In this work we report the oxidation of volatile organosulfur compounds (VOC) catalyzed by the enzyme chloroperoxidase from Caldariomyces fumago. VOC are regarded as atmospheric pollutants due to their unpleasant odor and low detection threshold. Diethyl sulfide, dimethyl disulfide, propanethiol, butanethiol and hexanethiol were found to be substrates for the enzyme in a liquid medium reaction at pH 6, under peroxidatic conditions. Product analysis showed that sulfides were oxidized to their respective sulfoxides whereas thiols were oxidized to the corresponding disulfides. The identified products showed significantly lower vapor pressure than the parental compounds; thus, the products are not considered atmospheric pollutants. A 70-mL two-phase reactor was assembled in order to determine the efficiency of the enzymatic treatment. The liquid phase, consisting of 15% organic solvent and 85% buffer, was contacted with the gaseous phase, consisting of a substrate-enriched air stream. Using dimethyl disulfide as model substrate, we found that only enzymatic oxidation occurred in this system; by controlling the enzyme and peroxide concentration, we found that the substrate is transferred to the aqueous phase where 1mol of enzyme converted approximately 12,400mol of substrate, thus highlighting the potential of enzymatic treatment of malodorous gaseous streams.
En este trabajo reportamos la oxidación de una serie de compuestos organoazufrados volátiles (COV) catalizada por la enzima cloroperoxidasa obtenida del hongo Caldariomyces fumago. Los COV se consideran contaminantes atmosféricos debido a su olor desagradable y a su bajo umbral de detección. El sulfuro de etilo, disulfuro de dimetilo, propanotiol, butanotiol y hexanotiol fueron transformados por la enzima en un medio de reacción acuoso a pH 6 y en presencia de peróxido de hidrógeno. El análisis de los productos demostró que los sulfuros fueron oxidados a sus respectivos sulfóxidos, mientras que los tioles fueron oxidados a sus correspondientes disulfuros. Los productos identificados tienen una presión de vapor significativamente menor que los compuestos originales, por lo que son mucho menos volátiles y por tanto no se consideran contaminantes atmosféricos. Se ensambló un reactor de dos fases de 70mL de volumen con el fin de determinar la eficiencia del tratamiento enzimático. La fase líquida, compuesta por 85% de amortiguador y 15% de solvente orgánico, se puso en contacto con la fase gaseosa, compuesta por aire enriquecido con el sustrato. Usando disulfuro de metilo como sustrato modelo, encontramos únicamente reacción enzimática en este sistema; al controlar la concentración de enzima y de peróxido en la fase líquida se logró transferir el sustrato a la fase acuosa en donde 1mol de enzima convirtió aproximadamente 12,400 moles de sustrato, resaltando el potencial de los tratamientos enzimáticos para las corrientes gaseosas con mal olor por COV.
Chloroperoxidase (CPO, EC 1.11.1.10) from Caldariomyces fumago is an attractive enzyme for application in several and diverse fields such as the fine chemical and pharmaceutical industry1, diagnosis2 as well as the oil-related industry3 and environment remediation4,5. The versatility of CPO is determined by its ability to catalyze different reactions under controlled conditions, including halogenation (chlorination, bromination and iodination), dehydrogenation, oxygen insertion and peroxide dismutation. In addition, low substrate specificity allows CPO to catalyze the transformation of a wide range of compounds of different chemical nature6. One of the most interesting catalytic modality of CPO is the peroxygenation activity, which catalyzes the oxidation of olefins and sulfur compounds to generate epoxides and sulfoxides, respectively1,6
The broad specificity displayed by CPO towards organosulfur compounds is well established. The enantioselective generation of (R)-sulfoxides catalyzed by CPO has been thoroughly studied. The enzyme is able to catalyze the oxidation of β-carbonyl sulfides, cycloalkyl methyl sulfides, alkyl aryl sulfides, dialkyl sulfides and cyclic sulfides to their respective (R)-sulfoxides7-9. The enantioselectivity is generally favored in the absence of halogen ions and pH near neutrality (pH 6). On the other hand, under acidic conditions (pH 3) and in the presence of chloride, the enzyme catalyzes the oxidation of sulfides to the racemic mixture of sulfoxides10. Under these conditions, the sulfoxidation of aromatic and heterocyclic compounds has been reported11. Furthermore, significant enzymatic transformation of the sulfur-containing fraction in straight diesel fuel has been demonstrated, thus highlighting the ability of CPO to catalyze the oxidation of chemically diverse organosulfur compounds12.
Short-chain, volatile organosulfur compounds (VOC) have low odor threshold and very unpleasant odor, characteristic of the putrid odor of rotten egg and vegetables13. Exposure to even low concentrations of these compounds leads to dizziness, vomiting, headaches and eye irritation14. Thus, these compounds are classified as atmospheric pollutants particularly affecting the area surrounding sources of emission, such as waste water treatment plants, composting sites, paper pulping process and thermal-sludge treatment plants15.
Considering the low specificity of CPO for sulfur compounds and the environmental problem that VOC represent, it was interesting to study the ability of the enzyme to catalyze the oxidation of short-chain, volatile sulfides and thiols. The results are focused on the kinetic behavior of the enzyme, the identification of products and the operation of a two-phase reactor for the removal of VOC from a gaseous stream.
Materials and methodsChemicals and enzyme. CPO was produced from Caldariomyces fumago UAMH 89362 and purified as reported elsewhere16 obtaining a preparation with a Rz (A398nm/A280nm) of at least 1.2. Hydrogen peroxide, diethyl sulfide, dimethyl disulfide, propanethiol, butanethiol, hexanethiol and phenyl sulfide were purchased from Sigma Co. Organic solvents and salts were obtained from J.T. Baker.
Kinetic characterization of CPO-catalyzed oxidation of VOC. Biocatalytic oxidation of organosulfur compounds was performed at 25°C in closed vials, to prevent volatilization of the substrates. Kinetic characterization was performed using substrate concentrations of up to 5mM, due to insolubility of the compounds. The reaction mixture contained 10% acetonitrile, 60mM phosphate buffer pH 6 and 1mM H2O2. The reaction was started by addition of the enzyme (0.05-0.1 nmol). Substrate conversion was monitored by UV absorbance (typically 220-250nm in a Perkin Elmer DAD) through reverse phase HPLC (Hypersil ODS 2.1 X 100μm column from Agilent), using a 30% acetonitrile/70% water isocratic phase. Reduction in the area of the UV signal was used to calculate substrate conversion. Reaction rate was calculated as the ratio of substrate conversion to reaction time. Controls with peroxide and without enzyme were also performed, in order to calculate the non-enzymatic conversion. Kinetic characterization was performed using substrate concentrations of up to 5mM, due to insolubility of the compounds. Given that saturation could not be achieved in all cases, the value of kcat/Km was calculated from the slope of the linear section in the initial rate vs substrate concentration plot. For the control reactions, in the absence of the enzyme and in the presence of peroxide, the rate constant of non-enzymatic conversion (knon) was also calculated from the slope of rate vs substrate concentration plot. Results shown are the average of at least three independent reactions.
Product identification. For product identification, 10-mL reactions were monitored until at least 70% substrate conversion was achieved. The reaction mixture was extracted with methylene chloride, dried through an anhydride Na2SO4 bead and analyzed through gas chromatography (Agilent 6890N) coupled to a mass selective detector (Agilent 5973). A nonpolar HP-5ms column (30m x 0.25mm x 0.25μm) operating at low temperature (typically 40°C) was used to separate and identify the products.
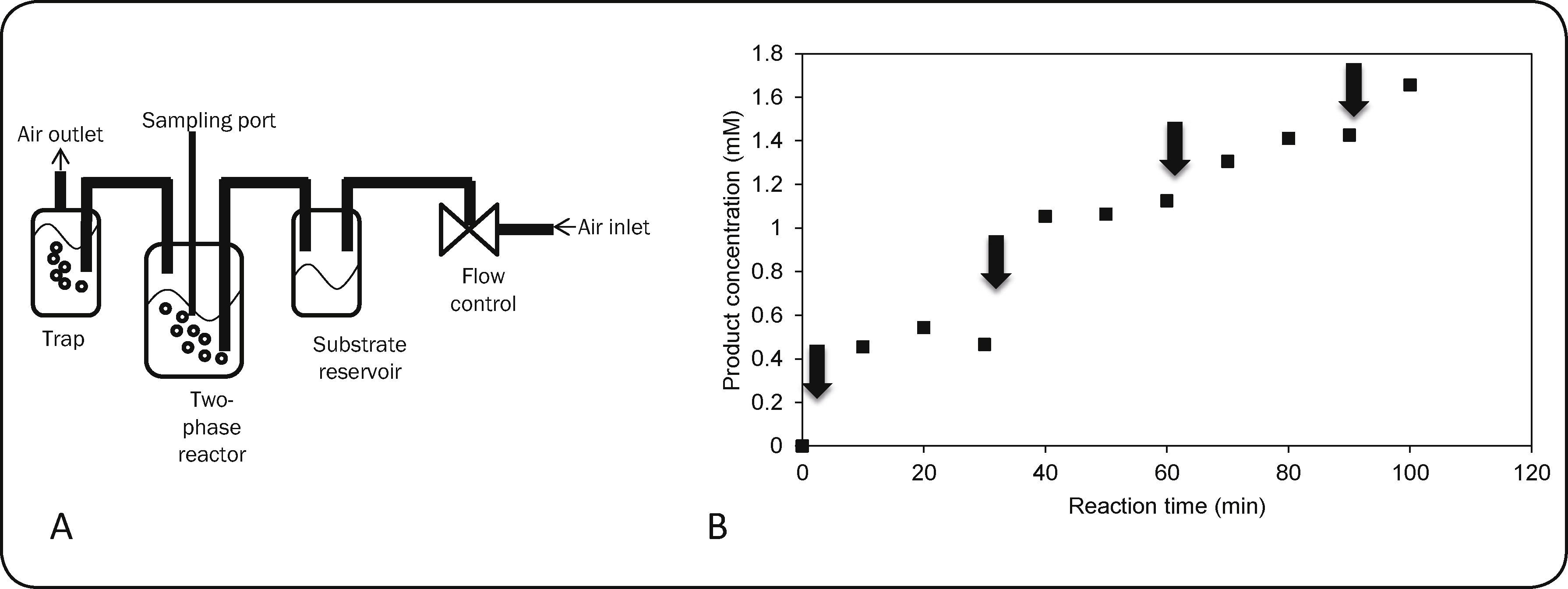
Reactor operation. A two-phase reactor was assembled as follows. An air stream was passed through a reservoir containing 2mL of 226.1mM of dimethyl disulfide (DMDS) in acetonitrile and the resulting DMDS-enriched stream was bubbled at the bottom of a glass vessel containing 70mL of the liquid reaction medium (15% tert-butanol and 85% 60mM phosphate buffer pH 6). Gas flow was maintained between 40-60mL/min, total duration of each experiment (i.e. until the reservoir was exhausted) was around 80-90min. Enzyme (1μmol CPO = 40,000 enzyme units) and peroxide were successively added and samples were taken from the liquid phase to be analyzed by HPLC. The reaction was monitored using 230nm for product detection, as substrate concentration in the liquid medium was extremely low and could not be accurately determined.
Conversion calculations of the enzymatic reaction in the reactor. Product concentration in the system was calculated form a calibration curve. The ratio of total product accumulated in the liquid phase to the total amount of enzyme added to the liquid phase was calculated for each condition tested in this study. Substrate conversion was referred to the theoretically limiting substrate, H2O2 in most cases. Experiments were performed at least in duplicate.
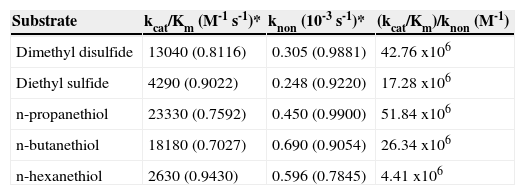
Results and discussionAll compounds tested in this work were transformed in the presence of CPO at pH 6 in a reaction medium containing 10% organic solvent. Under these conditions, non-enzymatic oxidation of the substrates also occurred. The rate of the enzyme-catalyzed reaction was calculated as the difference between the total rate in the presence of the enzyme and peroxide minus the control rate in the presence of only peroxide. In some cases, enzyme saturation could not be achieved due to low substrate solubility. However, the pseudo-first order rate constants of the catalyzed (kcat/Km) and non-enzymatic (knon) reactions were calculated from the linear section of the rate vs substrate concentration plot, as described in the experimental section, and are shown in Table I. The catalytic proficiency, also shown in Table I, is the ratio of these rate constants and has been interpreted by other authors as the ability of enzymes to lower the activation barrier of the reaction17. In units of concentration, the catalytic proficiency relates to the affinity between the enzyme and the chemically distorted substrate in the transition state. According to our data, the dissociation constant of VOCs is in the range of 100nM or less, suggesting a mild interaction within the active center of the enzyme.
Kinetic characterization of CPO-catalyzed (kcat/Km) and uncatalyzed (knon) oxidation of volatile organosulfur compounds in a system containing 10% acetonitrile.
| Substrate | kcat/Km (M-1 s-1)* | knon (10-3 s-1)* | (kcat/Km)/knon (M-1) |
|---|---|---|---|
| Dimethyl disulfide | 13040 (0.8116) | 0.305 (0.9881) | 42.76 x106 |
| Diethyl sulfide | 4290 (0.9022) | 0.248 (0.9220) | 17.28 x106 |
| n-propanethiol | 23330 (0.7592) | 0.450 (0.9900) | 51.84 x106 |
| n-butanethiol | 18180 (0.7027) | 0.690 (0.9054) | 26.34 x106 |
| n-hexanethiol | 2630 (0.9430) | 0.596 (0.7845) | 4.41 x106 |
*In parenthesis, the R2 of the linear correlation is shown.
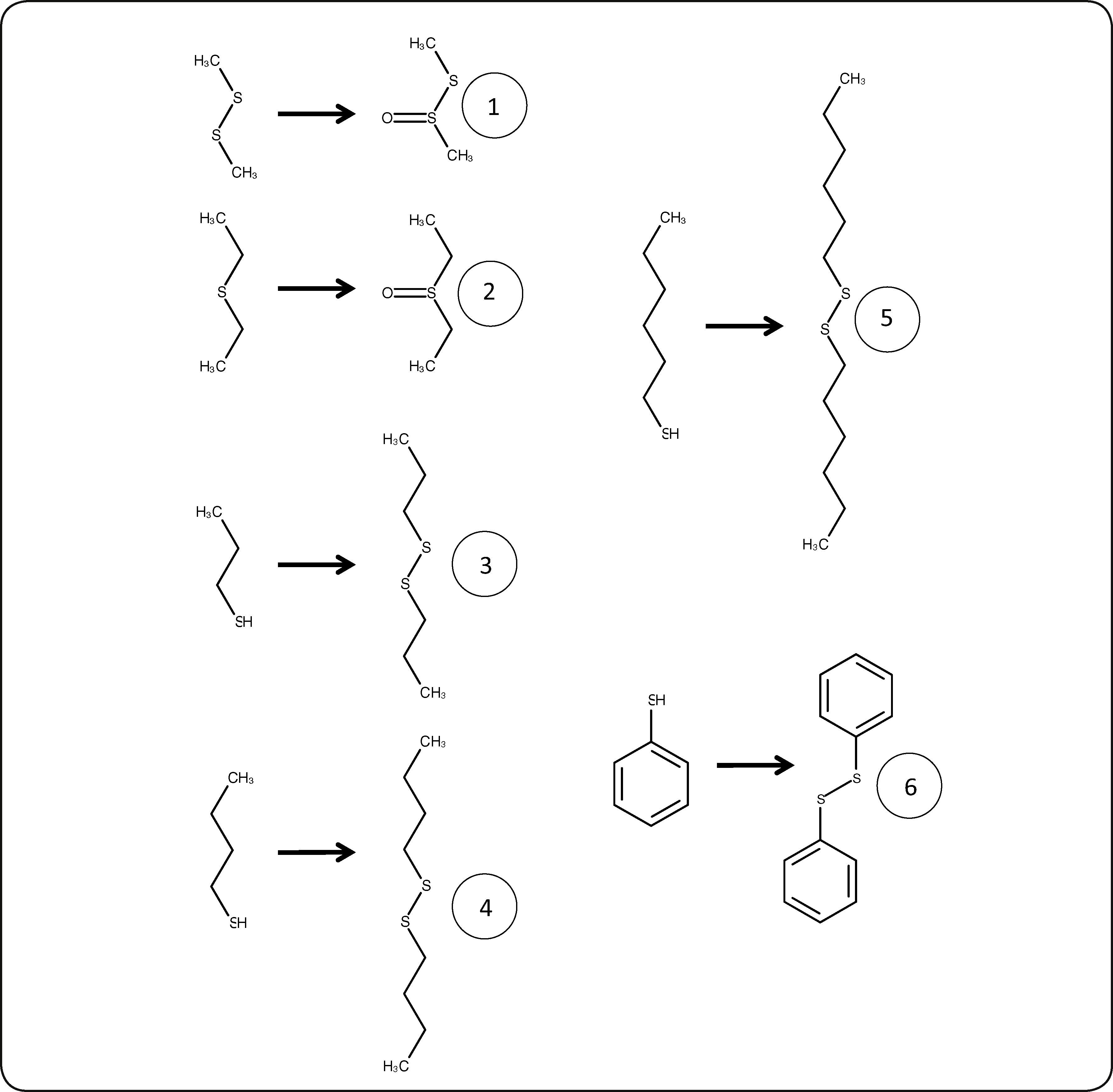
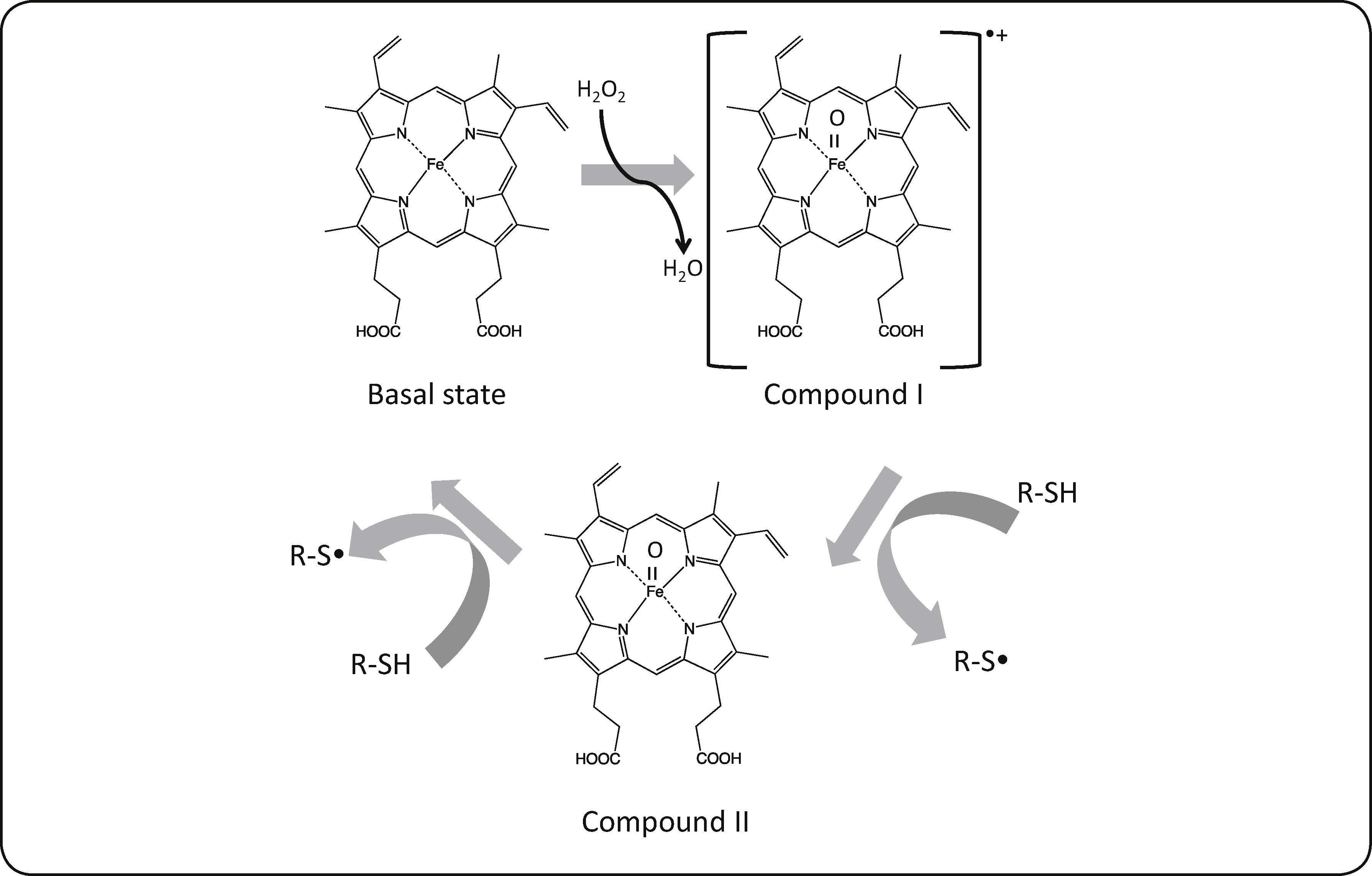
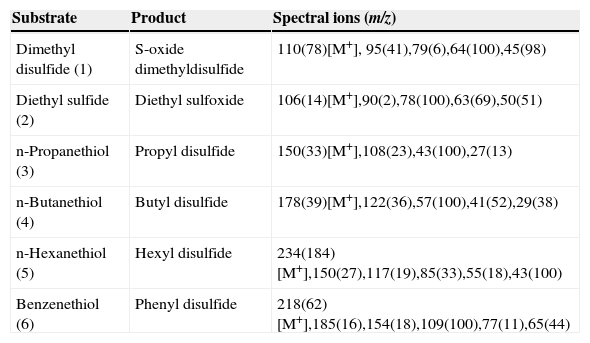
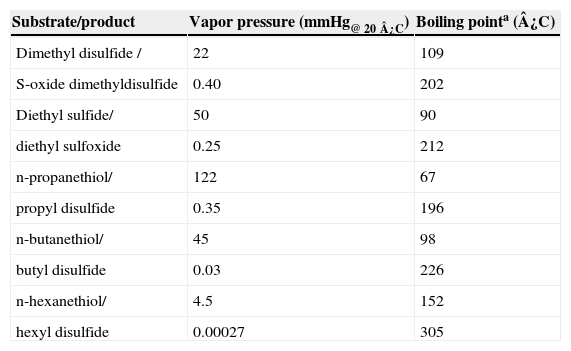
The reaction products, identified by mass spectrometry, are shown in Table II and Figure 1; no other products could be detected. Physical properties of substrates and products are shown in Table III. The oxidation generates compounds with higher boiling points, reduced vapor pressures and thus decreased volatility. Consequently, the oxidation decreases the atmospheric pollutant character of the studied sulfur compounds. We observed that the oxidation of sulfides generated the corresponding sulfoxides, as expected. It is well known that the CPO catalytic mechanism for the oxidation of sulfides involves a direct oxygen transfer within the active site of the enzyme, as evidenced by almost quantitative incorporation of labeled oxygen from H2O218 into the sulfoxide19. The formation of a short-lived sulfur-based free radical has also been invoked, but has not been demonstrated yet20. On the other hand, we observed that thiols were oxidized to the corresponding disulfides. This reaction has not been described before for CPO. The non-enzymatic formation of a disulfide has been reported as a consequence of CPO-catalyzed halogenation of thiols at acidic pH21, thus ruling out a similar mechanism under the peroxidatic conditions (i.e pH 6 and absence of KCl) used in the present study. In order to further confirm this type of reaction being catalyzed by CPO, we also identified diphenyl disulfide (see Table II) as the product of the oxidation of an aromatic thiol, benzenethiol. This reaction has been reported for other heme proteins such as myeloperoxidase (MPO) and horseradish peroxidase (HRP) using aliphatic thiols as substrates; it has been noted that oxidation rate is widely variable for these enzymes. For example, HRP-catalyzed oxidation of cysteine and cysteine derivatives showed rate constants in the range of 24-660 M-1 s-1; only cysteine methyl ester and 2,3-dimercaptopropanol showed higher oxidation rate constants, in the order of 103 and 104 M-1 s-1, respectively22. On the other hand, MPO seemed less sensitive to the nature and charge of the substrate, which were oxidized with rate constants in the range of 103-104 M-1 s-123. In the case of the substrates analyzed in this work, the rate constants of the CPO-catalyzed reaction are in the range of 103-104 M-1 s-1 (Table I), thus highlighting the potential of this versatile enzyme. Regarding the possible mechanism, the reaction could involve the formation of free radicals; it has been suggested that the first enzymatic intermediary of the catalytic cycle, an oxo-ferryl radical cation called Compound I, may be able to subtract an electron from the thiol group; the free radical, after losing a proton, combines with a second free thyl radical to form the disulfide (Figure 2).
Mass spectra data of the products generated from CPO-catalyzed oxidation of VOC.
| Substrate | Product | Spectral ions (m/z) |
|---|---|---|
| Dimethyl disulfide (1) | S-oxide dimethyldisulfide | 110(78)[M+], 95(41),79(6),64(100),45(98) |
| Diethyl sulfide (2) | Diethyl sulfoxide | 106(14)[M+],90(2),78(100),63(69),50(51) |
| n-Propanethiol (3) | Propyl disulfide | 150(33)[M+],108(23),43(100),27(13) |
| n-Butanethiol (4) | Butyl disulfide | 178(39)[M+],122(36),57(100),41(52),29(38) |
| n-Hexanethiol (5) | Hexyl disulfide | 234(184)[M+],150(27),117(19),85(33),55(18),43(100) |
| Benzenethiol (6) | Phenyl disulfide | 218(62)[M+],185(16),154(18),109(100),77(11),65(44) |
Values in parentheses are relative abundances. [M+], molecular ion.
Identified products of thiol oxidation catalyzed by chloroperoxidase in a reaction system containing 10% acetonitrile. Numbering corresponds with Table II.
Physical properties of the studied VOC and the generated products.
| Substrate/product | Vapor pressure (mmHg@ 20¿C) | Boiling pointa (¿C) |
|---|---|---|
| Dimethyl disulfide / | 22 | 109 |
| S-oxide dimethyldisulfide | 0.40 | 202 |
| Diethyl sulfide/ | 50 | 90 |
| diethyl sulfoxide | 0.25 | 212 |
| n-propanethiol/ | 122 | 67 |
| propyl disulfide | 0.35 | 196 |
| n-butanethiol/ | 45 | 98 |
| butyl disulfide | 0.03 | 226 |
| n-hexanethiol/ | 4.5 | 152 |
| hexyl disulfide | 0.00027 | 305 |
a, Ref. 18
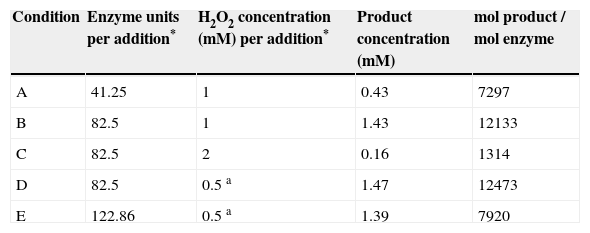
In order to test the ability of CPO to detoxify a gaseous effluent, an air stream was enriched with dimethyl disulfide (DMDS) as described in the experimental section and contacted with an aqueous reaction medium containing pH 6 phosphate buffer and 15% tert-butanol in order to favor the solubility of the substrate and stability of the enzyme; for this system tert-butanol was chosen as organic solvent because it has been reported to be less deactivating for the enzyme, compared to acetonitrile. The system is schematized in Figure 3A. DMDS was selected as model substrate due to a higher enzymatic conversion compared to the non-enzymatic conversion (Table I). Different enzyme and hydrogen peroxide concentrations were assayed and product formation was monitored through HPLC, obtaining profiles similar to the one shown in Figure 3B. Product formation was attributed solely to enzyme catalysis, as proper controls showed little chemical oxidation in the absence of enzyme (less than 0.1mM product), probably due to low substrate concentration in the system. The maximum amount of product found in the aqueous solution was 1.46mM. The ratio of mol of product per mol of added enzyme (P/E) was calculated under different conditions, as shown in Table IV. The P/E ratio increased with enzyme concentration, up to a maximum value near 12,100mol of product/mol of enzyme (condition A and B); further addition of enzyme resulted in lower P/E ratio (condition E). These observations suggest that the reaction is not limited by the amount of enzyme present in the reaction. If all of the substrate in the reservoir was transferred to the liquid phase and converted to product, a maximum concentration of 6.46mM would be expected. As total H2O2 is 4mM, the oxidant could be limiting the substrate conversion, thus successive additions of 2mM H2O2 (total 8mM) were tested (condition C); however, higher concentrations of H2O2 led to extremely low concentrations of product. It is known that in the absence of an efficient electron donor substrate, peroxidases are inactivated in a suicide-like fashion24,25. Finally, in order to test whether addition of a similar amount of H2O2 (3.5mM) but dosified in more additions (7 additions of 0.5mM) could lead to higher P/E ratios, the amount of H2O2 was dosified in condition D. However, similar product concentration and P/E ratio was obtained, although a smoother profile for product generation was noticed.
Product concentration and enzyme consumption for different CPO and H2O2 concentrations in the two-phase reactor.
| Condition | Enzyme units per addition* | H2O2 concentration (mM) per addition* | Product concentration (mM) | mol product / mol enzyme |
|---|---|---|---|---|
| A | 41.25 | 1 | 0.43 | 7297 |
| B | 82.5 | 1 | 1.43 | 12133 |
| C | 82.5 | 2 | 0.16 | 1314 |
| D | 82.5 | 0.5 a | 1.47 | 12473 |
| E | 122.86 | 0.5 a | 1.39 | 7920 |
* 4 additions a: 7 additions
In conclusion, CPO from C. fumago is able to catalyze the oxidation of short-chain sulfides and mercaptans to generate the corresponding sulfoxides and disulfides. The products are less volatile compounds, and its ability to pollute the atmosphere is thus reduced by oxidation. Based on limiting concentrations of peroxide, a ca. 40% conversion of the volatile compound contained in a gaseous stream was achieved, by controlling the concentration of enzyme in the reactor.
This example of a peroxidase able to catalyze the oxidation of volatile sulfur hydrocarbons highlights the versatility of low specificity enzymes in the environmental field; this reaction could be of practical application in the remediation of gaseous effluents. Further improvement of the system is necessary, through reactor engineering, in order to improve substrate transfer to the liquid medium.
The results presented in this work are part of the master thesis of Julio C. Cruz (UNAM) and bachelor thesis of Jesús García (Universidad Politécnica del Estado de Morelos). This work was supported by PAPIIT UNAM IN212510, BIOCATEM Network (Grant 245413) and CONACYT 179241. The authors thank Rosa Román from Institute of Biotechnology, UNAM and René Guardián Tapia from CIICAp-UAEM for technical support.