Neuropsychiatric and neurodegenerative disorders are frequently associated with gastrointestinal (GI) co-pathologies. Although the central and enteric nervous systems (CNS and ENS, respectively) have been studied separately, there is increasing interest in factors that may contribute to conditions affecting both systems. There is compelling evidence that serotonin (5-HT) may play an important role in several gut–brain disorders. It is well known that 5-HT is essential for the development and functioning of the CNS. However, most of the body's 5-HT is produced in the GI tract. A deeper understanding of the specific effects of enteric 5-HT on gut–brain disorders may provide the basis for the development of new therapeutic targets. This review summarizes current data focusing on the important role of 5-HT in ENS development and motility, with particular emphasis on novel aspects of 5-HT signaling in conditions where CNS and ENS comorbidities are common, such as Parkinson's disease and depressive disorders.
Serotonin (5-hydroxytryptamine, 5-HT) is an evolutionarily ancient molecule found across all domains of life.1–3 Although it was first detected in the gastrointestinal (GI) tract of rabbits,4 its subsequent discovery in the central nervous system (CNS) quickly attracted attention due to its key role in brain development, sleep–wake state, appetite, mood, as well as in many mental states such as anxiety, depression, impulsivity, among others.5,6 However, 5-HT is increasingly recognized for its paracrine and endocrine actions in the gut, where approximately 95% of the body's 5-HT is produced.7,8 Indeed, enteric 5-HT modulates the enteric nervous system (ENS) development and neurogenesis, motility, secretion, inflammation, sensation and epithelial development.9–11 Interest in the role of 5-HT in brain and gut disorders has also increased in recent years. Some of them include depressive disorders and Parkinson's disease (PD), both of which have a high comorbidity with GI dysfunction.12 Despite this, the precise role of 5-HT in such gut–brain disorders remains elusive.
One way in which 5-HT may affect brain and gut function is through its ability to influence the gut–brain axis. The gut–brain axis is defined as the bidirectional communication between the CNS and ENS.13 This interaction involve the brain modulation of GI functions such as immune responses, secretion, and motility, as well as the transmission of sensations (e.g., nausea) to the CNS.14 Interactions between the CNS and ENS occur at neural, endocrine, immune, and metabolic levels15 and can also be strongly influenced by the gut microbiome.16,17 Dysregulation of the microbiome–gut–brain axis has been implicated in the etiology of several disease processes in which CNS and GI dysfunction often coexist. It is likely that overlapping functional and developmental processes affected by 5-HT in the CNS (e.g., mood and cognition)18 and the gut (e.g., motility)9 may also occur simultaneously, manifesting as concurrent gut–brain disorders.
In this review, we will provide an insight into the role of 5-HT in ENS development and function. We will focus on novel aspects of 5-HT signaling in disorders where CNS and ENS comorbidities are common, such as PD and depression. As gut microbiota can modulate host 5-HT system and 5-HT gut–brain axis functions, we will summarize how these interactions may contribute to the progression of PD and depression. Finally, we will discuss how mechanistic findings into the 5-HT gut–brain pathway may lead to the development of new therapeutic options for PD and depression, as well as other disorders involving the gut–brain axis.
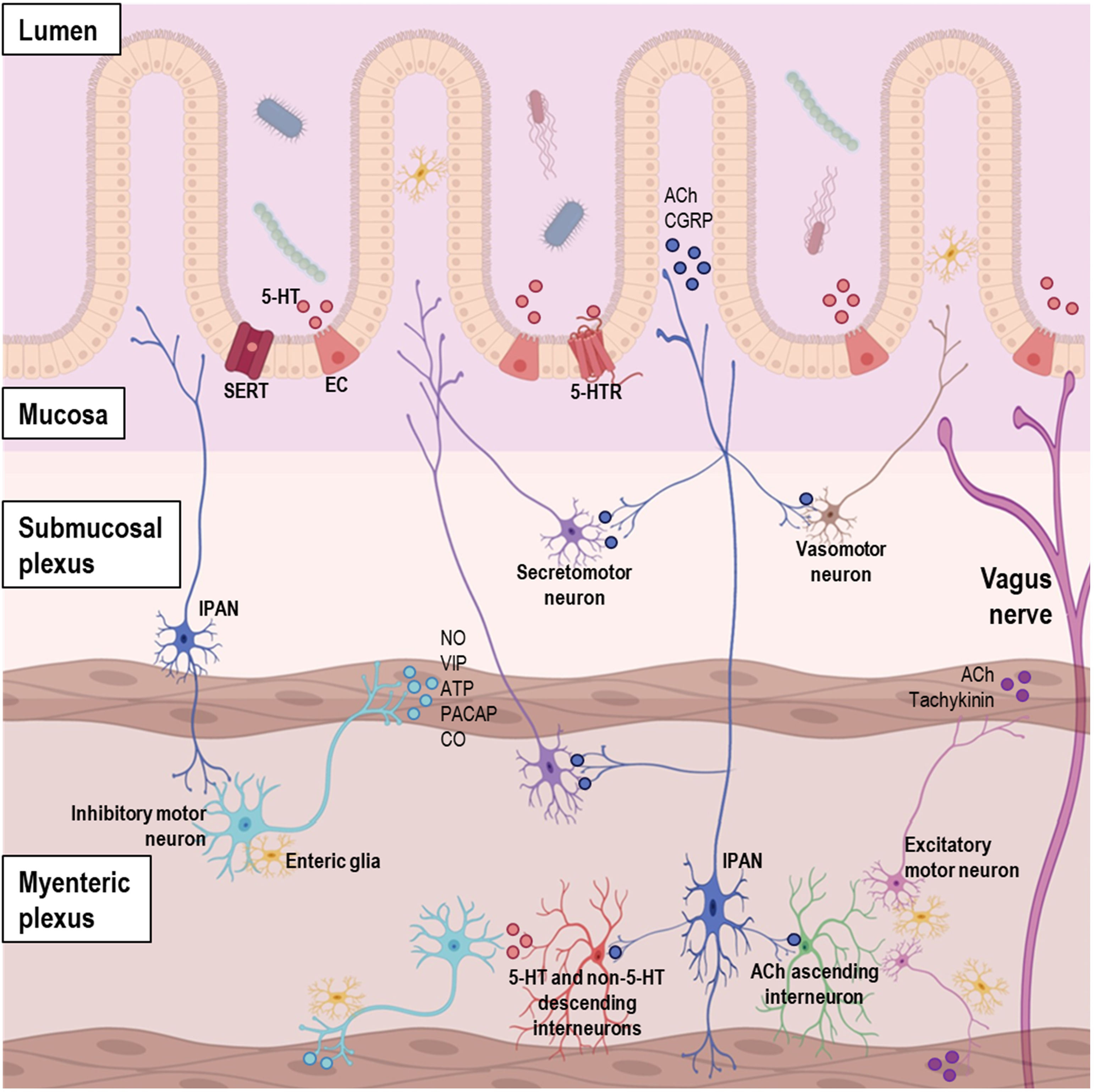
Cellular organization and physiological function of the ENSThe GI tract is innervated by an extensive intrinsic network of ganglion-rich nerve connections known as the ENS.19 The ENS, together with the sympathetic and parasympathetic nervous system, is one of the main divisions of the autonomic nervous system. The human ENS contains about 400–600 million neurons (∼100 million neurons in mouse ENS), which are found in two major networks, the myenteric and submucosal plexuses, also known as Auerbach's and Meissner's plexus, respectively.20 Often referred to as the “second brain”, ENS plays a crucial role in the regulation of digestive processes, intestinal motility, and barrier defense.21
Although the ENS receives CNS input via the vagus nerve and the thoracolumbar and lumbosacral spinal cord, it may act independently of the CNS.19 The ENS regulates peristaltic, secretory and immunological functions, as well as more complex processes such as non-propulsive mixing or segmentation, slow orthograde propulsion via the migrating myoelectric complex, retropulsion of noxious substances, modification of nutrient handling and alteration of local blood flow.22 The ENS also comprises enteric glial cells, which maintain the integrity of the epithelial barrier and play a role in gut inflammation and interaction with the microbiome.23 Emerging data show that enteric glia is one of the most dynamic signaling components of the ENS and have challenged the concept of enteric glia as passive cells. Rapid and reciprocal communication between glial and neuronal cells in the gut controls enteric reflexes24–26 and communication between extrinsic and intrinsic neurons24 that innervate the GI tract. Similarly, enteric glia contributes to several disease processes, including neuroinflammation, cancer, and infection,24,26 in which glia acquire pro-inflammatory or pro-tumorigenic phenotypes. In these cases, both loss and gain of glial function contribute to altered ENS homeostasis and GI pathophysiology. Indeed, it has been reported that neuroinflammatory circuits may be involved in PD, such as activation of type 2 innate lymphoid cells in GI tract.27,28
The ENS consists of up to 20 different types of neurons, including motor neurons, interneurons, and intrinsic primary afferent neurons (IPANs) (Fig. 1).19,29,30 These neurons are further classified based on their morphological, electrical, neurochemical, and functional properties. The main transmitters involved in the different neuronal types of the ENS are described as follows.19
Diagram of the organization of gastrointestinal (GI) tract showing the complex network of the enteric nervous system (ENS), entero-endocrine cells, and neurotransmitters. Abbreviations. 5-HT: serotonin; ACh: acetylcholine; ATP: adenosine triphosphate; CGRP: calcitonin gene-related peptide; CO: carbon monoxide; EC: enterochromaffin cell; IPAN: intrinsic primary afferent neuron; NO: nitric oxide; PACAP: pituitary adenylyl cyclase-activating peptide; SERT: serotonin transporter; VIP: vasoactive intestinal peptide.
Motor neurons control muscle contractions in the GI tract. Functionally, five main types of motor neurons have been identified: (1) excitatory, (2) inhibitory, (3) secretomotor, (4) vasomotor, and (5) neurons innervating entero-endocrine cells, such as those innervating the gastrin-secreting cells of the stomach.29 Excitatory motor neurons predominantly use acetylcholine (ACh) as neurotransmitter, and to lesser extent tachykinin peptides (e.g., substance P).22 Inhibitory motor neurons primarily use nitric oxide (NO) as the neurotransmitter, with vasoactive intestinal peptide (VIP), adenosine triphosphate (ATP), pituitary adenylyl cyclase-activating peptide (PACAP), and carbon monoxide (CO) as secondary neurotransmitters.19 The excitatory motor neurons stimulate smooth muscle contraction, while the inhibitory neurons discharge continuously; consequently, the inactivity of the inhibitory neurons results in propulsive contractions towards the anus.31
Secretomotor neuron cell bodies are located in the submucosal and myenteric plexuses, but are part of secretomotor circuits involving IPANs with nerve endings in the mucosa.19,31 Their activity is triggered by the interaction of luminal contents, such as glucose, with the mucosa or by toxins, such as cholera and enterotoxins.31 Secretomotor neurons include cholinergic and non-cholinergic types, and their main function is to secrete chloride ions into the intestinal lumen, pulling water molecules with them. The non-cholinergic type uses VIP or related peptides as its main neurotransmitter and mediates most of the local reflex response, in contrast to the cholinergic neurons, which act on muscarinic receptors on the mucosal epithelium.19,31
Similar to the secretomotor neurons, the vasomotor neuron cell bodies are located in the submucosal plexus ganglia and their activity is also thought to be mediated by IPANs, although less significantly.31 They are the least studied type of motor neurons, but some evidence suggest that they are divided into cholinergic and non-cholinergic neurons, with ACh as the likely primary neurotransmitter and VIP as the secondary neurotransmitter.19 Secretomotor and vasomotor neurons work in tandem to regulate epithelial secretion and blood flow, and they are under extrinsic modulation via the sympathetic pathway.29
In addition, the mucosa of the GI tract contains entero-endocrine cells, and because the mucosa is densely innervated, most of these cells have nerve fibers in close proximity. Functional evidence that motor neurons innervate entero-endocrine cells includes data on the control of gastrin secretion, which is under the influence of vagal and of intrinsic gastric pathways.31 Major transmitters include 5-HT, cholecystokinin (CCK), secretin, somatostatin (SST), corticotrophin-releasing factor, gastrin, leptin, ghrelin, and glucagon-like peptide.29 These transmitters interact with afferent nerve fibers in the lamina propria, which in turn communicate with excitatory and inhibitory motor neurons.31
InterneuronsInterneurons connect neurons within the ENS, facilitating communication and coordination of local GI functions. There are two main types of interneurons, ascending and descending interneurons.30 Ascending interneurons are cholinergic and, like the descending interneurons, form networks that extend along the entire length of the gut.32 There are three types of descending interneurons: (1) ACh/NO/VIP±bombesine (also known as GRP – gastrin-releasing peptide)±GABA (gamma-aminobutyric acid)±NPY, (2) ACh/SST, or (3) ACh/5-HT. Intercellular connectivity studies showed that the ACh/NO/VIP interneurons are involved in the local motility reflexes, while ACh/SST type is involved in the guidance of migrating myoelectric complexes in the small intestine, and ACh/5-HT interneurons regulate secretomotor reflexes.29
IPANsIPANs function as sensory neurons that detect changes in the GI environment and play a role in reflexes within the ENS. IPANs are located in the submucosal7 and myenteric plexuses.22 Neurotransmitters associated with IPANs include ACh, calcitonin gene-related peptide (CGRP), and substance P.19,29 The latter two play a role in sensory signaling and are involved in transmitting information related to pain and other sensations.33
Role of the 5-HT system in the GI tractAs mentioned above, about 95% of the body's total 5-HT is found in the GI tract, while only 5% is found in the brain, clustered in the raphe nuclei involving groups B1–B9, which mediate the descending (B1–B3) and ascending (B4–B9) serotonergic projections.5,34 Nevertheless, until recently, most studies on 5-HT have focused on its role in the CNS, including the control of anxiety, depression, sleep, appetite or temperature, among others. Indeed, brain 5-HT gets more respect and certainly more press than the much larger gut 5-HT store. However, GI 5-HT also plays a vital role as a growth factor, endocrine hormone, paracrine factor, and neurotransmitter.7,9
At the GI level, about 90% of 5-HT is localized to the intestinal epithelium in enterochromaffin cells (EC), while the remaining 10% is produced by enteric neurons.7 EC and enteric neurons synthesize 5-HT from l-tryptophan (TRP), through a first rate-limiting step in which the enzyme tryptophan hydroxylase (TPH) converts this amino acid to 5-hydroxy-l-tryptophan (5-HTP) (Fig. 2). The enzyme l-amino acid decarboxylase then produces 5-HT by 5-HTP decarboxylation. The enzyme TPH exists in two isoforms, TPH1, which is mainly present in EC, and TPH2, which is expressed in CNS and enteric neurons.35,36 After being released by EC lining the gut lumen in response to chemical or mechanical stimulation or at the synaptic cleft,37 5-HT is taken up intracellularly by the serotonin reuptake transporter (SERT), mainly expressed in EC and enteric 5-HT neurons.38 There it can either be recycled to vesicles or converted to the metabolite 5-hydroxyindoleacetic acid (5-HIAA) by the action of monoamine oxidase A (MAO-A).
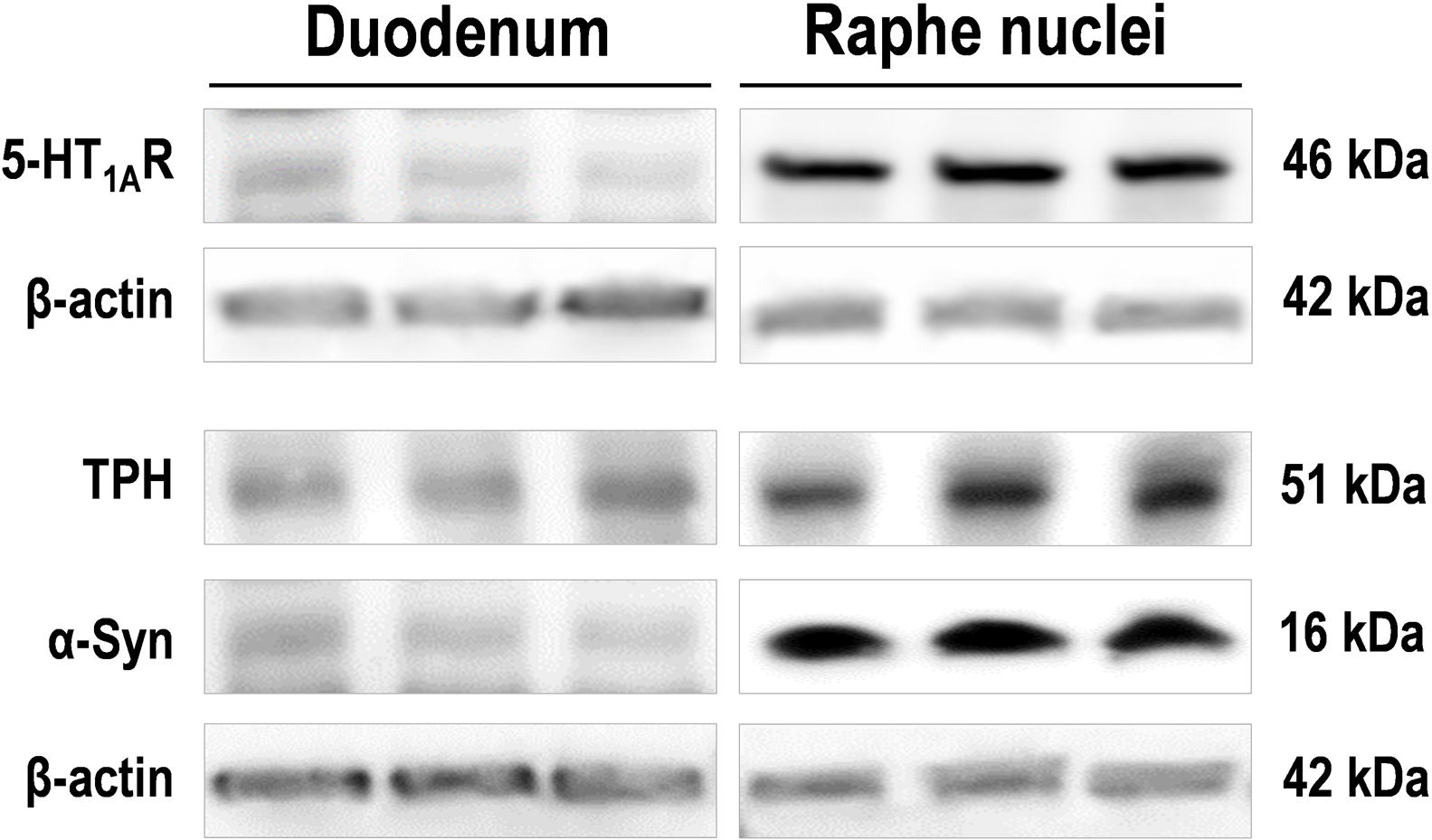
Images of Western blot of 5-HT1AR, tryptophan hydroxylase (TPH), and monomeric α-Syn protein in duodenum and raphe nuclei lysates from the same wild-type mice (n=3). For detection, immunoblots for 5-HT1AR, TPH, and α-Syn were performed using different antibodies against: 5-HT1AR (Ref: ab85615; 1:2000), TPH1/2 (Ref: AB1541, 1:1000), and mouse and human α-Syn (Ref: ab212184; 1:1000). β-Actin used as loading control (Ref: A3854; 1:50,000).
The availability of 5-HT can be affected not only by SERT function, but also by changes in the TRP metabolic pathway. This typically occurs at the point where TRP is converted to 5-HT or, alternatively, degraded to kynurenine via the enzyme tryptophan 2,3-dioxygenase or the indoleamine 2,3-dioxygenase (IDO)39 (Fig. 2). Although beyond the scope of this review, it is important to note that these different pathways, and thus the amount levels of 5-HT and kynurenine synthesized, are often regulated by pro-inflammatory mediators and are therefore relevant in inflammatory conditions such as inflammatory bowel disease.40
The actions of 5-HT in the GI tract are mediated by several 5-HT receptor (5-HTR) subtypes expressed on enterocytes, smooth muscle cells, enteric neurons, and sensory nerve terminals of vagal afferent neurons (Fig. 2 and Table 1). The most abundant 5-HTR subtypes expressed in the GI tract are 5-HT1A, 5-HT3, 5-HT4, and 5-HT7; however, all known variants, except for 5-HT5 and 5-HT6, are expressed in the gut,41,42 including 5-HT2AR.43 Interestingly, recent data support that the gut 5-HT2AR is a potential target for the peripheral actions of psilocybin, a classic psychedelic compound, which has recently emerged as a novel therapeutic intervention for depressive disorder and others mental health conditions.44,45
Expression and function of 5-HT receptors in GI tract.
| Receptor | Subtype | GI distribution | Proposed GI function | References |
|---|---|---|---|---|
| 5-HT1 | 5-HT1A | Enteric motor neurons, enterocytes | Gastric fundus relaxation | Boeckxstaens et al., Neurogastroenterol. Motil. 2006, 18:919–926; Gershon and Tack, Gastroenterology 2007, 132:397–414; Dickson et al., Am J Physiol Gastrointest Liver Physiol. 2010, 299:G144–57; Javid et al., Eur J Pharmacol. 2018, 821:79–87. |
| 5-HT1B | Enteric motor neurons, smooth muscle, extrinsic nerves | Pro-kinetic intestinal stimulation | ||
| 5-HT1D | Enteric neurons, smooth muscle, extrinsic nerves | Contraction of intestinal muscle | ||
| 5-HT1P | Enteric neurons | Participate in peristalsis | ||
| 5-HT2 | 5-HT2A | Enteric neurons, smooth muscle, enterocytes | Contraction of smooth musclesStimulate secretion | Pytliak et al., Physiol Res. 2011, 60:15–25; Morita et al., World J Gastroenterol. 2013, 19:6604–6612; Forcén R et al., Exp Physiol. 2016, 101:1064–1074; Buey et al., Life (Basel). 2023;13:1085. |
| 5-HT2B | Enteric neurons, smooth muscle, interstitial cells of Cajal, connective tissue | Contraction of smooth muscles in stomach fundusRelaxation of longitudinal muscle in the intestineRegulate enteric nerve growth | ||
| 5-HT2c | Enteric neurons | Participate in GI motility | ||
| 5-HT3 | Enteric neurons, interstitial cells of Cajal, enterocytes, extrinsic nerves, EC | Chloride secretion and 5-HT release from ECIncrease intestinal motilityEmesis | Glatzle et al., Gastroenterology 2002, 123:217–226; Kapeller et al., J. Comp. Neurol. 2011, 519:420–432; Kato, Biol Pharm Bull 2013, 36:1406–1409; Johnston et al., Eur J Pharmacol. 2014, 722:13–25; Aikiyo S et al., Tract. Digestion. 2021, 102:516–526. | |
| 5-HT4 | Enteric neurons, smooth muscle, interstitial cells of Cajal, enterocytes, EC | Increase intestinal motilityContraction of esophagusRelaxation of colonChloride secretionEnhance neuronal survival | Taniyama et al., J Gastroenterol. 2000, 35:575–582; Liu et al., J Neurosci. 2009, 29:9683–9699; Hoffman et al., Gastroenterology 2012, 142:844–854.e4; Reigstad et al., Neurogastroenterol Motil. 2016, 28:1443–1448; Spohn et al., Gastroenterology. 2016 151:933–944.e3; Mawe et al., Adv Exp Med Biol. 2022; 1383:329–334; Cui et al., J Neuroimmune Pharmacol. 2023, 18:610–627. | |
| 5-HT5 | Not described | Not described | ||
| 5-HT6 | Not well characterized | Not well characterized | ||
| 5-HT7 | Enteric neurons, smooth muscle | Excitatory effectAnti-inflammatory activityRegulate visceral sensation | Monro et al., Neuroscience. 2005, 134:975–986; Tonini et al., Neurogastroenterol Motil. 2005, 17:637–642; Zou et al., Chin Med J (Engl). 2007, 120:2069–2074; Prause et al., Res Vet Sci. 2009, 87:292–299; Kim et al., J Immunol. 2013, 190:4795–4804; Guseva et al., Inflamm Bowel Dis. 2014, 20:1516–29; Yaakob et al., J Neurogastroenterol Motil. 2015, 21:361–369; Guzel and Mirowska-Guzel, Molecules 2022, 27:1680; Osman Cureus. 2023, 15:e42532. | |
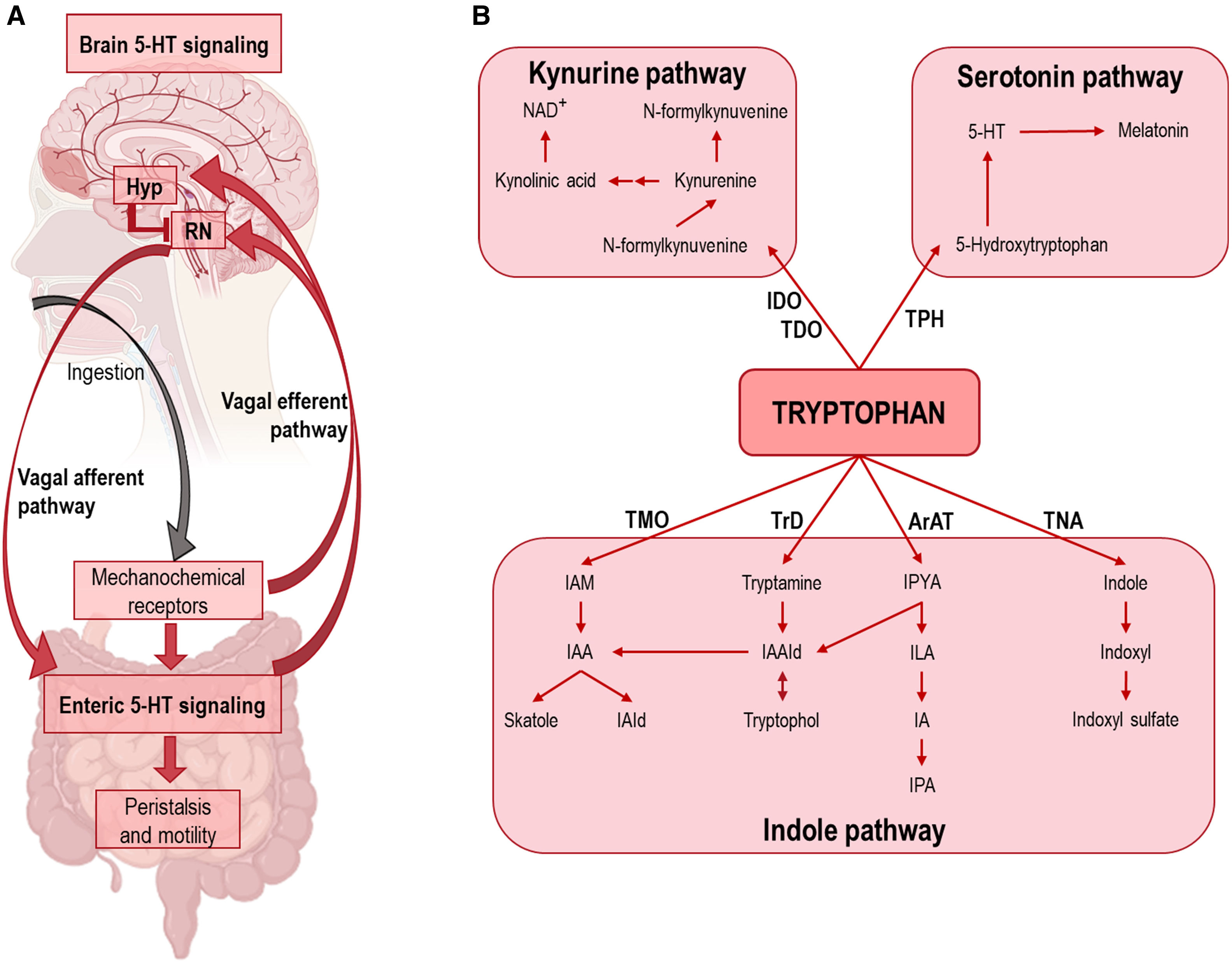
One of the major roles of 5-HT in the GI tract is the control of motility and peristalsis, primarily through the activation of 5-HT3R and 5-HT4R, which are the fast acting ionotropic and slow acting metabotropic receptors, respectively.8,46 Both receptor subtypes are present on neurons within the myenteric and submucosal plexuses of the ENS, IPANs, and EC.47,48 Both, motility and peristalsis, are complex and highly coordinated processes involving local circuits in the GI tract, as well as 5-HT neurons located in the midbrain raphe nuclei8,13 (Fig. 3). At the local level in the GI tract, preclinical studies with genetic mouse models have helped to elucidate specific mechanisms involving 5-HT derived from EC and enteric neurons.9,49 It has been reported that in vivo motility throughout the GI tract is significantly slower in TPH2 knockout (KO) mice compared to the controls,9 indicating an important role for neuronal 5-HT in GI motility. Although enteric 5-HT neurons are estimated to represent less than ∼10% of the neurons in the myenteric plexus, they have abundant projections – comparable to the anatomical organization of the brain 5-HT system5 – that innervate the major enteric inhibitory neurons (e.g., those expressing NO synthase), responsible for the processes controlling motility and peristalsis.50
(A) Schematic showing the integration of 5-HT signaling in GI tract and midbrain raphe nuclei. After ingestion, GI 5-HT interneurons activate enteric inhibitory neurons controlling motility and peristalsis. In addition, mechanochemical signals travel via the vagus nerve to the spinal cord, where descending 5-HT signals from the raphe nuclei (RN) stimulate peristalsis and subsequent ingestion. The release of leptin, ghrelin, insulin, etc., into the blood stimulates transcription of the POMC gene in the hypothalamus (Hyp), resulting in reduced activity of raphe 5-HT neurons via α-melanocyte-stimulating hormone (α-MSH)-induced stimulation of melanocortin 4 receptor (MC4R), ultimately leading to satiety. In the absence of appropriate negative feedback signals, the positive feedback loop between the vagus nerve and the colon can lead to an increase in peristaltic frequency. (B) Diagram of the three main pathways of tryptophan metabolism. Abbreviations. ArAT: aromatic amino acid aminotransferase; IDO: indoleamine 2,3-dioxygenase; IA: indole-acrylic acid; IAA: indole-3-acetic acid; IAAId: indole-3-acetaldehyde; IAld: indole-3-aldehyde; IAM: indole-3-acetamide; ILA: indole-3-lactic acid; IPA: indole-3-propionic acid; IPYA: indole-3-pyruvate; NAD: nicotinamide adenine dinucleotide; TDO: tryptophan 2,3-dioxygenase; TMO: tryptophan 2-monooxygenase; TNA: tryptophanase; TPH: tryptophan hydroxylase; TrD: tryptophan decarboxylase.
In addition to studies focusing exclusively on enteric 5-HT, the role of the brain 5-HT system in the regulation of GI motility is also noteworthy. As noted above, the GI tract is innervated by a dense network of neurons that form the ENS via the vagus nerve. The presence of food in the large intestine – signaled through mechanical stretch receptors or the presence of enteric peptides, such as CCK7,51 – is transmitted via the vagus nerve to descending serotonergic projection brain areas, such as the raphe magnus. This leads to activation and axonal release of 5-HT in the GI tract, with subsequent modulation of local 5-HT release from EC.51 Therefore, it has been proposed that GI 5-HT levels are under the control of a well-defined circuit, in which increased blood levels of peptides (e.g., leptin, insulin, glucose, and ghrelin) following food intake and satiety activate the arcuate nucleus of the hypothalamus, thereby inhibiting the 5-HT raphe nuclei by terminating the descending signals that mediate peristalsis (Fig. 3). The reader will find detailed information on the control of enteric peptide-mediated mechanisms on the 5-HT system in the GI tract in the following reviews.8,13,37
Another critical GI 5-HT function is the modulation of gut neurodevelopment, as occurs in the brain. During CNS development, 5-HT is required for neuronal differentiation and migration, neurite outgrowth, myelination and synapse formation.52 Similar to the CNS, enteric neuronal 5-HT also drives the development of enteric neurons through its actions as a growth factor, both prenatally and throughout the lifespan.53 Notably, the main source of 5-HT required for the development of enteric neurons is neuronal. In TPH1-KO mice, which lack the 5-HT produced by the EC, no obvious abnormalities were observed in neural subpopulations.9 During ENS development, 5-HT neurons represent one of the first neuronal populations that influence neurogenesis and drive the development and survival of other enteric neuronal types.13 Consistent with these observations, in vivo studies have revealed that TPH2-KO mice showed enteric neuronal hypoplasia accompanied by a significant decrease in dopaminergic (DA), GABAergic, and CGRP-expressing neurons.9 In contrast, mice lacking SERT (SERT-KO), which have a higher availability of 5-HT for neurotransmission, showed enteric neuronal hyperplasia.11 5-HT promotes enteric neurogenesis, at least partially, through its actions on 5-HT2BR and/or 5-HT4R.54,55 Importantly, 5-HT enteric neurons have also been shown to influence their own subsequent development.11 Although the mechanisms are not fully understood, the ability of 5-HT neurons to shape the ENS suggests that environmental stimulus that alter the activity of 5-HT neurons, such as psychosocial trauma, stress and inflammation, may lead to long-lasting changes in ENS structure and function. In support of these observations, it has been shown in animal models that colon irritation during development or exposure of offspring to stressful situations, e.g., maternal separation, induces structural/functional changes in GI tract and brain, similar to those observed in human with irritable bowel syndrome and psychiatric pathologies.56–58
Intestinal microbiota and 5-HT pathwayIn recent years, the role of the microbiome in the etiology of various pathological processes, such as PD and depression, has attracted considerable interest. In particular, the interactions between 5-HT and the gut microbiome are increasingly recognized for their ability to alter the physiological and pathological state in the host.59–61 The gut microbiota interacts with 5-HT in a bidirectional manner; changes in the gut microbiome lead to changes in host 5-HT homeostasis, and manipulations of the 5-HT pathway affect the composition of gut microbiota communities. The resulting microbial composition may be then a modifier of GI function, as well as the gut–brain axis.
Gut microbiota plays direct and indirect roles in GI-derived 5-HT production.60 Indeed, it has been shown to be a key regulator of TRP metabolism (5-HT precursor) (Fig. 3). The colon harbors the densest and most metabolically active microbial community, comprising more than 1013 individual microbial cells and expressing various types of enzymes capable of using TRP as a precursor.59,62,63 For instance, germ-free (GF) mice exhibit increased TRP levels in both feces and plasma, along with reduced 5-HT concentration in the GI tract and plasma, respectively compared to control mice. This was associated with reduced expression of TPH1, the regulatory enzyme of 5-HT synthesis in EC, suggesting that primary disruptions in host TPH1 expression result in TRP accumulation. Oral supplementation of GF mice with 5-hydroxytryptophan (5-HTP) ameliorated 5-HT deficits in GI tract and plasma. Thus, it is now widely accepted that the gut microbiota plays a key role in regulating the host serotonergic system, primarily through direct alteration of TPH1 transcription, promoting 5-HT production in the host, and indirectly influencing SERT expression.60,63,64 In addition, gut microbiota composition may also alter expression of host 5-HT3R, implicated in intestinal secretion and peristalsis.65 Likewise, modulation of the gut microbiota can also induce maturation of the adult ENS through 5-HT release and 5-HT4R activation, as reported in GF mice. In fact, the authors identified a mechanistic link between the microbiota and enteric neurons as the initiation of 5-HT release and subsequent 5-HT4R activation.66 These studies open up new avenues of research into bidirectional microbiota–gut–brain interactions.
Insights into host–microbiota dynamics also show that gut bacteria actively produce microbial metabolites that can affect the host. Emerging evidence suggests that these metabolites are key in the microbiota–host communication and are implicated in physiological functions and diseases. Specifically, microbiota-mediated changes in TRP availability may have an impact on local 5-HT availability and 5-HT-mediated intestinal functions. Certain bacterial species like Clostridium sporogenes have been associated with increased TRP catabolism and production of indole metabolites, such as tryptamine.67 Conversely, restoring TRP levels has been shown to result in the expansion of Lactobacillus spp., which further led to the conversion of TRP to the intermediate indole-3-lactic acid (ILA).59,68 Furthermore, the gram-negative probiotic, Escherichia coli Nissle 1917, has also been shown to enhance 5-HT biosynthesis and bioavailability in GI tract.69 Despite the apparent effects of bacterial delivery on many functions, the underlying mechanisms of action of these bacteria remain poorly known. The need for better understanding of the specific effects of bacterial strains is becoming increasingly apparent. There is no doubt that this research is ongoing and will yield exciting results in the coming years.
Recent studies have shown that individuals with PD and depressive disorders possess a different gut microbiota than healthy populations.70–73 The microbiota may play a role in the onset or progression in both disorders. Consistent with these observations, colonization of α-synuclein-(α-Syn)-overexpressing mice with microbiota from PD patients impaired motor function compared to microbiota transplants from healthy individuals.74 Similarly, fecal microbiota transplantation from patients with depressive and anxiety disorders into rodents modulates mood-related behaviors.75,76 However, remains unknown whether the bacterial populations of the diseased individuals play a role in these disorders by interacting with the host GI 5-HT system. Although outside the scope of this review, it is important to note that the composition of the mammalian gut microbiome is of vital importance for the development of neural circuits involved in emotional processing, motor control, learning and memory.77 The gut microbiota appears to be crucial for the formation of brain circuits related to stress response, while emotional and physiological stress affects the gut functions.78 In this way, changes in gut microbiota, also known as dysbiosis,79 is involved in the pathogenesis of several brain disorders and at the same time, offering a novel therapeutic target including dietary interventions, fecal microbiota transplantation, probiotics, prebiotics, symbiotics, and post-biotics as novel and safe treatment options.
Parkinson's disease, co-pathology with depressive disorder, and the 5-HT gut–brain systemPD is a multisystem disorder clinically characterized by motor symptoms such as hypokinesia, rigidity, resting tremor, and impaired postural control.80 A wide range of disabling non-motor symptoms are also present over the course of the illness. Among these, GI disturbances and neuropsychiatric features have been shown to often precede the onset of neurological features characteristic to PD. More than 80% of PD patients report some degree of GI dysfunction during the course of the disease.81,82 Furthermore, the neuropsychiatric symptoms, including anxiety and depression, are inherent to the disease and are neither a result nor a side effect of the long-term dopaminergic treatment that PD patients receive.34,83 These co-pathologies are common and can be found in all stages of PD, from the premotor and the early untreated phases of the disease to the advanced stages of PD.84,85 Among the neuropsychiatric disorders, depression is one of the most prevalent symptoms and affects 35–50% of people with PD.86–88 A dysfunctional 5-HT system is generally considered a risk factor for depressive disorder.89 Consistent with this view, several reports suggest a positive correlation between reduced 5-HT neurotransmission and the severity of depressive/anxiety symptoms in PD,83,88,90–93 likely caused by pathological changes in the 5-HT system.34,94
Recent evidence from studies in humans and in animal models suggested that the GI tract and its neural, mainly vagal, connection to CNS may play an important role in the etiology of PD and its comorbidity with depression.12,81,95,96 Given the anatomical and functional organization of the central and peripheral serotonergic system, one would expect 5-HT to be a key neurotransmitter in the gut–brain communication system. Although the etiology of PD remains unclear, the accumulation of α-Syn protein plays a crucial role in the disease process and is considered the gold standard for establishing a definitive PD diagnosis.80,97 Aggregates of α-Syn protein represent one of the main components of Lewy bodies (LB) found in the PD pathology, although not the only one.98,99 High-resolution multicolor STED microscopy observations of post-mortem substantia nigra compacta (SNc) samples from PD patients reveal a localized enrichment of numerous proteins and lipids mainly in the central part of the α-Syn-immunopositive inclusions.100 In recent years, a new concept of α-Syn pathology has been incorporated that influence the course of the disease in each patient and comprises the anatomical location of the initial α-Syn inclusion and its propagation.101 In some patients, initial α-Syn pathology occurs within the CNS, leading to a brain-first subtype of PD. In others, the α-Syn pathology ascends via the vagus to both the left and right dorsal motor nuclei of the vagus owing to the overlapping parasympathetic innervation of the gut, leading to a body-first subtype.101 Importantly, the most caudal 5-HT clusters of the raphe nuclei (raphe pallidus, raphe obscurus, and raphe magnus) contain LB-related lesions in the early stages of PD,102 suggesting that α-Syn pathology spreads from GI tract to the brainstem, affecting the raphe nuclei before reaching the DA neurons in the SNc. The 5-HT neurons found in the caudal raphe nuclei play a role in a number of autonomic processes, including pain and decreased GI motility,8 which are recognized non-motor symptoms in PD. In addition, the rostral raphe nuclei, containing the dorsal raphe nucleus (DR) and median raphe nucleus (MnR), are also affected in PD showing accumulation of α-Syn protein in 5-HT-containing neurons.34 Some early studies showed a significant loss of the serotonergic marker TPH-positive, but others did not, in DR and MnR from post-mortem brain samples of PD patients, and this was even more pronounced in patients with PD and depression.103
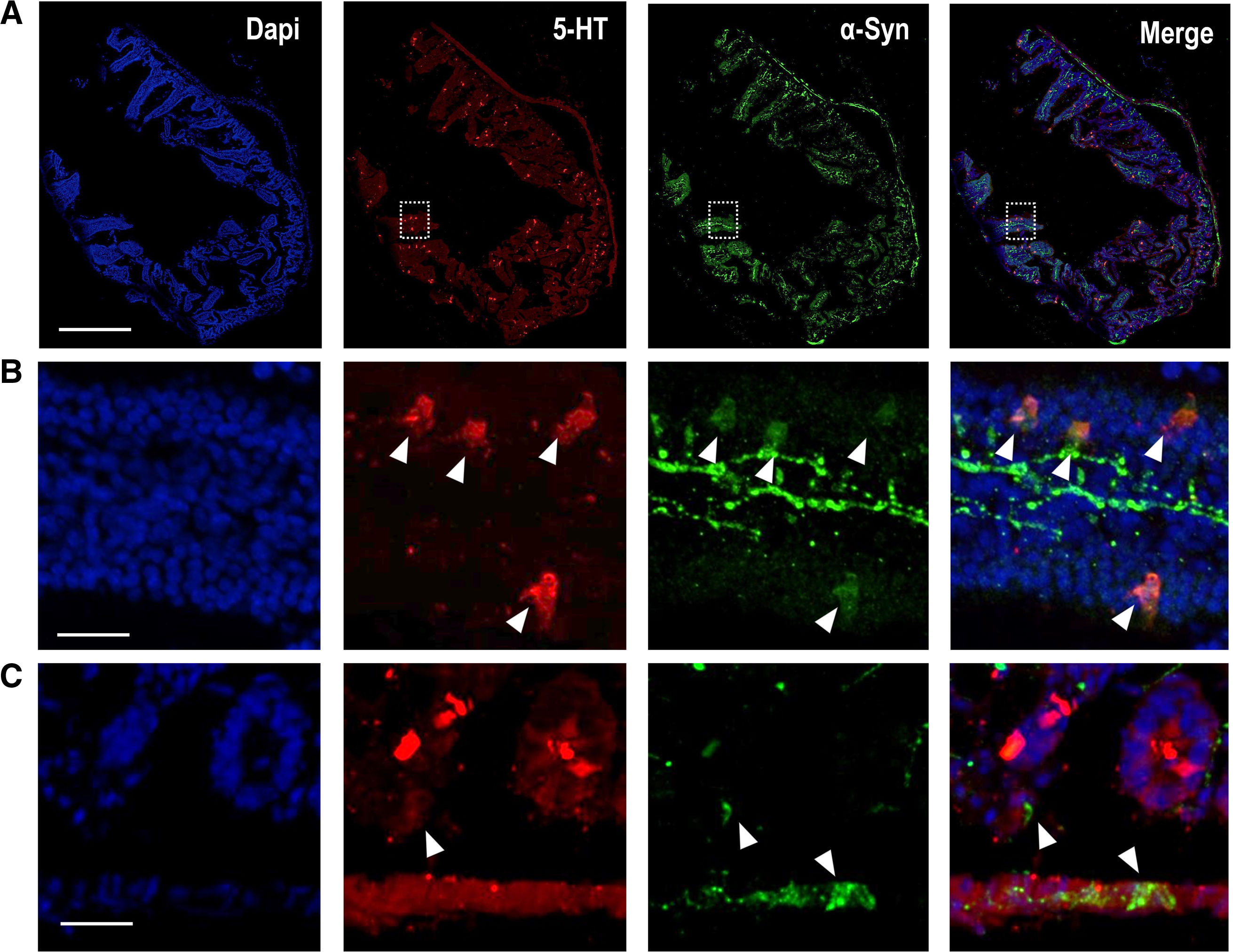
Although α-Syn-positive LBs were originally described in the brain, aggregated α-Syn has also been reported in the ENS of PD patients.104 The α-Syn protein is found both at the level of the ENS and in epithelial cells (e.g., EC, which are essential to form the interface between the lumen and the ENS) in the normal human and rodent GI tract,105–107 as shown in Fig. 4. Recent studies showed co-localization of α-Syn with 5-HT-positive EC, but not with ACh-positive epithelial cells, in the human jejunum,105 suggesting that α-Syn may play a role in synaptic transmission in the ENS and contribute to maintaining the integrity of the small intestinal epithelial barrier via EC. In support of this observation, enteric nerves do not reach the intestinal lumen and do not have direct contact with the intestinal contents, but do it so through these epithelial cells, specifically the EC, which contain the highest levels of 5-HT in the GI tract.7 In addition, the presence of α-Syn was also confirmed in entero-endocrine cells positive for CCK and peptide YY (PYY).106 Using scanning electron microscopy, recent studies showed that EC have axon-like basal processes referred neuropodia with neurofilaments, which are typical structural proteins of axons.108 In addition to receptors for neurotrophins, presynaptic and postsynaptic proteins were identified in EC, increasing the likelihood that EC connect the intestinal lumen with the nervous system.105 Furthermore, 5-HT-rich EC are involved in the inflammatory process, linking the immune, endocrine and nervous systems in the GI tract. Indeed, colonic inflammation is critically correlated with changes in α-Syn expression.109 Taken together, it could be hypothesized that EC, because of their precise distribution at the interface, may be exposed to pathogens or toxins that could affect soluble α-Syn levels and their conformational states. If α-Syn is misfolded in EC, its transport to enteric neurons containing α-Syn could be the first step in a propagation cascade leading to PD. Further research is needed to find out whether 5-HT in the GI tract plays a role in these processes. For instance, some studies using PC12 cells conditionally overexpressing α-Syn have shown that 5-hydroxyindoleacetaldehyde (5-HIAL), a 5-HT metabolite product produced by MAO-A, increases α-Syn oligomerization, indicating that 5-HT itself may affect the formation of α-Syn protein fibrils.110 In any case, evidence indicates that GI α-Syn propagates through the vagus nerve and causes pathological changes in the brainstem, affecting the integrity of the 5-HT system before the DA system.102,111
(A) Representative confocal images showing α-Syn protein (green; Ref: ab212184, 1:250) co-localized with 5-HT-positive cells (red; Ref: Immunostar 20079, 1:1000) in mouse duodenum. Scale bar: 1mm. (B) Higher magnification confocal images identified in (A) with inset, showing 5-HT-positive mouse intestinal epithelium cells containing α-Syn protein. Scale bar: 250μm. (C) Representative confocal images showing α-Syn-positive fibers close to 5-HT-positive epithelium cells in the mouse duodenum. The ascending pathology hypothesis of α-Syn proposes that uptake of the toxin by epithelium cells in contact with nerves and glia in the GI tract, may cause aggregation of α-Syn within these cells and this aggregated protein may migrate to enteric nerves, thus initiating a pathogenic cascade leading to α-synucleinopathies.106 Scale bar: 20μm.
In addition to the comorbidity of depression in PD, major depressive disorder (MDD) is a critical challenge to global mental health and the leading cause of mental health-related disability worldwide.112 MDD is a highly prevalent mood disorder that negatively affects education, social relationships, and employment and is prospectively associated with obesity, cardiovascular disease, GI disorders, and early death, including suicide.113 This disorder involved depressed mood, energy changes, sleep disorder, poor concentration, and lack of interest or excitement.114
Neuroimaging studies have identified structural and functional brain changes including volume reductions in cortical and subcortical structures, enlarged lateral ventricles, and white matter microstructural differences suggestive of compromised myelin integrity.115–117 In addition, histopathological studies in human and mouse models have shown changes in neuron and glia density, and reduced expression of neuroplasticity and synaptic proteins.118–120 Importantly, deficits in the brain 5-HT signaling are also implicated in the neuropathology of MDD and have been the neurochemistry substrate for antidepressant treatments.121 Imbalances in the production and transmission of several neurotransmitters, especially 5-HT and low levels of its precursor TRP, are commonly observed in the CNS of patients suffering from depressive disorder.122–124 In fact, a widely accepted etiological theory is the “monoamine hypothesis of depression”, which postulates that depression disorder is associated with a decreased monoamine function (NE, DA, 5-HT) in key brain areas, such as the ventromedial prefrontal cortex, hippocampus, amygdala, among others.89,125
TRP, an essential amino acid, is critical in the synthesis of multiple metabolites including 5-HT, kynurenine, melatonin, and for protein synthesis.126,127 It is important in both GI tract and CNS functions, and recently has emerged as a key player in the microbiota–gut–brain axis.59Fig. 4 shows the three main pathways of TRP metabolism: (1) the unique 5-HT precursor in the brain and GI tract; (2) the kynurenine pathway whose metabolites are involved in the systemic inflammatory cascade and also in the regulation of neuronal activity; and (3) the indole pathway that is mainly produced in the gut microbiota.59,128 Accordingly, dysregulation of TRP metabolites plays a central role in the pathogenesis of many neurologic and psychiatric disorders, including MDD. Previous studies have reported a shift in the composition of gut microbes with the capacity to synthesize 5-HT and engage with the gut–brain axis. Although not the focus of this review, it was noted in session 4 that the composition of the gut microbiota affects host TRP metabolism leading important changes in the functional availability of GI 5-HT, and recently MDD has been associated with significant changes in the microbiota. The reader is referred to59,127,129 for more on this topic.
Therefore, depressive disorder appears to involve dysfunction of the 5-HT system in both the brain and the GI tract. In support of this, a model has recently been proposed showing some symptoms that illustrate an analogy of a dysregulated 5-HT system in the brain and the GI tract.8 It has been described that anxiety and depressive disorders, although two distinct symptoms, may be partly due to the disruption of a common system (i.e. 5-HT) and may co-occur in the same person.130 In a compromised 5-HT system, it is conceivable that a given context can drive 5-HT levels out of their optimal range in either direction, contributing to certain facets of anxiety or depression disorders manifesting in the same individual. A similar situation occurs in the GI 5-HT system, where diarrhea and constipation coexist when the same compromised system is pushed out of its optimal range in both directions.42 GI motility disorders occur due to an imbalance in the interaction of the gut–brain axis, which is regulated by 5-HT.131 Patients with MDD are more likely to suffer from GI motility disorders, which raises the hypothesis that changes in the physiology of the 5-HT GI system lead to CNS symptoms, while the reverse is also true. Altered 5-HT levels in the GI tract are regulated by SERT, hence antidepressants that act on SERT, such as selective serotonin reuptake inhibitors (SSRIs), may be effective in alleviating mood symptoms and comorbidity with GI disorders. However, GI side effects have also been reported in MDD patients treated with SSRIs that may lead to treatment discontinuation.132 Further research focusing on the integration of 5-HT system function in the gut–brain axis will be needed to understand why there is such a strong link between serotonergic pathology, symptoms of psychiatric disorders, and comorbidity with GI disorders.
New evidence for therapeutics targets in PD and depression based on 5-HT gut–brain pathwayAs noted above, GI and brain 5-HT signaling has been implicated in a number of GI disorders, as well as neurological and neuropsychiatric disorders such as PD and depression. Although many of these cases are also associated with changes in the composition of the microbiome, which is an important factor in the progression of these disorders, our understanding of the relationship between the microbiota and the gut–brain 5-HT pathway is still in its infancy. Further mechanistic data will be required to fully understand the magnitude of this impact and, ultimately, to suggest new targets for therapeutic intervention.
In “Intestinal microbiota and 5-HT pathway” section, we described that 5-HT signaling occurs bidirectionally between the host and microbiome, and may contribute to physiological functions and pathology. Bacteria residing in the GI lumen can sense 5-HT levels in the lumen, which may induce and/or inhibit the expression of bacterial genes related to biofilm formation, adhesion, motility or virulence. In addition, 5-HT levels in the lumen may also confer a selective advantage to certain types of microorganisms. Some species of microbiota may also increase host 5-HT levels through increased expression of TPH1, or decreased expression of SERT, and possibly other less-known mechanisms (e.g., changes in 5-HT3R and 5-HT4R activity). These complex effects may even be mediated by secreted bacterial secondary metabolites or by direct interaction between microbes and receptors/transporters in the host.59–61,133 In PD, many of the associated motor and non-motor symptoms are related to the loss of integrity of the 5-HT system.34,83,88,90–94 Recently, PD patients have been reported to have significant reductions of short-chain fatty acid (SCFA)-producing bacteria in the gut microbiome.134 Furthermore, SCFA have been shown to stimulate 5-HT production by ECs.135 Therefore, it could be hypothesized that the decrease in GI 5-HT levels in PD patients is due to a reduction in SCFA-producing bacteria. In support of this observation, new data showed that exposure to the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) not only increased α-Syn levels in the ileum, but also produced changes in the composition of the gut microbiota in a mouse model of PD. Notably, fecal transplants from these PD-like mice to healthy mice resulted in decreased 5-HT content and impaired motor function, and vice versa, fecal transplants from healthy mice to PD-like mice restored 5-HT levels and recovered motor function.136 In addition, pre-treatment with GR125487 (5-HT4R antagonist) was shown to exacerbate MPTP-induced neurodegenerative processes, possibly via the JAK2/PKA/CREB pathway, and inflammation by altering gut microbiota composition.137 Therefore, both GI and brain 5-HT4R should be further explored and may serve as targets for PD diagnosis and treatment, given their recent increased interest.138
Similarly, a growing body of evidence suggests the contribution of 5-HT4R in the pathophysiology of chronic depression and associated gut abnormalities. Thus, emotional stress and hyperactivity of hypothalamic–pituitary–adrenal axis alter ENS response to physiological and noxious stimuli, which in turn leads to onset of GI disorders.139 Several preclinical and clinical studies demonstrated the beneficial role and mechanism of 5-HT4R-based therapeutics for the treatment of depressive disorder and possible consequences for the gut via brain–gut axis interactions.140
Current data have also illustrated that the gut microbiota is strongly associated with the pathology of depression and anxiety.141 Indeed, pioneering preclinical studies conducted over the last two decades have also suggested a link between early-life psychological stress and gut microbiota dysbiosis.142 Supporting this idea is the finding that the supplement lycopene (a lipophilic carotenoid hydrocarbon pigment) can simultaneously reduce colitis, depression, and anxiety-like behavioral effects in a mouse model by reducing certain microbiota species, suppressing neuroinflammation, and upregulating neurotrophic factor and postsynaptic density protein.143 However, there is little information on the causality between these effects and 5-HT signaling in the gut–brain axis. Some studies have reported that anxiety-like behavior is reduced in Bifidobacterium dentium monocolonized mice compared to germ-free mice, probably due to induction of 5-HT synthesis and increased expression of SERT, 5-HT2AR, and 5-HT4R in the gut and brain.144 Another recent example shows that chronic unpredictable mild stress (CUMS)-induced alteration of the gut microbiota in rats leads to marked changes in community diversity, taxon abundance and functional profiles, as well as depressive-like behaviors. Administration of fluoxetine, a selective serotonin reuptake inhibitor (SSRI), reverses some of the gut microbiota profile and functions, consistent with antidepressant effects.141 Taken together, this evidence strengthens the basis for future research to identify metabolites that mediate host responses and to validate the causal relationship between gut microbiota dysbiosis and depression by demonstrating the role of specific bacteria in hosts. Furthermore, the remission of depression induced by antidepressant treatment remains an unsolved puzzle in the therapy of the disease. Understanding the interaction between antidepressants and the gut microbiota may open the door to innovative interpretations and potentially targeted interventions.
Although no attempt has yet been made to modulate host 5-HT levels by microbiome engineering, much of what is now known about the influence of bacteria on the host 5-HT system has been discovered through the study of probiotic strains. As we advance our understanding of the relationship between 5-HT in the gut–brain axis and the microbiome in PD and depression, tools to precisely modulate the composition of the microbiome will be essential. To date, microbial therapies have been designed to detect and respond to a range of biomarkers. For example, SYNB1618 is an engineered probiotic that is being used to reduce levels of the host metabolite phenylalanine in patients suffering from the metabolic disorder phenylketonuria.145 A similar concept could be used to degrade or synthesize 5-HT without affecting the levels of other metabolites, which could have unintended consequences. For example, we could consider designing a microbe-based therapy that can sense host 5-HT levels and respond accordingly by secreting secondary metabolites to up- or down-regulate synthesis, metabolism, transport or receptor gene expression. Even being able to imagine that a probiotic can be manipulated in the gut–brain axis by photo-stimulation. Indeed, this strategy was used to restore cognitive alterations in chronically stressed mice through the regulation of Sirt1 and neuroinflammation.146 This strategy may also be advantageous in the context of local 5-HT manipulation, where, for example, increased serotonin production may be beneficial in the gut, but detrimental elsewhere. Therefore, although the use of engineered probiotics is still in its early stages, significant advances in this field are expected as our basic scientific understanding of 5-HT in the context of the gut–brain axis increases for therapeutic interventions in PD and depression.
Looking forwardIn recent years, it has become increasingly clear that bidirectional communication between the CNS and the body has important implications for the maintenance of homeostasis and brain function. Dysregulation of the gut–brain axis may contribute to or aggravate a variety of neurological and psychiatric disorders. The 5-HT system is emerging as a key player, in addition to its role in the CNS, and the role of 5-HT in the neurogenesis of the ENS and function in the GI is increasingly recognized. This makes 5-HT more than just a modulator of GI motility and secretion. In this review, we aim to provide a critical overview of the role of 5-HT system in ENS and GI tract under physiological conditions and in PD and MDD, the etiology of which is not fully elucidated. One of the main obstacles to understanding the physiological functions of enteric 5-HT and its regulatory pathway is the multiplicity of responses to applied 5-HT. The vast majority of receptors described for 5-HT at the level of the brain are also expressed in the GI tract, however, unlike in the brain, less is known about the distribution and specific functions of these receptors in the GI tract. The study of these receptors may lead to a better understanding of gut–brain interactions and treatments for disorders of the gut–brain axis. In addition, important areas of ongoing research include (1) 5-HT signaling in enteric epithelial homeostasis and its mechanistic role in ENS-CNS α-synucleinopathy, (2) microbiota composition and TRP metabolism, and (3) immune-inflammatory pathways. Thus, the ENS and 5-HT signaling pathway may be a nexus between the gut, microbiota, immune system and brain, and dysfunction in the ENS may exacerbate CNS diseases.
Authors’ contributionsWrote or contributed to the writing of the manuscript: María Sancho-Alonso, Unai Sarriés Serrano, Lluis Miquel Rio, and Analia Bortolozzi. Revised the manuscript: All the others.
Conflicts of interestThe authors declare that they have no conflict of interest.
This work was supported by MCIU/AEI/FEDER, UE grants (PID2019-105136RB-100, PID2022-141700OB-I00, MCIN/AEI/10.13039/501100011033 to AB) and AGAUR 2021-SGR-01358, Catalan Government. CB/07/09/0034 and CB/07/09/0008 Center for Networked Biomedical Research on Mental Health (CIBERSAM). USS is a recipient of a fellowship from the Non-Doctor Researcher Formation Pre-doctoral Program of Basque Government, Spain. MSA has a Margarita Salas Grant (MS21-132) from the University of Valencia (requalification of the Spanish University System of the Ministry of Universities of the Government of Spain, financed by the European Union, Next Generation EU). Figures 1 and 3 were generated using online application “created with BioRender.com”