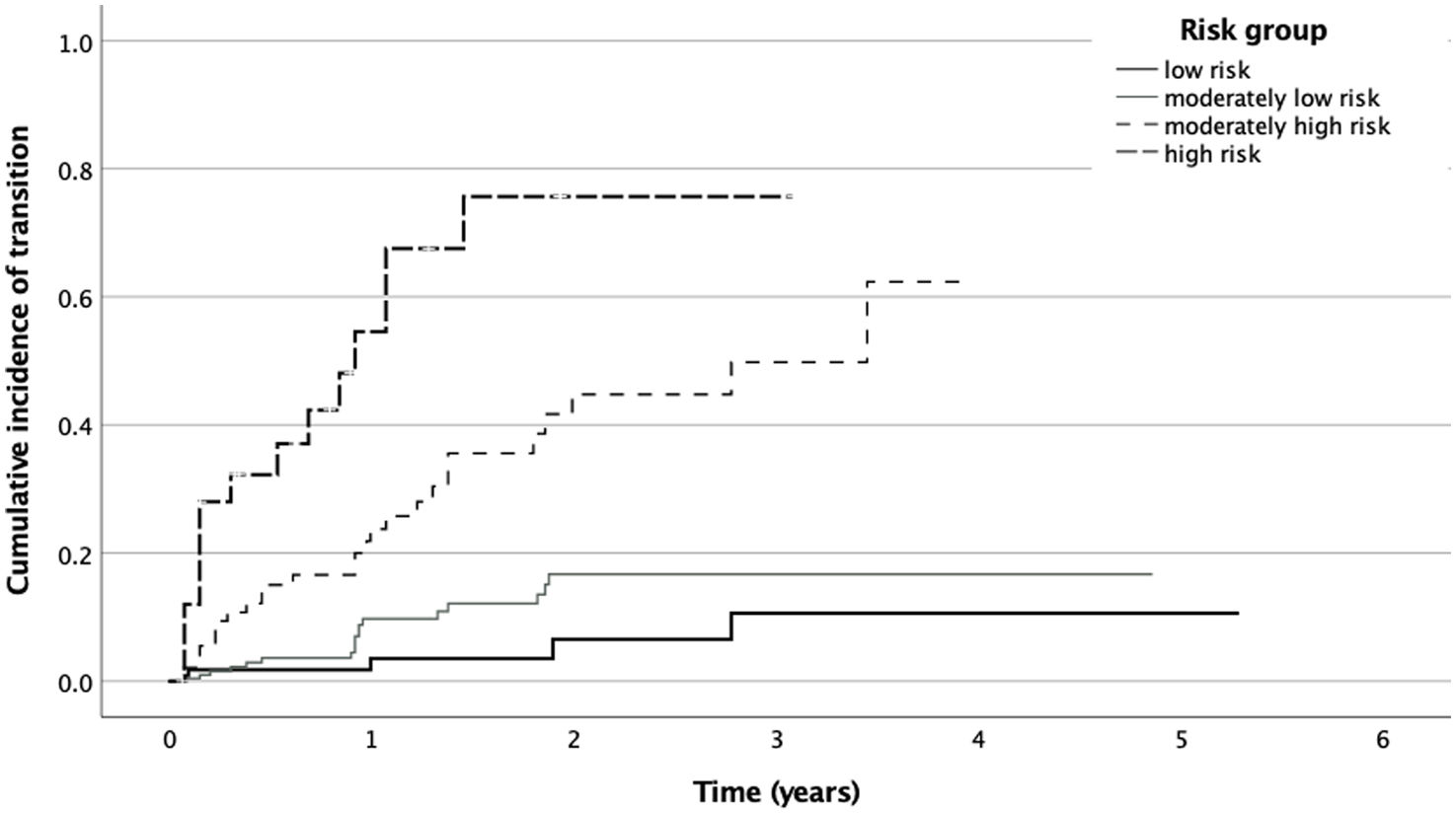
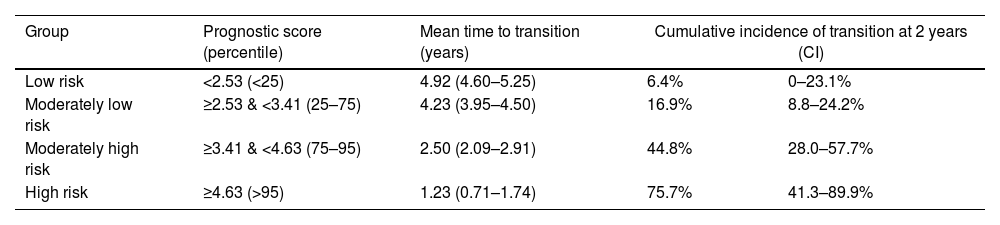
Stratification into risk groupsKaplan–Meier curves of the four risk groups created using PI as the stratification criterion are displayed in Fig. 2. The cumulative incidence of transition at 2 years in the different risk groups is shown in Table 3. All contrasts between higher- vs. lower-risk levels were significant (see Supplement, Table S2).
Transition outcomes at 2 years depending on risk group, defined according to the prognostic score of the study pretest risk stratification model.
| Group | Prognostic score (percentile) | Mean time to transition (years) | Cumulative incidence of transition at 2 years (CI) | |
|---|---|---|---|---|
| Low risk | <2.53 (<25) | 4.92 (4.60–5.25) | 6.4% | 0–23.1% |
| Moderately low risk | ≥2.53 & <3.41 (25–75) | 4.23 (3.95–4.50) | 16.9% | 8.8–24.2% |
| Moderately high risk | ≥3.41 & <4.63 (75–95) | 2.50 (2.09–2.91) | 44.8% | 28.0–57.7% |
| High risk | ≥4.63 (>95) | 1.23 (0.71–1.74) | 75.7% | 41.3–89.9% |
Including CHR status as an additional predictor improved model performance, with moderate discrimination (Harrell's c index=0.76), an integrated Brier score of 0.097, and a regression slope of 1.018 (aggregate values in hold-out samples). All predictor variables but two (marital status: divorced or separated; age×sex interaction) had a non-zero regression coefficient in the new model (see Table 2).
DiscussionThe aim of the present study was to develop and test the performance of a pretest risk stratification model to better identify youth at high risk for psychosis and offset risk dilution resulting from outreach campaigns.
Cumulative transition incidenceIn the whole sample, we observed a transition rate of 24.5% at 3 years after the first assessment of CHR among help-seeking population. The cumulative transition incidence for our sample of referred patients is similar to that previously reported for pure CHR samples,34 and substantially higher than the one reported for referrals to OASIS24 (15% of within 3 years). Thus, we can consider our sample to be substantially risk-enriched, also evidenced by the fact that almost 2/3 of referred patients met CHR criteria.
Pretest risk modelOur attempt to externally validate the pretest risk model by Fusar-Poli et al. (2016)24 model in a Swiss population was not successful. Apart from the different case mix, there are other potential explanations for this. First, ‘events’ were defined differently in the two studies: Whereas Fusar-Poli et al. defined their outcome as an ICD-10 diagnosis of psychotic disorder in the electronic health record, we used instead psychotic transition as defined by CHR screening instruments (i.e., SIPS, CAARMS or BSIP). Second, differences between the models might be explained by differences in the structure of the public health care systems in London and in Switzerland. In contrast to the UK universal National Health Service, in which access to care is free of charge, the Swiss healthcare system is characterized by large variation in healthcare policies across cantons, and a certain reliance on healthcare payments by households.35 Conceivably, these differences might affect the ways sociodemographic variables interact with each other to impact pathways to care and clinical outcomes. For example, ethnicity, and particularly black ethnicity, has been consistently reported to affect rates of psychotic disorders and access to mental health care in London.36 Although a direct comparison with Switzerland is difficult due to the paucity of related studies in the field of mental health, studies of access to health care in Switzerland have focused more on migration and/or citizenship status, and socioeconomic factors, than ethnicity.35,37
Risk stratification and clinical applicationsThe intended purpose of our pretest risk stratification model was to use it in the usual care setting as a ‘gatekeeper’, i.e., to guide decisions on referrals for specialized assessment in cases of suspected CHR. Our model achieved a discrimination capacity of 67%, i.e., similar to the model by Fusar-Poli et al.24 Given the low-moderate discrimination, it is unlikely that our stratification model, in its current form, is suitable as a pre-screening tool to exclude low-risk patients from the burden of specialized screening. Still, the model significantly differentiated between higher- (high and moderately high) and lower- (low and moderate) risk groups; moreover, adding CHR status as a predictor substantially improved model performance. Thus, an alternative use of our model, currently implemented in PsyYoung, could be for identifying individuals with high pretest risk, i.e., those with the highest potential benefit from early intervention.
Further analyses are needed to determine if other variables, available at referral, could increase the discrimination capacity of a genuine pretest risk stratification model (i.e., without the need to perform CHR screening). For example, recent reviews have identified further potential predictors of psychotic transition such as employment, living status, or cannabis dependence,38,39 which could be used to refine models with data on environmental risk factors.40–42 Moreover, some populations have been reported to be at higher risk to develop psychosis (e.g., individuals with two relatives with psychosis or suffering from 22q11.2 deletion syndrome).43
It should be noted that there are other potential applications for clinical risk prediction models in this population, for example diagnostic or prognostic models.44 The former category includes models that detect patients in secondary care who might have high psychosis risk, i.e., those who should be referred for specialized assessment. One such model,45 based on ICD-10 diagnosis at index presentation, age, sex and ethnicity, has shown consistent moderate to good discriminability in independent validation samples,46–48 and its feasibility of clinical implementation has been demonstrated.41 On the other hand, prognostic models stratify patients with an established CHR concerning their risk for transition to psychosis. For example, Cannon et al. (2016)49 used symptom severity, functionality and neuropsychological performance to predict transition risk. Their model showed moderate discrimination performance both in the original sample and in external validation samples.50–52
LimitationsOur results should be considered in view of certain limitations. The outcome of interest – transition to psychosis – was defined using different instruments at each site. For example, SIPS and CAARMS apply different criteria for the definition of FEP with regard to frequency and duration of symptoms, administered medication, and dangerousness to self or others. Even though these differences appear to affect a small minority of referrals,53 harmonization of CHR and transition criteria54 might improve precision psychiatry approaches intended for multicenter populations. Another limitation to be noted is that the modest number of transition events (n=65) entails a risk of overfitting, which we attempted to control by using LASSO shrinkage. Moreover, our sample was characterized by a very high proportion of CHR among referrals, and thus it is unclear if our model is generalizable to less enriched patient populations. Finally, even though we used nested cross-validation to provide an unbiased assessment of model performance, there was no external validation of the model, which would have been important to establish its generalizability. A prospective evaluation of the model in an independent sample is currently under way within the project PsyYoung.
ConclusionThe present study aimed to train an individualized pretest risk stratification model based on data from patients referred for CHR screening to psychosis early detection services of four Swiss cantons. Even though the developed model achieved adequate discrimination, the absence of a ‘zero-risk’ group makes it unsuitable for use as a ‘gatekeeper’ for specialized CHR assessment. Other applications of the model, such as use in combination with CHR assessment results to guide specialized treatment decisions, show more promise.
FundingThis research was funded by Promotion Santé Suisse grant number 19.313.
Conflicts of interestThe authors declare no conflicts of interest.
Data availability statementThe data collected within the project PsyYoung are available upon request to the senior author. The data are not publicly available for reasons of personal data protection.
The PsyYoung project is funded by Gesundheitsförderung Schweiz in the context of the funding program of the Swiss Health Department ‘Prevention in Health Care’. Dr. Luis Alameda thanks the Foundation Adrian and Simone and Carigest SA Foundation for their support.