Deep brain stimulation (DBS) is an effective alternative to treat severe refractory obsessive-compulsive disorder (OCD), although little is known on factors predicting response. The objective of this study was to explore potential sex differences in the pattern of response to DBS in OCD patients.
MethodsWe conducted a prospective observational study in 25 patients with severe resistant OCD. Response to treatment was defined as a ≥35% reduction in Yale-Brown Obsessive Compulsive Scale (Y-BOCS) score. Logistic regression models were calculated to measure the likelihood of response at short and long-term follow-up by sex as measured by Y-BOCS score. Similar analyses were carried out to study changes in depressive symptomatology assessed with the Hamilton Depression Rating Scale (HDRS). Additionally, effect sizes were calculated to assess clinical significance.
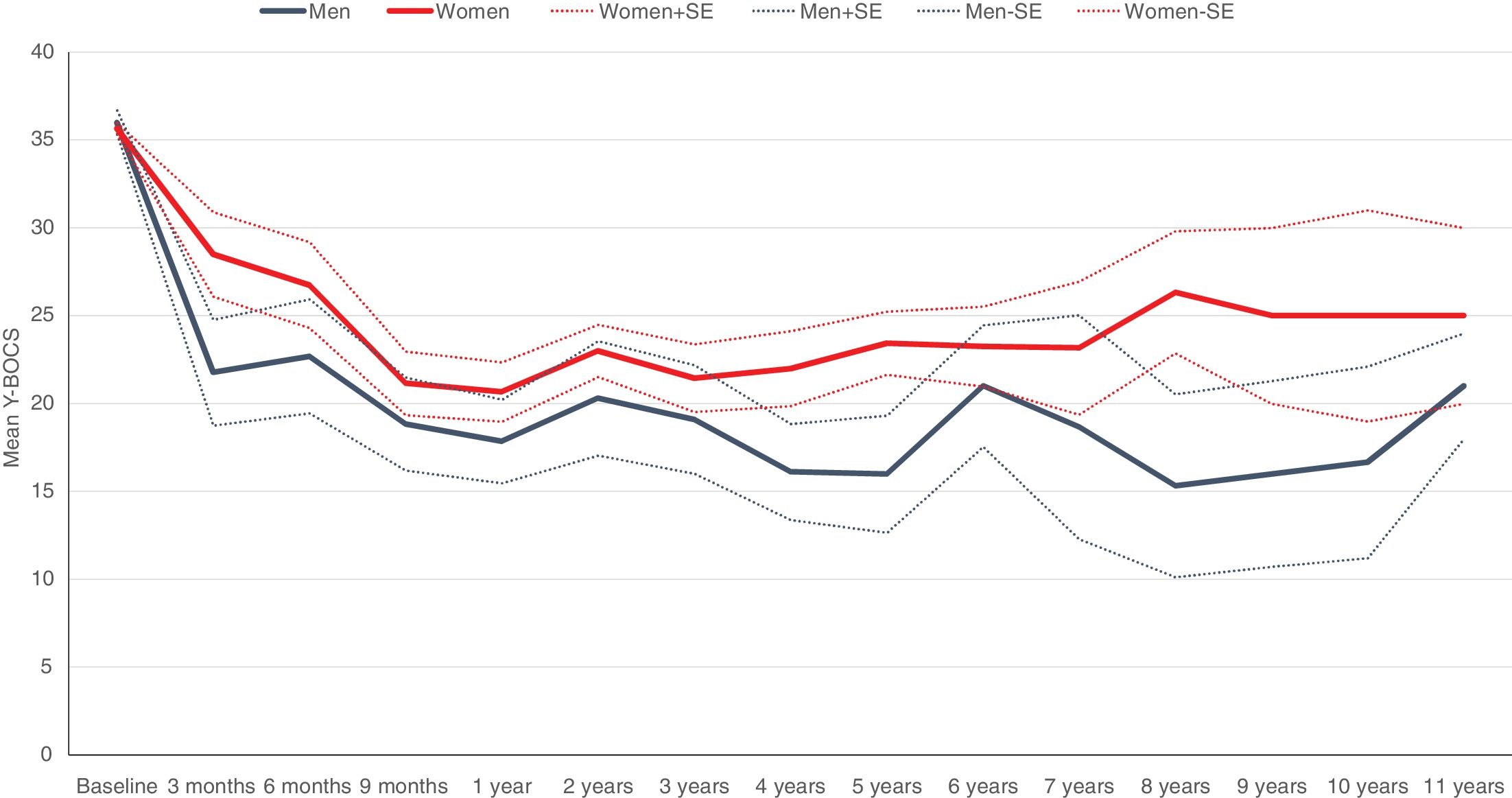
ResultsWe did not observe significant clinical differences between men and women prior to DBS implantation, nor in the response after one year of stimulation. At long-term follow-up, 76.9% of men could be considered responders to DBS versus only 33.3% of women. The final response odds ratio in men was 10.05 with significant confidence intervals (88.90–1.14). No other predictors of response were identified. The sex difference in Y-BOCS reduction was clinically significant, with an effect size of 3.2. The main limitation was the small sample size.
ConclusionsOur results suggest that gender could influence the long-term response to DBS in OCD, a finding that needs to be confirmed in new studies given the paucity of results on predictors of response to DBS.
Obsessive-compulsive disorder (OCD) is characterized by intrusive and repetitive thoughts, images or impulses (obsessions) that generate anxiety, followed by mental or motor actions (compulsions) performed to alleviate it. OCD usually begins in childhood, adolescence or the first years of adult life, and can create major difficulties in social, academic, work and family adaptation.1
There are effective approaches to treat OCD, selective serotonin reuptake inhibitors (SSRIs) and behavioral therapy, in particular, exposure with response prevention, being first-choice options.2 In patients refractory to these first-line approaches, other alternatives include potentiation with second-generation antipsychotics, dual-action antidepressants such as venlafaxine, and cognitive therapy.2 Drugs with glutamatergic action have also been described to be useful in refractory forms of OCD.3 Between 60% and 75% of patients with OCD respond well to the standard combination of psychopharmacological treatment and cognitive-behavioral therapy (CBT), although some show just a partial response.2 Nevertheless, around 5–10% of patients with OCD are refractory to all available treatments and develop extremely severe, disabling forms of the disease,4 this being accompanied by great suffering for both patients and their families.5,6 Over the last two decades, new treatment options have been proposed for these patients, including deep brain stimulation (DBS).7
Treatment of severe and disabling OCD using DBS was proposed in 1999 by Nuttin et al.,8 and has been shown to be effective in ameliorating symptoms in over half of patients undergoing it.9 Nearly 300 DBS devices have been implanted worldwide in various anatomical targets including the ventral capsule/ventral striatum, anterior limb of the internal capsule, subthalamic nucleus, and bed nucleus of the stria terminalis.10–12 Meta-analyses studying the response to DBS in the short term indicate that 60% of patients achieve symptom relief as indicated by ≥35% reduction in the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) score.11–13 Recent studies on the response to DBS in the long term confirm that improvement achieved in the first years is maintained over time.9,10 The uncertainty about individual outcomes, its invasive nature, the possible surgical complications and adverse effects make it fundamental to identify clinical predictors of response to improve patient selection.13,14 Nevertheless, the study of this topic has yielded few results to date. So far, the only factors associated with good response in some studies have been a late age at OCD onset, comorbidity with personality disorder,13,14 sexual and religious obsessions and good insight.14
It has been described that some clinical characteristics of OCD, such as age at onset and symptom dimensions, might be affected by an influence of sex on the disorder.15 Specifically, a higher percentage of patients with early-onset forms of OCD are men, and they are more likely to have order/symmetry symptoms, comorbid tic disorders, and a family history of OCD.15 Women, on the other hand, show later-onset forms of OCD, and are more likely to experience comorbid depression and contamination/cleaning symptoms as their primary symptom dimension.16 Further, the influence of sex on OCD is not limited to clinical characteristics, but could also be related to the etiological bases of the disease. In particular, sex differences have been described in some of the polymorphisms associated with an increased genetic risk of developing OCD17 and numerous studies demonstrate the influence of female sex hormones on the onset or worsening of the disease, at different reproductive stages (menarche, pregnancy, postpartum).18 Different patterns of neuropsychological performance depending on sex among OCD patients have been also described, supporting the hypothesis of sexual dimorphism associated with the disorder.19 Sex does not appear to influence the response to CBT or pharmacological treatment in OCD.2,20 Given the limited number of OCD patients undergoing DBS, to date, it has not been explored neither whether there are sex differences in the pattern of response to neurostimulation at short- and long-term, nor whether such differences could modulate the influence of other predictive variables.
The objective of this study was to analyze whether there are sex differences in the pattern of response and tolerance to DBS in the short- and long-term in patients with severe refractory OCD and whether sex could be a predictor of response to DBS or mediate the predictive value of other clinical variables.
MethodsWe analyzed differences in response to DBS by sex in a sample of twenty-five patients participating in a prospective observational study conducted between 2007 and 2021 to establish long-term efficacy of DBS in patients with severe and refractory OCD.9 Patients were recruited through the OCD Clinical and Research Unit at the Department of Psychiatry in Bellvitge Hospital (Barcelona) between 2007 and 2020 and followed up until 2021. Diagnosis was assigned by two psychiatrists with extensive clinical experience in OCD (P.A. and C. S.), following DSM-IV-TR (from 2007 to 2013) and subsequently DSM-5 criteria for OCD, using the Spanish version of the Mini International Neuropsychiatric Interview (MINI) 6.0 and 7.0.2 versions. The study protocol was approved by the Hospital Clinical Research Ethics Committee. To be eligible, patients had to meet the following inclusion criteria: 1. Diagnosis of severe OCD understood as a total Y-BOCS21 score over 32; 2. OCD refractory to standard treatment defined as (a) failure to respond to a minimum of six attempts with first- and second-line medications, including at least three SSRIs, clomipramine and the addition of two different antipsychotics and (b) failure to respond to CBT (twenty 1-h sessions of in vivo exposure with response prevention); 3. Severe impairment of daily functioning with a Global Assessment of Functioning (GAF)22 score <45; 4. Diagnosis of disabling OCD documented in their medical record more than 5 years earlier; 5. Age over 18 years; and 6. Ability to understand and follow instructions and provide their own written informed consent to participate in the study. The surgical procedure was carried out in the Neurosurgery Department, as described in detail in the annex.
VariablesFor each patient, we collected information on the following variables: age at DBS device implantation, age at OCD onset, sex, years of education, most severe OCD symptom dimension (considering the following categories: 1. Aggressive; 2. Sexual/religious; 3. Symmetry/ordering; 4. Contamination/cleaning; 5. Hoarding; or 6. Miscellaneous), psychiatric comorbidities, adverse effects, and DBS treatment duration. Current and lifetime prevalence of psychiatric comorbidities were assessed through SCID-I.
To assess response to treatment over the follow-up, the Spanish version of the Y-BOCS was used, which measures the severity of obsessive-compulsive symptoms (score range: 0–40).23 The questionnaire was administered at the beginning of the study, at 3, 6, 9 and 12 months after implantation and annually thereafter up to 12 years. Following standard criteria,24 patients were considered responders if their Y-BOCS score decreased by 35% or more.14,24 Given that depressive disorders are the most common comorbidity in patients with OCD, we also used the Hamilton Depression Rating Scale (HDRS) questionnaire to measure the severity of depressive symptoms (score ranging from 0 to 54).25 Global functioning was evaluated at each visit using the GAF. This tool measures social, occupational and psychological functioning (score range: 1–100).22 Patients were assessed for adverse events occurrence at each visit using a checklist containing the most common DBS side effects.
Statistical analysisDifferences in social and clinical characteristics between men and women were assessed using Student's t-test for normally distributed data and Wilcoxon's nonparametric test for ordinal and non-normally distributed data. The significance level was set at p=0.05. Chi-square tests were used to analyze contingency tables. All the statistical analysis were carried out using R, version 3.6.1.
The likelihood of response to DBS by sex was calculated using binary logistic regression. As previously stated, the cut-off for a positive response was set at a ≥35% reduction in Y-BOCS score.24 Considering this cut-off, response to treatment was used as the dependent variable, sex as the independent variable, and age at OCD onset, main OCD symptom dimension, age at DBS surgery and initial Y-BOCS score as adjustment covariates. To compare the early and final response, two different models were built by measuring the level of Y-BOCS score reduction at 1 year (early reduction) and at the end of the follow-up (long-term reduction). The analyses were repeated considering only sex as the independent variable and treatment response as the dependent one, to assess whether the results were maintained when the covariates were not introduced into the model. For HDRS and GAF, logistic models were built to assess the likelihood of a ≥50% reduction in the score of HDRS and a GAF score >60. To compare the logistic regression models, we used the Akaike information criteria (AIC). The lower the AIC is, the better the model. To assess whether the magnitude of any observed sex difference in the reduction in Y-BOCS score was clinically significant, the effect size was calculated,26 dividing the difference between the means of men and women by the pooled standard deviation of their values. The effect is considered negligible when the effect size is less than 0.2, and small, moderate or large when it is between 0.2 and 0.5, between 0.5 and 0.8, or greater than 0.8 respectively.26
A sample size of 17 patients in each group would have been necessary to detect a difference equal or greater than 15% in the percentage of reduction on Y-BOCS scores, assuming a common standard deviation of 15 (data derived from meta-analysis on the response to DBS in OCD13), an estimated rate of follow-up losses of 5%, an alpha risk of 0.05 and a beta risk of less than 0.2 in a bilateral contrast. Our sample size is consequently limited, so results from this study must be confirmed in larger groups of OCD subjects treated with DBS.
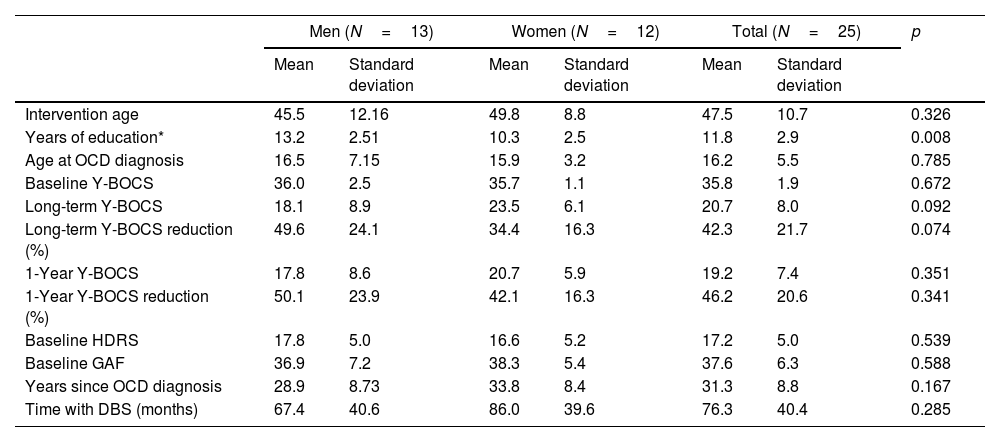
ResultsTable 1 shows the characteristics of the 25 patients who underwent DBS implantation, differentiated by sex: 13 men versus 12 women. The mean age was slightly higher among women, without reaching statistical significance. No statistically significant differences were found by sex in other characteristics such as age at OCD onset, baseline Y-BOCS score, main OCD dimension, years since the neurosurgical technique was performed, and years from OCD onset to DBS implantation. Only years of education were significantly higher in men. Patients were followed for 76.3 months (±40.4; range: 12–156). We observed no significant sex difference in early response (defined as response at 12 months after the start of stimulation) with rates of 61.5% in men and 58.3% in women. On the contrary, significant sex differences emerged in variables associated with the long-term response to DBS, namely, the reduction in Y-BOCS score, mean percentage of Y-BOCS reduction, and final response to treatment, applying the cut-off of a ≥35% reduction in Y-BOCS (see Fig. 1). Notably, 76.9% of men could be considered long-term responders versus only 33.3% of women. A similar pattern of sex influence was observed in changes on comorbid depressive symptoms. While no significant differences were detected between men and women on reductions on HDRS scores on the short term, men showed higher HDRS long-term improvement than women (46.2% vs. 16.7%), although this difference did not reach statistical significance. No specific individual stressor (either due to personal factors or related to the device – battery depletion –) was associated with the long-term evolution of the patients.
Characteristics of OCD patients treated with deep brain stimulation and response to DBS by sex.
| Men (N=13) | Women (N=12) | Total (N=25) | p | ||||
|---|---|---|---|---|---|---|---|
| Mean | Standard deviation | Mean | Standard deviation | Mean | Standard deviation | ||
| Intervention age | 45.5 | 12.16 | 49.8 | 8.8 | 47.5 | 10.7 | 0.326 |
| Years of education* | 13.2 | 2.51 | 10.3 | 2.5 | 11.8 | 2.9 | 0.008 |
| Age at OCD diagnosis | 16.5 | 7.15 | 15.9 | 3.2 | 16.2 | 5.5 | 0.785 |
| Baseline Y-BOCS | 36.0 | 2.5 | 35.7 | 1.1 | 35.8 | 1.9 | 0.672 |
| Long-term Y-BOCS | 18.1 | 8.9 | 23.5 | 6.1 | 20.7 | 8.0 | 0.092 |
| Long-term Y-BOCS reduction (%) | 49.6 | 24.1 | 34.4 | 16.3 | 42.3 | 21.7 | 0.074 |
| 1-Year Y-BOCS | 17.8 | 8.6 | 20.7 | 5.9 | 19.2 | 7.4 | 0.351 |
| 1-Year Y-BOCS reduction (%) | 50.1 | 23.9 | 42.1 | 16.3 | 46.2 | 20.6 | 0.341 |
| Baseline HDRS | 17.8 | 5.0 | 16.6 | 5.2 | 17.2 | 5.0 | 0.539 |
| Baseline GAF | 36.9 | 7.2 | 38.3 | 5.4 | 37.6 | 6.3 | 0.588 |
| Years since OCD diagnosis | 28.9 | 8.73 | 33.8 | 8.4 | 31.3 | 8.8 | 0.167 |
| Time with DBS (months) | 67.4 | 40.6 | 86.0 | 39.6 | 76.3 | 40.4 | 0.285 |
| Men | % | Women | % | Total | % | ||
|---|---|---|---|---|---|---|---|
| 1-Year response (Y-BOCS reduction) | 0.870 | ||||||
| Y-BOCS ≥35% | 8 | 61.5% | 7 | 58.3% | 15 | 60.0% | |
| Y-BOCS <35% | 5 | 38.5% | 5 | 41.7% | 10 | 40.0% | |
| Long-term response (Y-BOCS reduction)* | 0.028 | ||||||
| Y-BOCS ≥35% | 10 | 76.9% | 4 | 33.3% | 14 | 56.0% | |
| Y-BOCS <35% | 3 | 23.1% | 8 | 66.7% | 11 | 44.0% | |
| Patterns of long-term response (Y-BOCS reduction) | 0.032 | ||||||
| Sustained good responders | 8 | 61.5% | 4 | 25.0% | 11 | 44.0% | |
| Fluctuating responders | 2 | 15.4% | 4 | 8.3% | 3 | 12.0% | |
| Persistent non-responders | 3 | 23.1% | 4 | 66.7% | 11 | 44.0% | |
| 1-Year response (HDRS reduction) | 0.543 | ||||||
| HDRS ≥50% | 7 | 53.8% | 5 | 41.7% | 12 | 48.0% | |
| HDRS <50% | 6 | 46.2% | 7 | 58.3% | 13 | 52.0% | |
| Long-term response (HDRS reduction) | 0.053 | ||||||
| HDRS ≥50% | 6 | 46.2% | 2 | 16.7% | 8 | 32.0% | |
| HDRS <50% | 7 | 53.8% | 10 | 83.3% | 17 | 68.0% | |
| 1-Year response GAF | 0.404 | ||||||
| GAF >60 | 7 | 53.8% | 8 | 66.7% | 15 | 60.0% | |
| GAF ≤60 | 6 | 46.2% | 4 | 33.3% | 10 | 40.0% | |
| Long-term response GAF | 0.404 | ||||||
| GAF >60 | 7 | 53.8% | 8 | 66.7% | 15 | 60.0% | |
| GAF ≤60 | 6 | 46.2% | 4 | 33.3% | 10 | 40.0% | |
| OCD dimension | 0.125 | ||||||
| Aggressive obsessions | 6 | 46.2% | 5 | 41.7% | 11 | 44.0% | |
| Religious/sexual obsessions | 0 | 0.0% | 2 | 16.7% | 2 | 8.0% | |
| Symmetry/ordering | 4 | 30.8% | 0 | 0.0% | 4 | 16.0% | |
| Contamination/cleaning | 3 | 23.1% | 4 | 33.3% | 7 | 28.0% | |
| Miscellaneous | 0 | 0.0% | 1 | 8.3% | 1 | 4.0% | |
OCD: obsessive-compulsive disorder; DBS: deep brain stimulation; Y-BOCS: Yale-Brown Obsessive Compulsive Scale; HDRS: Hamilton Depression Rating Scale; GAF: Global Assessment of Functioning.
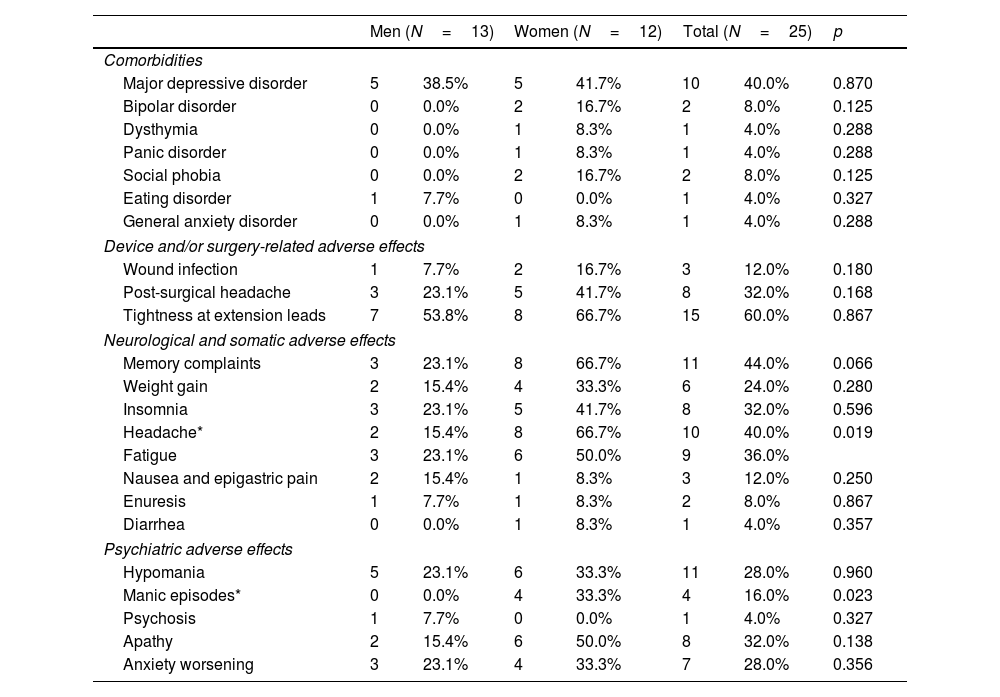
Adverse effects and comorbidities are reported in Table 2. There were no major sex differences in adverse effects, only headache and manic episodes were significantly more common in women than in men. The most common psychiatric side effect was hypomania which appeared in six women and five men. Significant differences were observed for manic episodes that required hospitalization, these occurring in four women. Two of them were patients with a previous comorbid diagnosis of bipolar disorder on mood stabilizer treatment with lithium carbonate. All manic episodes resolved completely with pharmacological treatment (risperidone or olanzapine) and adjustment of stimulation parameters, and did not recur with new stimulation trials. Antipsychotic treatment in these four patients was discontinued less than four weeks after discharge and it was not considered necessary to initiate a mood stabilizer in the two patients who were not previously taking one. There were no manic episodes among men, but one did have a psychotic episode that required hospitalization and remitted with antipsychotic treatment (risperidone).
Comorbidities associated with the obsessive-compulsive disorder diagnosis and adverse effects of deep brain stimulation treatment by sex.
| Men (N=13) | Women (N=12) | Total (N=25) | p | ||||
|---|---|---|---|---|---|---|---|
| Comorbidities | |||||||
| Major depressive disorder | 5 | 38.5% | 5 | 41.7% | 10 | 40.0% | 0.870 |
| Bipolar disorder | 0 | 0.0% | 2 | 16.7% | 2 | 8.0% | 0.125 |
| Dysthymia | 0 | 0.0% | 1 | 8.3% | 1 | 4.0% | 0.288 |
| Panic disorder | 0 | 0.0% | 1 | 8.3% | 1 | 4.0% | 0.288 |
| Social phobia | 0 | 0.0% | 2 | 16.7% | 2 | 8.0% | 0.125 |
| Eating disorder | 1 | 7.7% | 0 | 0.0% | 1 | 4.0% | 0.327 |
| General anxiety disorder | 0 | 0.0% | 1 | 8.3% | 1 | 4.0% | 0.288 |
| Device and/or surgery-related adverse effects | |||||||
| Wound infection | 1 | 7.7% | 2 | 16.7% | 3 | 12.0% | 0.180 |
| Post-surgical headache | 3 | 23.1% | 5 | 41.7% | 8 | 32.0% | 0.168 |
| Tightness at extension leads | 7 | 53.8% | 8 | 66.7% | 15 | 60.0% | 0.867 |
| Neurological and somatic adverse effects | |||||||
| Memory complaints | 3 | 23.1% | 8 | 66.7% | 11 | 44.0% | 0.066 |
| Weight gain | 2 | 15.4% | 4 | 33.3% | 6 | 24.0% | 0.280 |
| Insomnia | 3 | 23.1% | 5 | 41.7% | 8 | 32.0% | 0.596 |
| Headache* | 2 | 15.4% | 8 | 66.7% | 10 | 40.0% | 0.019 |
| Fatigue | 3 | 23.1% | 6 | 50.0% | 9 | 36.0% | |
| Nausea and epigastric pain | 2 | 15.4% | 1 | 8.3% | 3 | 12.0% | 0.250 |
| Enuresis | 1 | 7.7% | 1 | 8.3% | 2 | 8.0% | 0.867 |
| Diarrhea | 0 | 0.0% | 1 | 8.3% | 1 | 4.0% | 0.357 |
| Psychiatric adverse effects | |||||||
| Hypomania | 5 | 23.1% | 6 | 33.3% | 11 | 28.0% | 0.960 |
| Manic episodes* | 0 | 0.0% | 4 | 33.3% | 4 | 16.0% | 0.023 |
| Psychosis | 1 | 7.7% | 0 | 0.0% | 1 | 4.0% | 0.327 |
| Apathy | 2 | 15.4% | 6 | 50.0% | 8 | 32.0% | 0.138 |
| Anxiety worsening | 3 | 23.1% | 4 | 33.3% | 7 | 28.0% | 0.356 |
The results of the logistic regression model analyzing Y-BOCS long-term response (Tables 3 and 4) were highly significant, yielding a final response odds ratio (OR) in men of 10.05 with confidence intervals (CIs) of 88.90 and 1.14. No other variables were statistically significant. Although the OR for Y-BOCS response at 1 year in men was 7.58, it did not reach statistical significance (CI: 263.38, 0.22). Similarly, the OR for the 1-year (OR: 3.89, CI: 29.14, 0.52) and long-term (OR: 5.49, CI: 44.01, 0.68) HDRS changes were non-significant and also for the 1-year (OR: 4.03, CI: 141.93, 0.11) and long-term (OR: 0.88, CI: 1.62, 0.48) GAF changes. The model without covariates yielded the same outcome (Table 4).
Likelihood of long-term and 1-year response to deep brain stimulation based on Yale-Brown Obsessive Compulsive Scale score (≥35% reduction), on Hamilton Depression Rating Scale score (≥50% reduction) and on Global Assessment of Functioning (≥60).
| Long term Y-BOCS | Odds ratio | Upper CI | Lower CI | p |
|---|---|---|---|---|
| Male sex | 10.05 | 88.90 | 1.14 | 0.038 |
| Age at OCD diagnosis | 1.11 | 1.42 | 0.86 | 0.423 |
| Age at DBS surgery | 1.02 | 1.15 | 0.91 | 0.705 |
| Baseline HDRS | 0.91 | 1.13 | 0.74 | 0.403 |
| Baseline Y-BOCS | 0.95 | 1.58 | 0.57 | 0.848 |
| Y-BOCS 1 year | Odds ratio | Upper CI | Lower CI | p |
|---|---|---|---|---|
| Male sex | 7.58 | 263.38 | 0.22 | 0.263 |
| Age at OCD diagnosis | 1.89 | 3.98 | 0.90 | 0.091 |
| Age at DBS surgery | 1.06 | 1.21 | 0.92 | 0.412 |
| Baseline HDRS | 1.23 | 1.68 | 0.91 | 0.183 |
| Baseline Y-BOCS | 1.54 | 3.49 | 0.68 | 0.299 |
| Long term HDRS | Odds ratio | Upper CI | Lower CI | p |
|---|---|---|---|---|
| Age at DBS surgery | 1.04 | 1.17 | 0.92 | 0.524 |
| Male sex | 5.49 | 44.01 | 0.68 | 0.109 |
| Baseline HDRS | 1.02 | 1.24 | 0.83 | 0.856 |
| Age at OCD diagnosis | 0.91 | 1.13 | 0.74 | 0.396 |
| HDRS 1 year | Odds ratio | Upper CI | Lower CI | p |
|---|---|---|---|---|
| Age at DBS surgery | 0.61 | 0.95 | 0.40 | 0.030 |
| Male sex | 3.89 | 29.14 | 0.52 | 0.186 |
| Baseline HDRS | 1.02 | 1.24 | 0.84 | 0.851 |
| Age at OCD diagnosis | 1.00 | 1.21 | 0.83 | 0.981 |
| Long term GAF | Odds ratio | Upper CI | Lower CI | p |
|---|---|---|---|---|
| Age at DBS surgery | 1.04 | 1.15 | 0.94 | 0.484 |
| Male sex | 0.69 | 3.91 | 0.12 | 0.671 |
| Baseline GAF | 0.97 | 1.11 | 0.85 | 0.699 |
| Age at OCD diagnosis | 0.91 | 1.11 | 0.75 | 0.361 |
| GAF 1 year | Odds ratio | Upper CI | Lower CI | p |
|---|---|---|---|---|
| Age at DBS surgery | 1.08 | 1.21 | 0.96 | 0.197 |
| Male sex | 0.74 | 4.42 | 0.13 | 0.745 |
| Baseline GAF | 0.95 | 1.09 | 0.83 | 0.494 |
| Age at OCD diagnosis | 0.93 | 1.14 | 0.76 | 0.483 |
DBS: deep brain stimulation; OCD: obsessive-compulsive disorder; Y-BOCS: Yale-Brown Obsessive Compulsive Scale; HDRS: Hamilton Depression Rating Scale; CI: confidence interval; GAF: Global Assessment of Functioning.
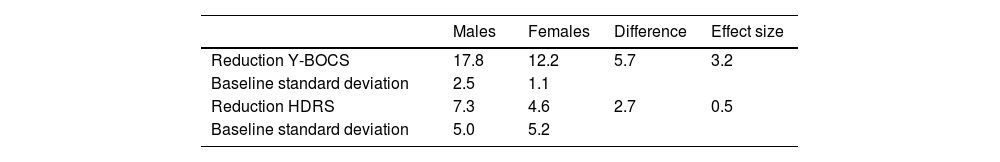
The effect sizes are listed in Table 5 and also indicate that the sex difference in Y-BOCS reduction was clinically significant since it was associated with an effect size of 3.2, which according to Cohen's criteria can be classified as very large.
Effect size of the sex differences in reductions in Yale-Brown Obsessive Compulsive Scale and Hamilton Depression Rating Scale scores.
| Males | Females | Difference | Effect size | |
|---|---|---|---|---|
| Reduction Y-BOCS | 17.8 | 12.2 | 5.7 | 3.2 |
| Baseline standard deviation | 2.5 | 1.1 | ||
| Reduction HDRS | 7.3 | 4.6 | 2.7 | 0.5 |
| Baseline standard deviation | 5.0 | 5.2 |
Y-BOCS: Yale-Brown Obsessive Compulsive Scale; HDRS: Hamilton Depression Rating Scale.
The main finding of this study is that sex seems to significantly influence the long-term response to DBS in severe refractory OCD, an association being found between a better and more sustained response to DBS and male sex, regardless of other variables such as age at OCD onset or main OCD symptom dimension. Although these results should be interpreted with caution given the small sample size of our study, they could help in the task of identifying predictors of response to DBS, a field of research with very limited results to date.
Even though the results are not conclusive, some studies in patients with Parkinson's disease treated with DBS have described a different response by sex. Accolla et al.,27 detected a smaller reduction in some symptoms of the disease, like bradykinesia, after one year of DBS in female patients. In the same vein, Romito et al.,28 observed a poorer initial response of motor symptoms in female patients in the first year of stimulation, with more lower limb akinesia and poorer gait score, though sex differences become insignificant after five years of treatment. On the other hand, Dietrich et al.,29 described that female patients with Parkinson's disease treated with DBS improved significantly more than their male counterparts in levels of depression associated with the disease, showed greater gains in quality of life and managed to significantly reduce the doses of levodopa necessary to control their motor symptoms. Finally, Golfrè Andreasi et al.,30 did not find significant sex differences in response to DBS, but did note that female patients might require different adjustments to stimulation parameters and specific modifications of comorbid treatments to achieve as good outcomes as male patients. This last finding seems especially important given that the limited clinical experience in the use of DBS in OCD leads to the use of the same stimulation parameters, at least initially, for both sexes, while this might not be the best therapeutic alternative. Moreover, it would be extremely interesting to explore whether there are differences by sex in the stimulation parameters associated with the optimal response and take this into account in future studies.
The Parkinson's disease literature has tended to attribute variations in response to DBS by sex to differences in the structure, function, and connectivity of the basal ganglia between men and women, as well as to the possible influence of hormones on the neurodevelopment and functioning of some brain regions.27 In fact, a marked sex dimorphism has been described in brain regions with a high number of estrogen receptors.28 Specifically, after correcting for global brain volume, men have larger gray matter volumes in the amygdala and hippocampus, while women tend to have larger volumes in the orbitofrontal cortex, hippocampus, and caudate nucleus.31 It is noteworthy that many of these areas with marked sex dimorphism have been implicated in the etiopathogenesis of OCD and are part of the cortico-striatal-thalamo-cortical circuit that is postulated to be dysfunctional in OCD, this being precisely what DBS seeks to regulate.32 Supporting the view that there are sex differences between brain regions involved in OCD, a study in twins by den Braber et al.,33 described that regions that were related to obsessive symptoms, including the left middle temporal gyrus, right middle temporal gyrus, and right precuneus, had different volumes between men and women. Studies analyzing sex differences in brain structure in patients with OCD are very scarce. In most cases, the influence of sex tends to be included as a confounding variable, with no direct analysis of differences between men and women.32 In a recent study, Ma et al.,34 described sex differences in resting brain functional connectivity in patients with OCD. Compared to males, female OCD patients showed higher amplitude of low-frequency fluctuations in the right parahippocampal gyrus, a part of the limbic system related to executive control and emotional regulation. Female OCD patients also showed a significantly decreased functional connectivity between the right parahippocampal gyrus and several brain regions including the right posterior central gyrus/precentral gyrus/superior temporal gyrus/barycentric lobule and left anterior cuneus.34 Bearing in mind that the beneficial effect of DBS is based precisely on its ability to modulate the connectivity between different brain regions, it would be crucial in future studies to ascertain whether there are pre-surgical sex differences in brain connectivity in patients with severe refractory forms of OCD as this might allow us to predict which patients would benefit most from DBS.
There are also notable similarities between our findings related to age at surgery and those from studies on the use of DBS in Parkinson's disease.35 Although the difference did not reach statistical significance, in our sample, women seem to take longer to receive DBS than men, being older at the time of DBS implantation and having a longer history of illness, a factor that might be potentially associated with a poorer response to DBS. In the case of Parkinson's disease, numerous studies indicate that women tend to be more fearful of the possible side effects of an invasive neurosurgical procedure such as DBS and more likely to reject this type of approach than men.36 Future work should explore whether this same pattern of differential access to DBS by sex also occurs in the case of OCD, and if so, explore the underlying reasons.
Sex does not seem to significantly influence the overall response to pharmacological or cognitive-behavioral treatments in OCD, unlike other factors such as age, family history of OCD, baseline severity, age at onset, insight, and certain symptom dimensions.37–39 Nonetheless, the subgroup of patients with particularly severe forms of OCD who are refractory to all other types of treatment and receive DBS might have distinct characteristics at the brain level that might explain an influence of sex on the long-term response to DBS.
Although DBS was generally a well-tolerated treatment, it is important to note that women showed more adverse effects of all kinds than men except for nausea and epigastric pain. These differences were especially evident in the appearance of manic symptoms and headache. The small sample size does not allow us to hypothesize about the reasons for this poorer tolerance, but future studies should address whether the factors that explain it are the same ones that underlie poorer long-term response. Although DBS is an effective therapy for a significant percentage of patients with refractory OCD and tends to be well tolerated, it is not exempt from potentially serious complications and has high financial and human resource costs. For these reasons, it is very important to identify variables that might help predict which patients are likely to respond best. To date, this search has yielded no conclusive results. A late onset of OCD and sexual/religious obsessions were associated with a better response to DBS in the meta-analysis by Alonso et al.,13 while Graat et al.,14 described better insight as the only variable significantly associated with a better response to stimulation. In our study, we did not observe clear predictors of short-term response to DBS, although there was a non-significant trend toward a better response in patients with more advanced age at OCD onset, consistent with what has been found in some meta-analyses.40 On the other hand, in our sample, patterns of long-term response differed significantly between the sexes. Specifically, men showed a good initial response that was maintained and even strengthened over time, with two out of three patients meeting the criteria for response, while women showed a similar initial level of improvement, but their response decreased significantly over the years and only one in three women could be considered responders. The few studies published on the long-term response to DBS in OCD have not analyzed potential sex differences.14 If our results are confirmed, future studies should evaluate what changes at the brain level might underlie the long-term loss of efficacy of DBS in women. There is also a need to explore the possible importance of considering sex in optimizing stimulation parameters, to achieve the best possible response to DBS.
Regarding clinical implications, with the current level of evidence, it is not possible to make recommendations concerning sex to be applied in patient selection for DBS. Patient selection still must be based on the severity of the obsessive-compulsive symptoms, a lack of response to conventional treatment, a long history of OCD, and functional impairment that is disabling in daily life.13,14 Further, the late improvement in a subset of men in our sample support the importance of keeping the device active beyond one year despite a lack of initial response.9
The main limitation of our study is the small sample size. This limitation is systematically commented on in publications on DBS and can only be overcome when international collaborations are achieved allowing individual data of patients from different groups to be aggregated.11,12 This way, it would be possible to improve the level of evidence supporting the decision to apply DBS for the treatment of severe, refractory and disabling OCD.
ConclusionsThe conclusion of our study is that male sex is associated with a better long-term response to DBS treatment in severe resistant OCD, a finding that should be replicated in future studies. It remains essential to study what factors can predict the short- and long-term response to DBS in OCD, given that the technique, although effective in a significant number of patients, is not risk-free and entails a high economic and human resource cost.
FundingPA, ER, SB, CS and JMM acknowledge Carlos III Health Institute (PI 18/00856 and PI 22/00752) and FEDER founds (“A way to build Europe”). SB was supported by a Río Hortega grant (CM21/00278) from the Carlos III Health Institute (co-funded by the European Social Fund. Investing in your future). We thank the CERCA Programme/Generalitat de Catalunya for institutional support.
Conflict of interestPA, ER, CS, MAA, GP and JMM participated in a clinical trial sponsored by Medtronic to monitor the safety and performance of electrical stimulation of the AIC in patients with chronic, severe, treatment-resistant OCD from 2014 to 2017 (ClinicalTrial.gov identifier: NCT01135745). The other authors report no biomedical financial interest or potential conflicts of interest.
Authors’ contributionsLorea Mar-Barrutia: Conceptualization, Investigation, Writing – original draft; Oliver Ibarrondo: Methodology, Software, Formal analysis, Writing – review & editing; Javier Mar: Methodology, Formal analysis, Writing – original draft; Eva Real: Investigation, Validation, Writing – review & editing; Cinto Segalàs: Investigation, Validation, Writing – review & editing; Sara Bertolín: Investigation, Validation, Writing – review & editing; Marco Alberto Aparicio: Investigation, Validation, Writing – review & editing; Gerard Plans: Investigation, Validation, Writing – review & editing; José Manuel Menchón: Investigation, Validation, Writing – review & editing; Pino Alonso: Conceptualization, Investigation, Writing – original draft. All authors revised the manuscript for important intellectual content and approved the final version. Further, they all had full access to all the data used in the study and accepted responsibility to submit for publication.