Obesity is a global pandemic associated with various cardio-metabolic and psychiatric disorders. Neurocognitive and functional deficits have been associated with several somatic and psychiatric disorders. Adiposity-related inflammation has recently emerged as a key risk factor for neurocognitive and functional impairments. This prospective transdiagnostic study aimed to investigate the role of adiposity-related inflammatory markers in neurocognitive and functional outcomes associated with weight changes.
MethodsPeripheral blood inflammatory and oxidative stress biomarkers and neurocognitive and functional performance were assessed twice over 1 year in 165 individuals, including 30 with schizophrenia, 42 with bipolar disorder, 35 with major depressive disorder, 30 with type 2 diabetes mellitus (T2DM), and 28 healthy controls. Participants were stratified by body mass index into categories of type 2 obesity (T2OB; n=30), type 1 obesity (T1OB; n=42), overweight (OW; n=53), and average weight (NW; n=40). Mixed one-way analysis of covariance and linear and binary logistic regression analyses were performed.
ResultsCompared with NW, T2OB and T1OB were significantly associated with impaired neurocognitive and functional performance (p<0.01; η2p=0.06–0.12) and higher levels of C-reactive protein and platelets (PLT) (p<0.01; η2p=0.08–0.16), with small-to-moderate effect sizes. IL-6, IL-10, and PLT were key factors for detecting significant weight changes in T1OB and T2OB over time. Regression models revealed that inflammatory and oxidative stress biomarkers and cellular adhesion molecules were significantly associated with neurocognitive and functional performance (p<0.05).
DiscussionObesity is characterized by neurocognitive and functional impairments alongside low-grade systemic inflammation. Adiposity-related inflammatory biomarkers may contribute to neurocognitive and functional decline in individuals with T2DM and psychiatric disorders. Our data suggest that these biomarkers facilitate the identification of specific subgroups of individuals at higher risk of developing obesity.
Obesity (OB) has emerged as a global pandemic over the past few decades. OB constitutes a major public health problem that causes a substantial societal and economic burden associated with a greater risk of morbimortality.1,2 Evidence suggests that OB and increased body fat/adiposity are bi-directionally associated with poor mental health outcomes.3 Indeed, several psychiatric disorders are strong risk factors for the development and maintenance of OB.4 Additionally, OB is frequently complicated by cardio-metabolic diseases, such as type 2 diabetes mellitus (T2DM). In this regard, T2DM appears to be related to body mass index (BMI), whereby individuals with severe OB have a higher risk of developing T2DM.5 Moreover, decreased quality of life and increased functional impairments are frequently observed in individuals with OB, predominantly owing to higher rates of treatment resistance and relapse.6
Psychiatric and cardio-metabolic multimorbidities may contribute to neurocognitive and functional decline in individuals with OB.7 In individuals with psychotic and mood disorders, more severe OB significantly increases neurocognitive deficits than average weight (NW).8,9 Of note, an extensive neurocognitive overlap has been observed between individuals with T2DM and psychotic and mood disorders.10 This overlap is associated with more significant functional impairment in individuals with comorbid OB. Fernando et al. recently reported that T2DM is associated with an increased risk of developing earlier neurocognitive impairments in individuals with overweight (OW)/OB.11 Furthermore, the presence of weight gain (WG) and high comorbidity rates among individuals with OB are associated with an increased risk of neurocognitive decline and poorer functional outcomes over time.12
Lifestyle factors, such as dietary patterns that are poor in nutrients and high in saturated fats and refined sugars, in conjunction with increased sedentary behaviors and reduced physical activity, have been shown to contribute significantly to increased weight, particularly in more vulnerable populations such as individuals with psychotic and mood disorders.13,14 Psychopharmacological treatments prescribed to individuals with psychotic and mood disorders have been associated with metabolic complications that also contribute to WG.15 Conversely, weight loss (WL) is associated with improved cognition. For instance, a recent review highlighted a positive effect of WL on neurocognitive performance in individuals with OW/OB over time.16
The association of weight changes with neurocognitive and functional performance may be mediated by inflammatory processes.17 OB is associated with chronic low-grade inflammation, which in turn is commonly observed in cardio-metabolic and psychiatric disorders.18 Recent meta-analyses have identified that increased concentrations of pro-inflammatory biomarkers, including C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6), alongside oxidative stress and mitochondrial dysfunction are associated with neurocognitive and functional impairments in a large proportion of individuals with OB and related comorbidities.19,20 Similarly, a meta-analysis that tracked weight changes revealed that heightened inflammation is consistently associated with WG, such that individuals with more severe OB and elevated inflammatory biomarkers have poorer neurocognitive and functional outcomes than those with OB alone.21 Collectively, increasing evidence suggests that adiposity-related inflammatory processes lead to neurocognitive and functional impairments across psychiatric disorders and T2DM. Therefore, regardless of the primary condition, inflammatory changes associated with excess body fat/adiposity may represent a transdiagnostic component with relevant implications for WG prevention and OB management.
To our knowledge, no study to date has evaluated the contribution of inflammatory markers to the detection of weight changes in individuals with OB and comorbid psychiatric disorders and T2DM. Moreover, more studies need to examine the ability of inflammatory biomarkers to predict neurocognitive and functional performance in individuals stratified by WG/WL from a transdiagnostic and longitudinal perspective. The aims of the present study were three-fold: (a) to compare inflammatory biomarkers and neurocognitive and functional performance across individuals with psychiatric disorders and T2DM stratified by BMI, (b) to examine the usefulness of inflammatory biomarkers for detecting significant weight changes, and (c) to explore whether baseline inflammatory biomarkers were significant predictors of neurocognitive and functional performance at the 1-year follow-up.
Materials and methodsStudy design and ethical considerationsThis article is part of a project that aims to identify and validate peripheral biomarkers for neurocognitive deficits in major depressive disorder (MDD), bipolar disorder (BD), schizophrenia (SZ), and T2DM. This prospective comparative cohort study was conducted between April 2015 and January 2018 to investigate the association and evolution of specific peripheral blood biomarkers and neurocognitive impairments in a unique longitudinal cohort of individuals with somatic and psychiatric disorders. Demographic and clinical data, neurocognitive and functional data, and information on peripheral blood biomarkers were collected at baseline (T1) and after one year (T2). Individuals with psychiatric disorders were recruited from mental health units (MHUs) in several towns in the province of Valencia, Spain (Gandía, Foios, Catarroja, Paterna, and Sagunto); the psychiatry outpatient clinic and endocrinology department of the University Hospital Dr. Peset; and Miguel Servet MHU in Valencia City. Healthy controls (HCs) were residents of the same areas as those of individuals with SMI. Participants were demographically matched. All participants provided written informed consent after the study procedures were fully explained. The ethics committees and institutional review boards at each participating center approved the study protocol, and the study was conducted in accordance with the ethical principles of the Declaration of Helsinki. For this study, only variables related to the present study aims were included in the analyses.
ParticipantsSZ, BD, and MDD were diagnosed according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders 5.22 T2DM was diagnosed based on the Standards of Care criteria of the American Diabetes Association.23 Participants with MDD and BD were required to meet the remission criteria24 of an acute affective episode. Individuals with SZ were required to be clinically stable.25 Individuals with T2DM were required to be free of severe diabetic neuropathy and kidney disease (serum creatinine <1.5mg/dL). For recruitment as HCs, the absence of physical illness, pharmacological treatments, and family history of psychiatric disorders in first-degree relatives was required. The ability to understand the study procedures and willingness to provide written consent were required for participation. General exclusion criteria for all groups included current hospitalization; documented cognitive impairment not secondary to psychiatric disorders such as intellectual disability or major neurocognitive disorders, i.e., dementia; disability or inability that prevented understanding of the protocol; current substance use disorders (except for nicotine); pregnancy; intake of steroids, corticosteroids, antioxidants, antibiotics, and immunologic therapies; fever with temperature over 38°C; and history of vaccination within 4 weeks of the evaluation. The same inclusion and exclusion criteria were used at T1 and T2.
Anthropometric variables of height (m) and body weight (kg) were measured using calibrated scales. The exposure of interest was BMI, calculated as kilogram per meter squared, as a measure of overall OB. BMI, measured at study baseline, was examined continuously and categorically. Considering the World Health Organization (WHO) criteria, the following references were used: NW (≤24.9kg/m2), OW (25–29.9kg/m2), type 1 obesity (T1OB) (30–34.9kg/m2), and type 2 obesity (T2OB) (35–39.9kg/m2).26,27 Accordingly, participants with a BMI ≥25kg/m2 were defined as having OW and different OB degrees (type 1 and type 2), and the remaining participants were classified as having NW. None of the participants were classified as having type 3 obesity (T3OB), since the criterion of BMI ≥40.0kg/m2 was not met. Thus, a valid measure was obtained to analyze the objective condition of the study (exposure or not to OW/OB). Considering the revised criteria of the Guidelines for the Management of Overweight and Obesity in Adults, WG and WL were defined as the difference per year in kilograms. Values were calculated as the difference between weights measured at T1 and T2 and were categorized into “weight gain >3kg,” “weight loss <3kg,” and “stable weight of 0.1–2.99kg”.28
Clinical and neuropsychological assessmentsAssessments were conducted by the same experienced psychologists and psychiatrists of the research group. Sociodemographic data, including sex, age, years of education, dependent and occupational status, and motor laterality (defined as manual, ocular, and crural dominance), were collected.
Clinical evaluations were conducted using the following instruments: (i) Kaplan–Feinstein Scale (KFS),29 (ii) Charlson Comorbidity Index (CCI),30 (iii) 17-item Hamilton Rating Scale for Depression,31 (iv) Young Mania Rating Scale,32 (v) Positive and Negative Syndrome Scale,33 and (vi) Clinical Global Impression Scale.34 The total number of prescribed psychopharmacological medications and other medications was also registered.
Cognitive performance was evaluated using a comprehensive battery of neuropsychological tests and subtests previously used by our group (CB/07/09/0021). The battery included the premorbid Intelligence Quotient, which was calculated using the WAIS-III Vocabulary subtest, considered a classical measure of intelligence level before the onset of a mental disorder.35 Seven cognitive domains were assessed: (i) verbal learning and memory: Complutense Verbal Learning Test (TAVEC) for total immediate recall, short-term free recall, and long-term free recall variables36; (ii) cognitive flexibility: Stroop Color and Word test (SCWT) color/word subtest37 and Wisconsin Card Sorting Test categories for completed and perseverative errors38; (iii) verbal fluency: FAS and animal-naming test for phonemic and semantic fluency, respectively39; (iv) working memory: Trail-Making Test (TMT) Part B39 and Wechsler Adult Intelligence Scale III edition (WAIS-III) digit span backwards40; (v) short-term memory: TAVEC immediate recall of the first learning trial, immediate recall of the interference list,36 and WAIS-III digit span forward40; (vii) visual memory: Rey–Osterrieth Complex Figure Test (ROCFT), Figure 2minutes after the copy (fRey2) and 20minutes after the copy (fRey20)41; and (vii) processing speed: finger-tapping test left unimanual, right unimanual, left bimanual, and right bimanual and average of the four scores39,42; WAIS-III digit symbol coding subtest40; SCWT color and word subtests37; and TMT Part A.39 A global cognitive score (GCS) was calculated by averaging the seven cognitive domain scores.
Functional performance was evaluated using the Functional Assessment Short Test,43 Short Form-36 Health Survey questionnaire (SF-36),44 and World Health Organization Quality of Life Brief Scale (WHO-QoL-Bref).45 A global functional score (GFS) was calculated by averaging total scores on the three scales.
Determination of biomarkers in peripheral bloodVenous blood extraction was performed, and serum and plasma samples were stored in a freezer at −80°C. Serum cytokine concentrations were determined using Luminex® X-MAP technology (Luminex Corp., Austin, TX, USA) based on flow cytometry. The following cytokines were analyzed: IL-6, IL-10, and TNF-α. Sample processing and data analysis were performed according to the manufacturer's instructions. CRP levels were determined using an immunonephelometric assay (Behring Nephelometer II, Dade Behring, Inc., Newark, DE, USA). Oxidative stress in leukocytes was evaluated using fluorimetry techniques with a fluoroscan (Synergy MX). In total, 100,000 cells were plated in each well of 96-well plates and were incubated for 30min at 37°C with the corresponding fluorochromes, as follows: dichlorofluorescein diacetate to measure reactive oxygen species (ROS) production (485nm excitation, 535nm emission), MitoSOX to measure mitochondrial ROS (mROS) (510nm excitation, 580nm emission), tetramethylrodamin methyl ester to assess mitochondrial membrane potential (552nm excitation, 574nm emission), nonylacridin orange mitochondrial mass (495nm excitation, 519nm emission), and 5-chloromethylfluorescein diacetate to measure intracellular glutathione (492nm excitation, 517nm emission). The monocyte cell line U-937 was used as an internal control to avoid potential fluctuations in fluorescence over time. Serum lipid peroxidation levels were measured using a commercial thiobarbituric acid reactive substances (TBARS) kit according to the manufacturer's instructions (Olympus, Hamburg, Germany). A Luminex 200 flow analyzer system (Austin, TX, USA) was employed to analyze adhesion molecules in serum. To measure immunological markers, citrated blood samples were incubated with dextran (3%) for 45min to isolate human polymorphonuclear leukocytes (PMNs). The supernatant was layered over Ficoll–Hypaque (GE Healthcare, Barcelona, Spain) and centrifuged for 25min at room temperature at 650× g. Lysis buffer was added to erythrocytes remaining in the pellet, which were incubated at room temperature for 5min and then spun at 240× g for 5min. PMNs were isolated. A 1.2-mL aliquot of PMNs was obtained from the peripheral blood of HCs and patients at a density of 106cells/mL in complete RPMI (RPMI 1640 medium supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, 1% glutamine, and 1% sodium pyruvate). Prior to this, primary cultures of human umbilical cord endothelial cells (HUVECs) were established. HUVECs were isolated. On the day of experimentation, PMNs were monitored through the endothelial monolayer at a speed of 0.3mL/min over a 5-min period. Activity was recorded, and the number, velocity, and adhesion to the endothelial monolayer of rolling PMNs were determined. The number of rolling PMNs was measured as those rolling for 1min. Velocity was assessed by determining the time in which 15 rolling PMNs covered 100μm. Adhesion was analyzed by counting the number of PMNs adhering to the endothelium for at least 30s in five fields. Platelet (PLT) levels were evaluated to be within the normal range in each participant at baseline and at the 1-year follow-up (150–400×109/L).
Statistical analysesData were analyzed using Statistical Package for Social Sciences version 26.0 for Windows.46 The sample size was calculated using Ene 2.0 software, which estimated that twenty-five individuals for each sample group were sufficient to ensure the representativeness.47 Descriptive analyses were conducted using a one-way analysis of variance (ANOVA) for continuous variables and the chi-square test for categorical variables. Differences between groups in neurocognitive and functional performance and biomarkers at T1 and T2 and their evolution over time were assessed using a mixed one-way analysis of covariance (ANCOVA), with diagnosis (psychiatric disorders and T2DM) and sex as co-variables. Normality was assumed for all continuous variables because the sample was sufficiently representative of the target population, which was statistically verified. This guaranteed that the variable groups for T1 and T2 could be assessed using ANOVA/ANCOVA. A post hoc analysis with Bonferroni-corrected pairwise t-test and the Mann–Whitney U were performed to examine differences between groups. The effect size was calculated with partial eta-squared (η2p), and the following values were used as reference: small=0.02, moderate=0.15, and large=0.35. The direct scores obtained for GCS and GFS were transformed into Z-scores. For calculation of Z-scores, the mean and standard deviation of individuals with NW at T1 were used as reference values. To test the predictive ability of biomarkers at T1 to discriminate individuals with significant WG/WL over time, binary logistic regression was performed using a predictive model that only included biomarkers that were significant for each group based on WG/WL. Similarly, to test the predictive capacity of biomarkers at baseline to explain the variance in neurocognitive and functional performance over time, linear regression analysis was performed using a predictive model that only included biomarkers and BMI that were significant for each group. Other variables relevant to neurocognitive and functional performance were not included because they were not the focus of this study, and the biomarkers were considered optimal predictors per se. For all analyses, p<0.05 indicated statistical significance. The procedure to generate the predictive models was performed as follows. First, a predictive analysis was performed with single biomarkers, then predictive models were generated that included and combined variables that were statistically more powerful. Finally, the optimal predictive combination was obtained. No more than five variables were included in each model, thus guaranteeing correct performance of the analysis.
ResultsSample descriptionAt T1, the sample consisted of 165 individuals, including 30 with SZ, 42 with BD, 35 with MDD, 30 with T2DM, and 28 HCs. The total sample was classified into four groups according to BMI categories, comprising 40 in the NW group (T2DM=3, MDD=9, BD=9, SZ=2, and HC=17), 53 in the OW group (T2DM=12, MDD=12, BD=15, SZ=8, and HC=6), 42 in the T1OB group (T2DM=9, MDD=9, BD=10, SZ=11, and HC=3), and 30 in the T2OB group (T2DM=6, MDD=5, BD=8, SZ=9, and HC=2). A summary of the baseline main outcomes for each diagnostic group (T2DM, MDD, BD, SZ, and HC) is presented in supplementary material 1.
In total, 40 participants were lost to follow-up at T2 (retention rate: 75.7%). The sample consisted of 27 individuals in the NW group (T2DM=1, MDD=6, BD=6, SZ=1, and HC=13), 43 in the OW group (T2DM=12, MDD=5, BD=12, SZ=9, and HC=5), 31 in the T1OB group (T2DM=8, MDD=8, BD=7, SZ=8, and HC=0), and 24 in the T2OB group (T2DM=4, MDD=6, BD=4, SZ=9, and HC=1).
Additionally, the sample was divided into three groups based on WG/WL over follow-up, comprising 34 individuals with WG (T2DM=6, MDD=8, BD=7, SZ=8, and HC=5), 24 individuals with WL (T2DM=2, MDD=6, BD=7, SZ=9, and HC=0) and 67 individuals with stable weight (SW) (T2DM=17, MDD=11, BD=15, SZ=10, and HC=14).
There were no differences by primary diagnosis (T2DM, MDD, BD, and SZ) in any of the groups related to obesity ([NW, OW, T1OB, and T2OB] and [WG, WL, and SW]) and the interaction between primary diagnosis and obesity was non-significant.
A summary of the baseline sociodemographic and clinical characteristics of participants in each group (NW, OW, T1OB, and T2OB) is presented in supplementary material 2. Of the total sample, 48% were women. The mean age of the total sample was 46.2 (SD: 12.3) years. The percentage of women was significantly higher in the NW group other groups. Age, years of education, dependent status, occupational status, and laterality were similar among groups. The NW group exhibited significantly lower levels of multimorbidity as measured with the KFS and CCI as well as lower prescriptions of psychopharmacological and non-psychopharmacological medications.
Between-group comparisons of neurocognitive and functional performanceNeurocognitive and functional performance of the four groups at T1 and T2 is presented in Table 1. Neurocognitive performance was significantly higher in the NW group than in the T1OB and T2OB groups at T1 (p<0.01; η2p=0.08) and in the OW group at T2 (p<0.01; η2p=0.09). Individuals with NW exhibited significantly higher functional performance than individuals with T2OB at T1 (p<0.01; η2p=0.06) and individuals with T1OB at T2 (p<0.001; η2p=0.12). Functional performance was significantly higher in individuals with OW than in those with T2OB (p<0.001; η2p=0.12). At both assessments, small-to-moderate effect sizes were observed for neurocognitive and functional performance in between-group comparisons. No significant within-group differences were observed in neurocognitive and functional performance over time.
Neurocognitive and functional performance at T1 and T2.
| Variablesa | NW | OW | T1OB | T2OB | Statistical analyses | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1(n=40) | T2(n=27) | T1(n=53) | T2(n=43) | T1(n=42) | T2(n=31) | T1(n=30) | T2(n=24) | T1(p)b | Post hoc testc | η2pd | T2(p)b | Post hoc testc | η2pd | |
| GCS | 0.0(1.0) | 0.1(0.9) | −0.5(1.2) | −0.6(1.1) | −0.9(1.1) | −0.7(0.9) | −0.9(1.2) | −0.7(1.1) | 5.0* | T2OB,T1OB | .08 | 4.2* | T2OB,T1OB,OW | .09 |
| GSFS | 0.0(1.0) | 0.1(0.8) | −0.3(0.9) | −0.1(0.8) | −0.3(1.0) | −0.4(1.1) | −0.6(1.1) | −0.8(1.2) | 3.4* | T2OB | .06 | 5.7** | T2OB,T1OBT2OB | .12 |
Abbreviations: T1: time 1; T2: time 2; NW: normal-weight; OW: overweight; T1OB: type 1 obesity; T2OB: type 2 obesity; GCS: global cognitive score; GSFS: global functional score; NS: not significant (NS=p>0.05; *p≤0.01; **p≤0.001). Effect size (η2p: small≈0.02; moderate≈0.15; large≈0.35).
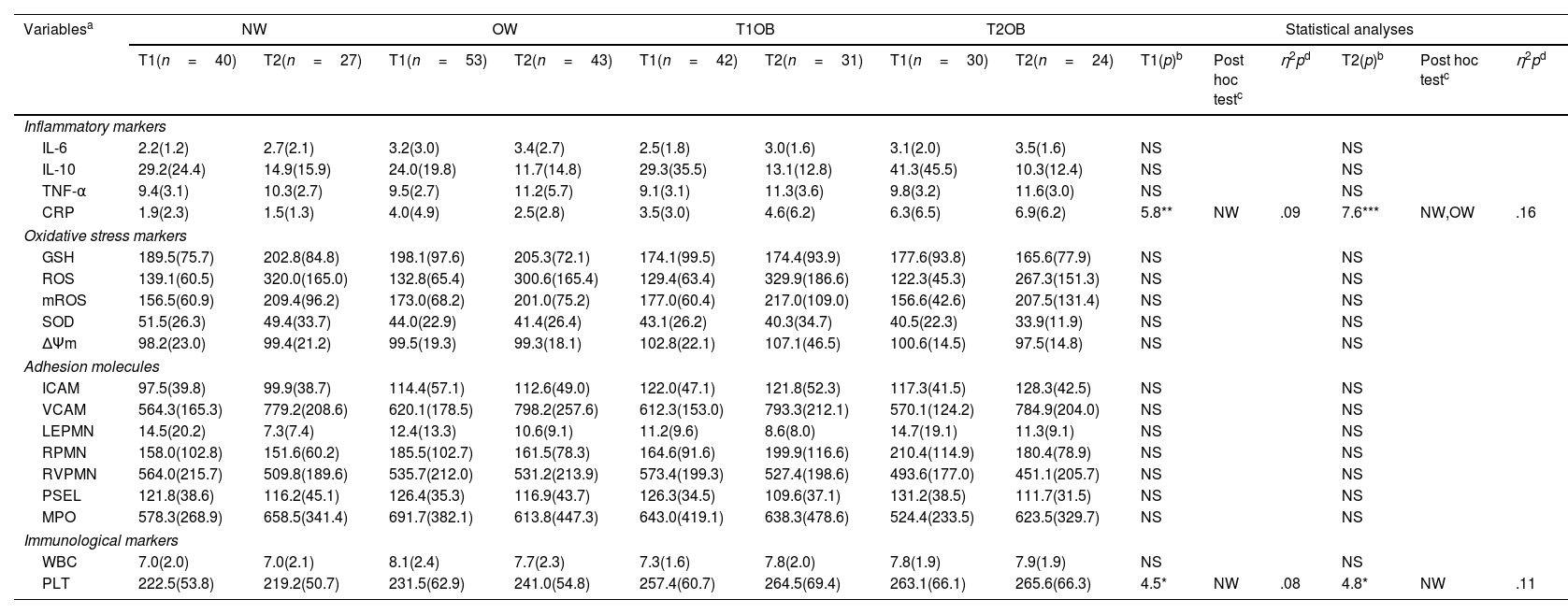
Levels of CRP were significantly higher in the T2OB group than in the NW group at T1 (p<0.001; η2p=0.09) and in the OW group at T2 (p<0.0001; η2p=0.16) (Table 2). Similarly, PLT concentrations were significantly higher in the T1OB and T2OB groups than in the NW group at both assessments (p<0.01; η2p=0.08–0.11). In all cases, the effect sizes were small-to-moderate. No significant within-group differences were noted over time.
Biomarkers at T1 and T2.
| Variablesa | NW | OW | T1OB | T2OB | Statistical analyses | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1(n=40) | T2(n=27) | T1(n=53) | T2(n=43) | T1(n=42) | T2(n=31) | T1(n=30) | T2(n=24) | T1(p)b | Post hoc testc | η2pd | T2(p)b | Post hoc testc | η2pd | |
| Inflammatory markers | ||||||||||||||
| IL-6 | 2.2(1.2) | 2.7(2.1) | 3.2(3.0) | 3.4(2.7) | 2.5(1.8) | 3.0(1.6) | 3.1(2.0) | 3.5(1.6) | NS | NS | ||||
| IL-10 | 29.2(24.4) | 14.9(15.9) | 24.0(19.8) | 11.7(14.8) | 29.3(35.5) | 13.1(12.8) | 41.3(45.5) | 10.3(12.4) | NS | NS | ||||
| TNF-α | 9.4(3.1) | 10.3(2.7) | 9.5(2.7) | 11.2(5.7) | 9.1(3.1) | 11.3(3.6) | 9.8(3.2) | 11.6(3.0) | NS | NS | ||||
| CRP | 1.9(2.3) | 1.5(1.3) | 4.0(4.9) | 2.5(2.8) | 3.5(3.0) | 4.6(6.2) | 6.3(6.5) | 6.9(6.2) | 5.8** | NW | .09 | 7.6*** | NW,OW | .16 |
| Oxidative stress markers | ||||||||||||||
| GSH | 189.5(75.7) | 202.8(84.8) | 198.1(97.6) | 205.3(72.1) | 174.1(99.5) | 174.4(93.9) | 177.6(93.8) | 165.6(77.9) | NS | NS | ||||
| ROS | 139.1(60.5) | 320.0(165.0) | 132.8(65.4) | 300.6(165.4) | 129.4(63.4) | 329.9(186.6) | 122.3(45.3) | 267.3(151.3) | NS | NS | ||||
| mROS | 156.5(60.9) | 209.4(96.2) | 173.0(68.2) | 201.0(75.2) | 177.0(60.4) | 217.0(109.0) | 156.6(42.6) | 207.5(131.4) | NS | NS | ||||
| SOD | 51.5(26.3) | 49.4(33.7) | 44.0(22.9) | 41.4(26.4) | 43.1(26.2) | 40.3(34.7) | 40.5(22.3) | 33.9(11.9) | NS | NS | ||||
| ΔΨm | 98.2(23.0) | 99.4(21.2) | 99.5(19.3) | 99.3(18.1) | 102.8(22.1) | 107.1(46.5) | 100.6(14.5) | 97.5(14.8) | NS | NS | ||||
| Adhesion molecules | ||||||||||||||
| ICAM | 97.5(39.8) | 99.9(38.7) | 114.4(57.1) | 112.6(49.0) | 122.0(47.1) | 121.8(52.3) | 117.3(41.5) | 128.3(42.5) | NS | NS | ||||
| VCAM | 564.3(165.3) | 779.2(208.6) | 620.1(178.5) | 798.2(257.6) | 612.3(153.0) | 793.3(212.1) | 570.1(124.2) | 784.9(204.0) | NS | NS | ||||
| LEPMN | 14.5(20.2) | 7.3(7.4) | 12.4(13.3) | 10.6(9.1) | 11.2(9.6) | 8.6(8.0) | 14.7(19.1) | 11.3(9.1) | NS | NS | ||||
| RPMN | 158.0(102.8) | 151.6(60.2) | 185.5(102.7) | 161.5(78.3) | 164.6(91.6) | 199.9(116.6) | 210.4(114.9) | 180.4(78.9) | NS | NS | ||||
| RVPMN | 564.0(215.7) | 509.8(189.6) | 535.7(212.0) | 531.2(213.9) | 573.4(199.3) | 527.4(198.6) | 493.6(177.0) | 451.1(205.7) | NS | NS | ||||
| PSEL | 121.8(38.6) | 116.2(45.1) | 126.4(35.3) | 116.9(43.7) | 126.3(34.5) | 109.6(37.1) | 131.2(38.5) | 111.7(31.5) | NS | NS | ||||
| MPO | 578.3(268.9) | 658.5(341.4) | 691.7(382.1) | 613.8(447.3) | 643.0(419.1) | 638.3(478.6) | 524.4(233.5) | 623.5(329.7) | NS | NS | ||||
| Immunological markers | ||||||||||||||
| WBC | 7.0(2.0) | 7.0(2.1) | 8.1(2.4) | 7.7(2.3) | 7.3(1.6) | 7.8(2.0) | 7.8(1.9) | 7.9(1.9) | NS | NS | ||||
| PLT | 222.5(53.8) | 219.2(50.7) | 231.5(62.9) | 241.0(54.8) | 257.4(60.7) | 264.5(69.4) | 263.1(66.1) | 265.6(66.3) | 4.5* | NW | .08 | 4.8* | NW | .11 |
Abbreviations: T1: time 1; T2: time 2; NW: normal-weight; OW: overweight; T1OB: type 1 obesity; T2OB: type 2 obesity; IL-6: interleukin-6; IL-10: interleukin-10; TNF-α: tumor necrosis factor-alpha; CRP: C-reactive protein; GSH: glutathione; ROS: reactive oxygen species; mROS: mitochondrial reactive oxygen species; SOD: superoxide dismutase; ΔΨm: mitochondrial membrane potential; CAM: cellular adhesion molecule; PMN: polymorphonuclear cells; I: inter; V: vascular; LE: leukocyte–endothelium adhesion; R: rolling; RV: rolling velocity; PSEL: P-selectin; MPO: myeloperoxidase; WBC: white blood cell; PLT: blood platelets; NS: not significant (NS=p>0.05; *p≤0.01; **p≤0.001; ***p≤0.0001). Effect size (η2p: small≈0.02; moderate≈0.15; large≈0.35).
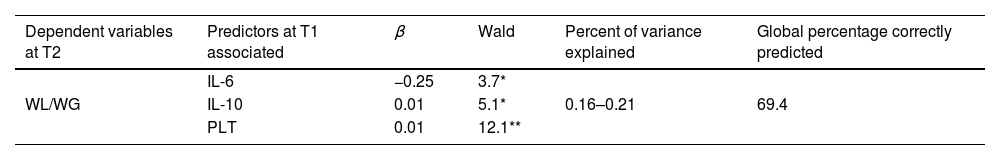
The ability of inflammatory biomarkers at T1 to detect significant weight changes at T2 was analyzed, and the results are presented in Table 3. The combination of two interleukins (IL-6 and IL-10) and PLTs resulted in a model that best discriminated individuals with WG/WL at T2, with a correct classification rate of 69.4%. There was a strong positive relationship between IL-10 (p<0.05) and PLT (p<0.0001) and a negative correlation with IL-6 (p<0.05).
Discriminatory capacity of baseline inflammatory markers to detecting significant weight changes.
| Dependent variables at T2 | Predictors at T1 associated | β | Wald | Percent of variance explained | Global percentage correctly predicted |
|---|---|---|---|---|---|
| WL/WG | IL-6 | −0.25 | 3.7* | 0.16–0.21 | 69.4 |
| IL-10 | 0.01 | 5.1* | |||
| PLT | 0.01 | 12.1** |
Abbreviations: T1: time 1; T2: time 2; WL: weight loss; WG: weight gain; IL-6: interleukin-6; IL-10: interleukin-10; PLT: blood platelets; NS: not significant (NS=p>0.05; *p≤0.05; **p≤0.0001).
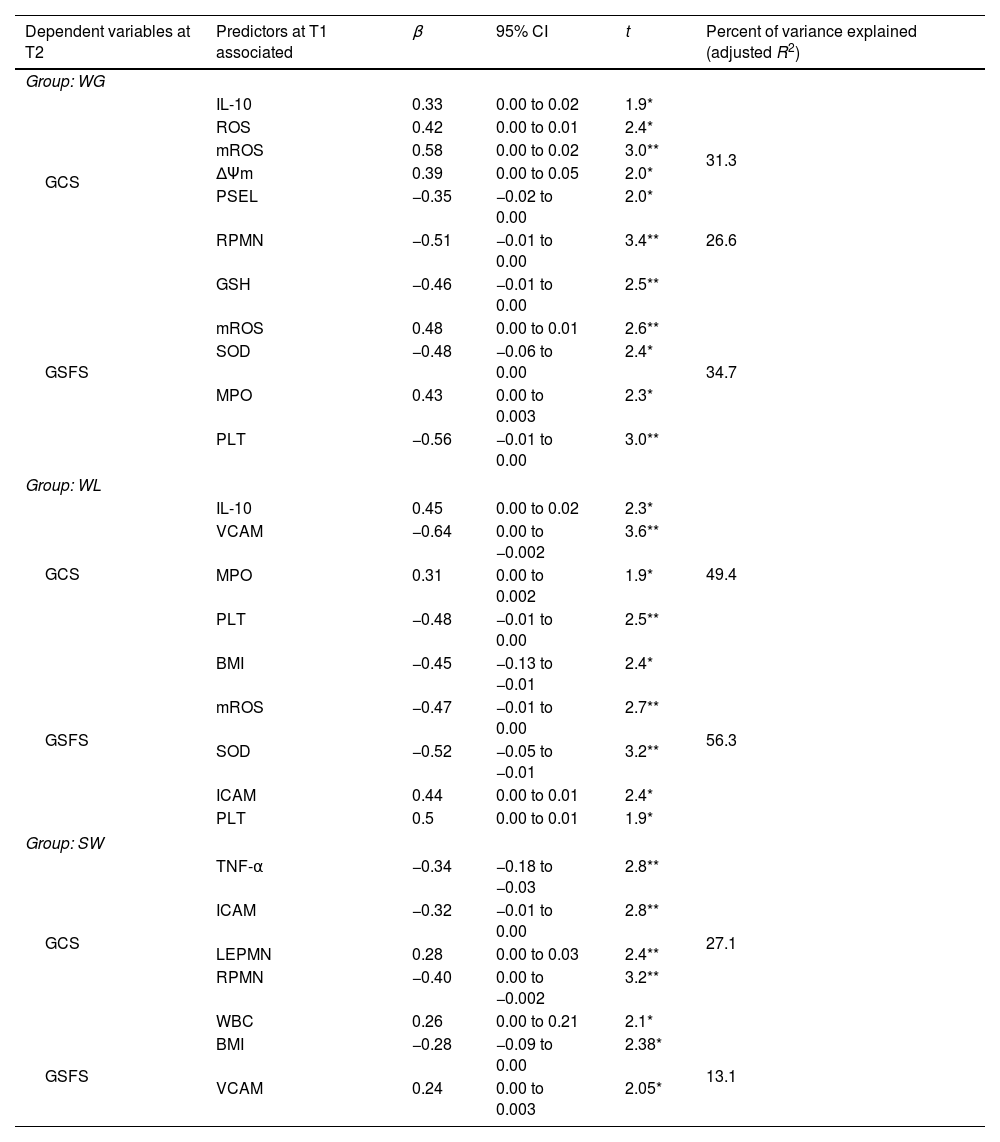
The results of the relative contributions of inflammatory markers at T1 to explain the variation in GCS and GFS scores at T2 are presented in Table 4. For individuals with WG, the combination of anti-inflammatory (IL-10) and oxidative stress biomarkers (ROS), mitochondrial reactive oxygen species (mROS), mitochondrial membrane potential (ΔΨm), and adhesion molecules (P-selectin [PSEL]) significantly predicted GCS at T2 and explained 31.3% of the variance. Of note, rolling polymorphonuclear cells (RPMNs) alone significantly predicted GCS at T2 and explained 26.6% of the variance. Moreover, oxidative stress biomarkers (glutathione [GSH], mROS, superoxide dismutase [SOD]), adhesion molecules (myeloperoxidase [MPO]), and PLT significantly predicted GFS at T2 and explained 34.7% of the variance.
Predictive biomarkers at T1 of neurocognitive and functional performance at T2.
| Dependent variables at T2 | Predictors at T1 associated | β | 95% CI | t | Percent of variance explained (adjusted R2) |
|---|---|---|---|---|---|
| Group: WG | |||||
| GCS | IL-10 | 0.33 | 0.00 to 0.02 | 1.9* | 31.3 |
| ROS | 0.42 | 0.00 to 0.01 | 2.4* | ||
| mROS | 0.58 | 0.00 to 0.02 | 3.0** | ||
| ΔΨm | 0.39 | 0.00 to 0.05 | 2.0* | ||
| PSEL | −0.35 | −0.02 to 0.00 | 2.0* | ||
| RPMN | −0.51 | −0.01 to 0.00 | 3.4** | 26.6 | |
| GSFS | GSH | −0.46 | −0.01 to 0.00 | 2.5** | 34.7 |
| mROS | 0.48 | 0.00 to 0.01 | 2.6** | ||
| SOD | −0.48 | −0.06 to 0.00 | 2.4* | ||
| MPO | 0.43 | 0.00 to 0.003 | 2.3* | ||
| PLT | −0.56 | −0.01 to 0.00 | 3.0** | ||
| Group: WL | |||||
| GCS | IL-10 | 0.45 | 0.00 to 0.02 | 2.3* | 49.4 |
| VCAM | −0.64 | 0.00 to −0.002 | 3.6** | ||
| MPO | 0.31 | 0.00 to 0.002 | 1.9* | ||
| PLT | −0.48 | −0.01 to 0.00 | 2.5** | ||
| GSFS | BMI | −0.45 | −0.13 to −0.01 | 2.4* | 56.3 |
| mROS | −0.47 | −0.01 to 0.00 | 2.7** | ||
| SOD | −0.52 | −0.05 to −0.01 | 3.2** | ||
| ICAM | 0.44 | 0.00 to 0.01 | 2.4* | ||
| PLT | 0.5 | 0.00 to 0.01 | 1.9* | ||
| Group: SW | |||||
| GCS | TNF-α | −0.34 | −0.18 to −0.03 | 2.8** | 27.1 |
| ICAM | −0.32 | −0.01 to 0.00 | 2.8** | ||
| LEPMN | 0.28 | 0.00 to 0.03 | 2.4** | ||
| RPMN | −0.40 | 0.00 to −0.002 | 3.2** | ||
| WBC | 0.26 | 0.00 to 0.21 | 2.1* | ||
| GSFS | BMI | −0.28 | −0.09 to 0.00 | 2.38* | 13.1 |
| VCAM | 0.24 | 0.00 to 0.003 | 2.05* | ||
Abbreviations: T1: time 1; T2: time 2; WG: weight gain; WL: weight loss; SW: stable weight; GCS: global cognitive score; GSFS: global functional score; IL-10: interleukin-10; TNF-α: tumor necrosis factor-alpha; GSH: glutathione; ROS: reactive oxygen species; mROS: mitochondrial reactive oxygen species; SOD: superoxide dismutase; ΔΨm: mitochondrial membrane potential; CAM: cellular adhesion molecule; PMN: polymorphonuclear cells; I: inter; V: vascular; LE: leukocyte–endothelium; R: rolling; PSEL: P-selectin; MPO: myeloperoxidase; PLT: blood platelets; NS: not significant (*p≤0.05; **p≤0.01).
Similarly, in the WL group, the combination of anti-inflammatory (IL-10) biomarker, adhesion molecules (vascular cellular adhesion molecule [VCAM] and MPO), and PLT significantly predicted GCS at T2 and explained 49.4% of the variance. Moreover, BMI, oxidative stress biomarkers (mROS and SOD), and adhesion molecules (intercellular adhesion molecule [ICAM] and PLT) significantly predicted GFS at T2 and explained 56.3% of the variance.
In the SW group, the combination of pro-inflammatory (TNF-α) biomarkers, adhesion molecules (ICAM, leukocyte–endothelium adhesion polymorphonuclear cells [LEPMN], and RPMN), and WBC explained 27.1% of the GCS variance at T2, while the combination of BMI and VCAM explained 13.1% of the GFS variance at T2.
DiscussionTo our knowledge, this is the first study to evaluate the predictive validity of inflammatory and oxidative stress biomarkers for neurocognitive and functional performance and their discriminatory ability to detect significant weight changes in individuals with psychiatric disorders and T2DM stratified by BMI using a longitudinal design and transdiagnostic perspective.
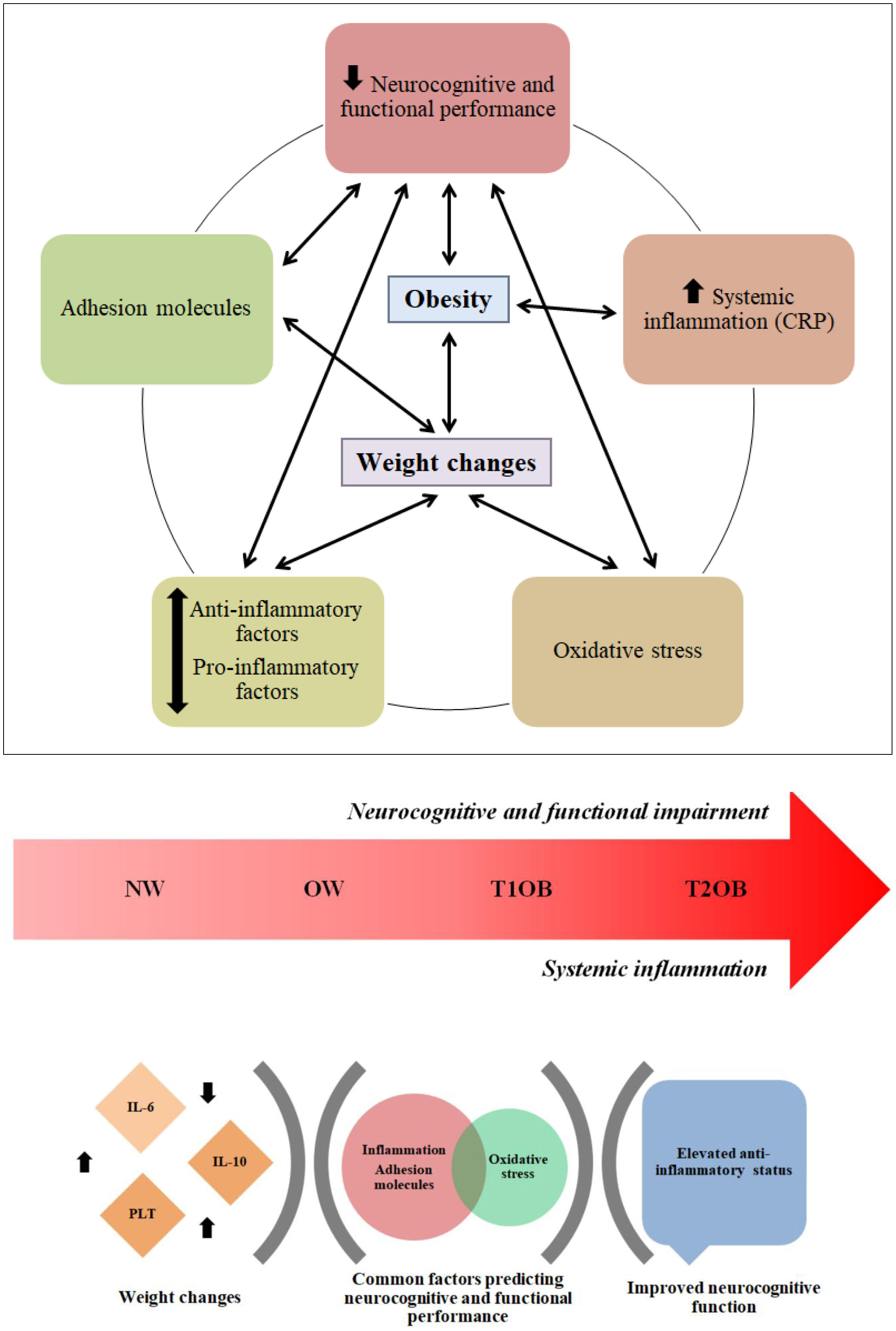
Individuals with OB exhibited an overall decrease in neurocognitive and functional performance alongside an increase in systemic inflammation. Increased anti-inflammatory and decreased pro-inflammatory factors were critical for detecting significant weight changes over time, considering different degrees of OB. Regarding WG/WL, inflammatory biomarkers, oxidative stress, and adhesion molecules were common factors for predicting the neurocognitive and functional performance of individuals with T2DM and psychiatric disorders, with higher reliability in the WL group. Specifically, elevated anti-inflammatory status significantly predicted improved neurocognitive function in individuals with WG/WL (Fig. 1).
Our findings build upon growing evidence suggesting that OB, T2DM, and psychiatric disorders share several pathogenic pathways, including adiposity-related systemic inflammation.3,5 The intricate relationship between weight status and inflammatory processes may influence neurocognitive and functional performance. The results of our study converge with previous findings suggesting that the chronic low-grade inflammation characteristic of OB increases the risk of neurocognitive and functional impairments over time.17,18 According to our findings, extant evidence indicates that inflammatory factors such as IL-6 and TNF-α modulate weight changes in individuals with psychiatric disorders and T2DM.19,48 Furthermore, the association between WG and systemic inflammation may increase the risk of neurocognitive and functional decline in individuals with OW/OB.21 Recent evidence suggests that better neurocognitive performance of individuals with OB is associated with WL and decreased inflammation.12 Therefore, from a transdiagnostic and comorbidity perspective, our findings provide evidence of overlapping inflammatory pathways that influence weight changes and lead to neurocognitive and functional impairments in OB.
Healthy lifestyles and nutritional strategies are associated with a lower frequency of WG, improved neurocognitive performance, and better functional outcomes.49 In our study, lower weight was associated with better neurocognitive and functional outcomes, and adiposity-related inflammatory processes potentially mediated this relationship. Mechanistically, the prevention and management of weight changes may contribute to normalizing adiposity-related inflammatory processes.50,51 Accordingly, an obesogenic diet rich in saturated fat and refined sugars is associated with systemic inflammation, leading to dysregulation of brain circuits involved in appetitive behaviors, thereby promoting WG.52 Thus, treatments to reduce systemic inflammation may improve neurocognitive and functional performance and reduce the risk of morbimortality in individuals with OB.53,54 In this regard, weight-loss interventions, including regular exercise, healthy lifestyles, and bariatric surgery, have been reported to reduce low-grade inflammation in OB and improve neurocognitive performance simultaneously.55
This study has some limitations. Although BMI is the most common measure of OB, it does not fully represent body fat distribution. More reliable anthropometric measures, such as waist circumference or waist-to-hip ratio, may have been preferable. The present study did not evaluate other parameters involved in OB pathophysiology, such as metabolic disruptions, changes in the microbiota gut–brain axis, and vascular dysfunction biomarkers. Moreover, after one year of follow-up, high sample mortality was observed. This may have led to a potential bias in retaining individuals who completed the assessments and were thus in a presumably better clinical condition. Despite these limitations, the strengths of this study include its novel transdiagnostic approach and comprehensive assessment of cognitive and functional outcomes in individuals stratified by BMI across populations with T2DM and psychiatric disorders. Moreover, the longitudinal design permits the establishment of potential causal relationships between adiposity-related inflammation and neurocognitive and functional outcomes. Finally, the study's multicenter nature increases the results’ external validity.
In conclusion, OB is associated with neurocognitive and functional impairments. The mechanisms underlying this association may involve the exacerbation of inflammatory pathways. Aberrant cellular adhesion molecule-related mechanisms in OB may trigger a pro-inflammatory response, impairing neurocognitive performance. Oxidative damage also plays a crucial role in maintaining inflammatory processes.19,56 A deeper understanding of shared pathways underscoring the link between OB, T2DM, and psychiatric disorders may contribute to the development of transdiagnostic anti-inflammatory therapeutic strategies for these disorders. For instance, a randomized cross-over trial reported a significant positive relationship between elevated cerebral blood flow and improved neurocognition following non-pharmacological, short-term supplementation with a ketone monoester.57 These data provide new clinical approaches for OB phenomenology in the context of cardio-metabolic and psychiatric disorders. The combined use of several biomarkers may facilitate the identification of individuals with an increased risk of developing OB and improve the management of specific patient subgroups with cardio-metabolic and psychiatric comorbidities. We propose that managing the biological bases of OB may contribute to maintaining healthy eating behaviors while identifying clinical phenotypes associated with different neurocognitive and functional trajectories.58 Therefore, combinations of inflammatory, oxidative stress and adhesion molecule biomarkers hold promise for improving innovative and personalized treatment strategies targeting cardio-metabolic and inflammatory processes in individuals with T2DM and psychiatric disorders with comorbid OB.
Authors’ contributionsJVS-O, VB-M, PC-G, VMV and RT-S: conception and design of the study; acquisition and analysis of data; drafting the manuscript and figures. GS-V, CS-M, IE-L, AH-M, JV-L, and BC-F: drafting the manuscript and figures. JV-F and RM-B: Formal analysis. All authors have read and agreed to the published version of the manuscript.
FundingThis work was supported by: Carlos III Health Institute (ISCIII) grants [grant number PI19/0838]. European Regional Development Fund (ERDF “A way to build Europe”) and Ministry of Education of the Valencian Regional Government [grant number PROMETEO/CIPROM/2022/58]. FISABIO [grant number UGP-14-184 Project]. Ministry of Science and Innovation [grant number PID2021-129099OB-I00].
Conflicts of interestNone declared.
We would like to thank the research participants and staff members of the mental health units in Foios, Catarroja, Paterna, Sagunto, and Gandía towns, and the psychiatry outpatient clinic of the University Hospital Dr. Peset and mental health center Miguel Servet, Valencia City.