Flexible ureterorenoscopy is increasingly used as a first-line treatment for patients with renal lithiasis and proximal ureteral calculi, with varying success rates among different groups of authors and with great heterogeneity in defining the minimum size to assess stone-free rate. The article presents a literature review on the state of the art in flexible ureterorenoscopy, as well as it clarifies different concepts, while offering tips and tricks that have been implemented in a reference center for the management of urolithiasis, aiming to reduce complications, while improving stone-free rate and the performance of the procedure for urologists.
La ureterorrenoscopia flexible es cada vez más utilizada como tratamiento de primera línea para pacientes con litiasis renal y del uréter proximal, con tasas de éxito variables entre los diferentes grupos de autores y con una gran heterogeneidad a la hora de definir el tamaño mínimo para valorar la tasa libre de cálculo. Se presenta una revisión de la literatura sobre el estado actual de la ureterorrenoscopia flexible, se aclaran diferentes conceptos y se exponen consejos y trucos que se han implementado en un centro de referencia para el manejo de la litiasis urinaria con el objetivo de disminuir las complicaciones, así como mejorar la tasa libre de cálculos y el desempeño del urólogo con respecto al procedimiento.
The prevalence of urolithiasis has been considerably increasing in recent decades, causing a rise in health care expenses, costing up to five billion dollars annually in the US. Two decades ago, one in every 20 adults in the US consulted a doctor for at least one episode of urolithiasis. Currently, one in 11 adults is diagnosed with urolithiasis during his or her lifetime.1 Additionally, the boom in the development of new technologies, primarily in the design of flexible equipment for the management of urolithiasis, has popularized their use in all centers of the world.
The first flexible ureterorenoscope (fURS) was used in 1983, developed by Bagley et al., leading to a number of technological advances, until achieving current equipment design with reduced caliber associated with a wide working channel that allows to use multiple instruments simultaneously, achieving an active deflection at its distal end. Similarly, there were significant improvements in resolution, until achieving a high-definition digital image.2,3
There is a clear and linear relationship between the number of cases and a decrease in surgical time, complication rate, and success rate; nevertheless, complications associated with the use of fURS remain an issue to consider. Described complications include ureteral avulsion, with an incidence of 0.5%, which has been decreasing since fURS were started to be used, and ureteral intussusception (defined as the enfolding of a segment of a hollow organ within the same organ) due to a circumferential lesion in the wall of the ureter. This complication has been associated with the presence of fibroepithelial polyps and transitional cell neoplasia; however, the incidence is so low that it cannot be estimated from the available literature. Perforation of the ureter is another frequent complication with an incidence range that goes from 15.4%, using a 12.5-Fr ureterorenoscope, to 1.7% with the use of 8.5- to 10.8-Fr ureterorenoscope. False passages are another common complication, which occurs when an instrument perforates the urethral mucosa without penetrating the whole ureteral wall. Its incidence range is low, between 0.4 and 0.9%. Finally, one of the most feared intraoperative complications is breakage or malfunctioning of the instruments; nevertheless, today such complications have become more anecdotal and their incidence is close to 0%. Among these factors, we find malfunctioning of a laser font, breakage of the fiber optics within the ureterorenoscope, and breakage of the extractor basket.4
This paper aims to review the tips and tricks described in literature, seeking to facilitate the use of fURS for the management of urolithiasis and thus to prevent possible complications, by clarifying concepts about the fURS technique and offering further tips based on our practice and clinical experience.
Methods and materialsA search was conducted in PubMed, MEDLINE, and SciELO databases, using the following keywords: “flexible ureterorenoscopy”, “laser lithotripsy”, “percutaneous nephrolithotomy”, “tips”, “tricks”, and “urolithiasis”. Based on the results found in literature and on surgical experience with more than 100 cases per year in the centers where the authors work, we describe the steps that must be followed when performing fURS, provide tips and tricks that are believed to facilitate for urologists the performance of fURS, in order to optimize results, while reducing surgical time and possible complications.
ResultsThe review is divided into three big groups:
- 1.
Changes in terminology today: stone-free rate, factors that affect it and tricks to decrease it. Diagnostic methodology of urolithiasis and monitoring of residual calculi, use of ureteral access sheath, yes or no?
- 2.
Laser: recommendations of use to reduce residual lithiasis and to improve fragmentation rate.
- 3.
Tips and tricks for the surgical procedure: flexible ureterorenoscopy and the use of devices.
Criteria used to define “stone-free rate” (SFR) vary. This is why previous and current consensus on residual fragments after surgical treatment of lithiasis is discussed. Different studies evaluating SFR used different techniques for measuring residual stone fragments: tomography, abdominal radiography, and intravenous urography. These techniques were combined or used individually.
The initial concept of SFR was described with techniques of extracorporeal lithotripsy, where clinically insignificant residual fragments were defined as noninfectious calculi less than 4mm remaining after the procedure. However, several publications showed that up to 40% of patients with SFR less than 4mm required additional interventions in the future. These findings justify the discussion on what the exact measure should be to define SFR after flexible ureterorenoscopy and lasertripsy (FURSL). Some experts believe that it is not acceptable to use the term “clinically insignificant residual fragments,” since residual fragments may act as nidus for new stone formation, resulting in high rates of symptomatic episodes and surgical re-interventions. It appears that the clinical significance of residual fragments depends on the shape, size, and initial treatment of calculi.5
According to the study by Beck et al., which followed-up infectious magnesium ammonium phosphate calculi (struvite) 27 months after extracorporeal lithotripsy, of 20 kidneys that were free of stones 16 remained stone-free. At 3 months follow-up after lithotripsy, of 18 kidneys with residual fragments 14 showed progression of urolithiasis.6 In another study, which followed-up 42 patients with residual stone fragments less than 5mm after percutaneous nephrostolithotomy, 71% of patients with residual fragments bigger than 2mm had symptomatic episodes, while in patients with residual calculi less than 2mm, only 24% had symptoms associated with renoureteral colic. Re-intervention rate was also significant, representing 53 vs. 8% respectively.7
With respect to the diagnostic method to determine stone-free rate, it is recommended to be standardized for each group; uro-CT scan is suggested as the most reliable method to assess residual stone fragments after intervention, because it allows to identify residual lithiasis less than 5mm and radiolucent lithiasis, in addition to standardizing an evaluation methodology for further studies.8
The right time for evaluating SFR is further discussed; in a review of 154 studies evaluating the treatment of urolithiasis, postoperative imaging evaluation of residual calculi was performed at an average of 49 days after treatment (range between 1 and 365 days).9 Our group determined an evaluation upon completion of a month after procedure, based on the concept of “ureteral evacuation” with an average of 30 days for ureteral stones.
Other strategies have been proposed to determine SFR after ureteroscopy or extracorporeal lithotripsy. The S.T.O.N.E. score is one of them, which takes into account five variables: size, location of calculus (ureter, upper or lower renal pole), obstruction by hydronephrosis, number of stones present, and evaluation of density in Hounsfield units. Although this score is not yet validated, it was possible to find statistically significant results with respect to size, location, degree of hydronephrosis, and SFR. In this study, SFR was 82%.10
Key pointsSFR is difficult to determine due to the diversity of imaging evaluation methods (plain abdominal radiography, ultrasound, CT). The best method to date is uro-CT scan; however, it is not always affordable due to its high cost.
Use of two cut-off values is suggested for SFR; <2mm and ideally 0% in a tomographic study.
Evaluation time for stone-free rate is not standardized. We suggest 30 postoperative days based on averages of international publications.
Factors affecting stone-free rateThere are different factors affecting SFR, starting with technological modifications and advances applied to this intervention every day, as well as the modification of the limits and indications for FURSL.
Lithiasic sizeTraditionally, treatment with FURSL has led to similar results in patients with stones <1.5cm. But increased possibilities for the treatment of larger kidney stones or individual conditions of patients (anatomical alterations, infundibular narrowing, blood dyscrasias) turn FURSL into a first-line treatment over percutaneous nephrolithotomy despite calculus size; due to this, SFR has been modified in this group of patients, which affects the final outcome of the intervention.
It has been shown that in case of stones larger than 1.5cm, multiple interventions are often required and initial approach should be considered as a multi-stage procedure.
Between 2007 and March 2015, only 10 studies on FURSL have evaluated results on stones that were larger than traditional sizes (larger than 1.5cm).8 Ten studies included 561 patients with an average stone size of 2.8cm (range between 2.4 and 3.1). These studies determined that SFR after the first procedure was 56.8%, and 86.3% after the second intervention, with SFR defined as less than 4mm.11–15 Stones larger than 1.5cm are associated with a failure to achieve SFR in a single surgical time of FURSL.16 Lithiasic volume is the strongest preoperative predictive factor for surgical success in terms of SFR.
Location of calculiThe location of stones in the lower pole of the kidney is an important factor that can affect the evacuation of residual stone fragments during endourological procedures, because it complicates the drainage of fragments. It is considered as the second most important factor affecting SFR after size.8 It has been suggested that the geometric and anatomical features of the lower calyx are of great importance in determining SFR (infundibulum-pelvic angle greater than 90°, infundibular length less than 30mm or infundibular diameter smaller than 5mm, and volume of the collecting system). Nevertheless, until today, evidence regarding this remains controversial. The opinion of the European Association of Urology on this aspect is that there is NOT enough evidence to determine whether the geometric features of the lower collecting system are useful in clinical practice to determine SFR.10,17,18
Surgical procedureIt has been discussed which is the method of choice to treat stones located in the proximal ureter and in the calyceal systems. Treatment options supported by the literature include FURSL, percutaneous nephrolithotomy, and extracorporeal lithotripsy. Definition of the most suitable method for each clinical scenario depends on SFR, complication risk, bleeding risk, surgical time, and hospitalization time.
A meta-analysis evaluated 10 studies, which sought to determine the best management option for nephrolithiasis, comparing fURS and percutaneous nephrolithotomy. It was found that SFR is higher in patients undergoing percutaneous nephrolithotomy with OR: 2.19, CI: 95% and p<0.00001, which means that there is a probability of 68.6% to remain without insignificant residual fragments with percutaneous nephrolithotomy compared to FURSL. Nevertheless, one of the limitations of this study is that it includes in the group of percutaneous nephrolithotomy the use of both semi-rigid and flexible nephroscopes; a subgroup analysis showed that percutaneous nephroureterolithotomy had better results in terms of SFR compared to fURS with OR: 3.07, CI: 95%, but when comparing retrograde fURS with percutaneous nephrolithotomy with flexible nephroscope, retrograde fURS had better results with OR: 1.70, CI: 95%. As for the other outcomes evaluated, percutaneous nephrolithotomy had higher complication rates, a larger decrease in postoperative hemoglobin, and increased hospital stay. Surgical time had no statistically significant difference.19
Another systematic literature review and meta-analysis compared the effectiveness of percutaneous nephrolithotomy, fURS, and extracorporeal lithotripsy regarding SFR at 3 months post-surgery, based on 8 studies, 7 of which were randomized clinical trials. As a result it was found that SFR was 89.5% with fURS vs. 70.5% with extracorporeal lithotripsy. When comparing percutaneous nephrolithotomy with extracorporeal lithotripsy, SFR was higher in the first procedure (96.2 vs. 46.1% respectively). Only one study compared retrograde fURS with percutaneous nephrolithotomy, thus meta-analysis was not possible in this group. Nevertheless, the study by Kuo et al. did not report a statistically significant difference in SFR between these two procedures.20,21
Key pointsFactors that directly affect the success of FURSL and SFR are: calculus size (which is the main determining factor), location (specifically, the lower calyx has the greatest impact), and instruments used during the procedure (baskets, laser parameters, irrigation, and washing).
Use of ureteral access sheathsUreteral access sheaths during FURSL have been widely used since the introduction of this surgical technique. Takayasu et al. first reported the use of Teflon tubing in 1974.22 However, Newman introduced the use of ureteral access sheaths two decades ago. But it was only until some years ago that the use of these access sheaths has gained importance thanks to new materials used for their manufacturing, which enabled a better performance with less ureteral damage.23,24 Nevertheless, there is much controversy worldwide regarding the need for their routine use.
The main justification for the use of ureteral access sheaths is that they allow to atraumatically dilate the ureter, as well as repeated insertion and withdrawal of the equipment when necessary. However, with the advent of laser lithotripsy, residual stone fragments are millimetric and the passage of the ureteroscope is reduced to a single insertion.25 This is why the routine use of ureteral access sheaths may seem redundant, unnecessary, and potentially dangerous.
Current indications for the management of lithiasic pathology of the upper urinary tract are based on calculus size. Given that stones >2cm have a worldwide trend to be treated by percutaneous access with minor implications and risks regarding ureteral injury and surgical time. This is why in case of smaller-sized stones, the need for the “insertion and withdrawal” of the equipment is limited, with a considerably high SFR with “dusting or pulverization” technique.
Another aspect on which the routine use of ureteral access sheaths is based is an improvement in the drainage of irrigation fluid around the ureteroscope and through the sheath. However, this aspect has no proven foundation, as the sheath is usually placed at a considerable distance from the lithiasic mass to be treated. While there is proximal urethral mucosa not covered by the sheath, it will coat around the lumen of the sheath, impeding better drainage.26 Additionally, this mucosa may have some degree of edema and inflammation, which, due to the repeated passage of the equipment, could lead to laceration and subsequent stricture of this urethral segment.
On the other hand, placement of the access sheath requires the use of a guidewire, which usually is a hydrophilic guidewire with a rigid end, which generates such a force that it is possible to damage the urethral mucosa during the positioning of the sheath, increasing the risk of ureteral injury.27
Buckling in the ureter can be a factor against the passage of the equipment through the ureter. Taking into account this, the use of ureteral access sheaths has been proposed to prevent buckling and to facilitate the insertion of the equipment. However, it is suggested that ureters presenting edema or inflammation do not have the flexibility to allow the access of a sheath and to correct possible buckling, which could cause damage to the ureter when introducing the sheath.28
Some surgeons have suggested that ureteral access sheaths help to control the performance of the ureteroscope. However, this has not been confirmed, as the longevity of the equipment can also be affected by the operator, his or her experience, the support staff, and sterilization techniques.25
In contrast, some argue that the use of ureteral access sheaths may cause greater ureteral damage, which could generate, in the long term, stricture of the lumen. So far there have been no prospective studies that would successfully document stricture formation rate after positioning the ureteral access sheath. Delvecchio et al.,29 reported a stricture rate of 1.7% in a retrospective report of 62 cases. The use of a ureteral access sheath may alter the perfusion of urethral mucosa by compression. While removing the access sheath, the process of mucosal reperfusion may cause the exposure of tissue to free radicals, leading to subsequent damage, especially in long procedures.
The prudent time for the use of ureteral access sheaths has not yet been adequately established. Therefore, surgical procedure time is directly related to the experience of the surgeon, the characteristics of the patient's ureter, and the size of the stone to be treated.
A study published in 2013 evaluated the safety of using ureteral access sheaths in FURSL, an issue that had sparked much controversy until then. The authors defined a classification scale for iatrogenic ureteral trauma associated with the passage of the ureteral access sheath, based on a retrospective review of data obtained from ureteroscopy at the end of the procedure. Endoscopic classification includes 5 degrees of injury (0–4) grouped into two categories: low (grade 0 and 1) and high grade (2, 3, and 4) lesions. The classification spectrum ranges from the absence of injury (grade 0) to total ureteral avulsion (grade 4).
The main objective of the study was to describe the incidence and nature of ureteral trauma, as well as possible risk factors for ureteral injury associated with the passage of the access sheath. Some degree of ureteral injury was found in 46.5% of patients reviewed, and severe trauma (involving the smooth muscle layers) was observed in 13.3% of patients. Male gender (p 0.024) and advanced age (p 0.018) were identified as statistically significant risk factors for severe trauma. Nevertheless, the most significant predictor was non-placement of ureteral JJ stenting prior to the procedure (p 0.001), indicating that pre-stenting significantly decreased the risk of severe ureteral injury associated with the passage of the access sheath. The authors recommend to perform an endoscopic evaluation of the status of the ureter at the end of the procedure to identify possible ureteral injuries.30 In our center, ureteral inspection after surgery is a routine procedure.
There are no data published on risk factors for ureteral injury associated with the passage of ureteral access sheaths during FURSL in our country. It is important to note that the use of ureteral access sheaths adds cost to the procedure.
A survey conducted by the Endourology Society,31 with the participation of urologist experts in endourology from different countries, established that 58.3% of participants preferred to use ureteral access sheaths in all cases. Among other indications evaluated in the questionnaire, ureteral access sheaths were used only in case of large stones by 30% of respondents. 10% use UAS only if the ureter has had a previous ureteral catheter (e.g. JJ stent); 20% only for fragment extraction; and 5% never use ureteral access sheaths.
Key pointsThe use of ureteral access sheaths depends on the preference of the operator. The conventional use seems to be associated with larger calculi and techniques of extraction, not pulverization.
In case of small-sized middle or upper calyceal stones, placement of preoperative ureteral JJ stenting is an option for not using ureteral access sheaths.
It is important to recognize ureteral injuries associated with ureteral access sheaths, as well as risk factors for high grade lesions. As far as possible, the use of preoperative ureteral JJ stenting must be sought, considering that it is a protective factor for ureteral injuries associated with the passage of the ureteral access sheath.
Laser fragmentationIt has been over 50 years since laser (light amplification by stimulated emission of radiation) was first used for intracorporeal lithotripsy. Laser energy is produced when an atom is stimulated by an external energy source, which creates a population of electrons that remain in an excited state. When energy levels go down, they release energy in form of photons.
The most widely disseminated and used laser, recognized as the gold standard for fURS, is the holmium:YAG laser,32 which has a wavelength of 2140nm and delivers energy pulses with a duration of 250–350μs. Its action mechanism is based on firing laser pulses. Each pulse heats up the surface of the calculus, causing vaporization of the water in its inside, as well as on its surface, weakening tensorial forces in the inside, which, when interacting with weak shock waves generated by a cavitation bubble produced by the laser pulse, fragments the stone.33,34
A very favorable characteristic of the holmium:YAG laser is that it is highly absorbed by water, from a distance of up to 3mm, and, at the same time, tissue penetration is minimal, which makes it ideal for the management of urolithiasis. It has been described that in order to injure the urothelium of the ureter, it must be at a distance of 1mm from it.32 There are several laser fiber sizes, ranging from 200μm to 1000μm. 200- and 365-μm fibers are ideal for fURS, because they do not limit the degree of deflection of the equipment. Larger fibers do not significantly improve stone fragmentation compared to smaller ones, in a configuration of constant energy ≤1J. This means that stone fragmentation depends more on energy density and not on fiber diameter. For this reason, it is recommended to base fiber diameter choice on required deflection degree for fURS and not on stone size.35,36
Ideal energy and frequency settings to start fragmentation with 200- and 365-μm fibers are 0.6J and 6Hz. For practical purposes, it is recommended to use the lowest possible settings to configure the laser and then gradually increase parameters. It is also recommended to initially increase energy and then to adjust frequency, because it has been found that high frequencies may affect visibility. For 200-μm fibers, it is recommended to use an energy setting of less than 1.0J, since higher energy density is more likely to damage the fiber and decrease the efficiency of fragmentation.35
Laser fragmentation techniques in lithiasic pathology can be divided into two types: (1) pulverization which seeks to micro-pulverize calculi, using high frequency and low energy in the laser generator, as well as to prevent passage of instruments used for fragment removal and to facilitate spontaneous drainage of residual fragments; (2) partial fragmentation, which seeks to fragment calculi into smaller fragments allowing them to be removed with baskets. This technique uses low frequency and high energy.
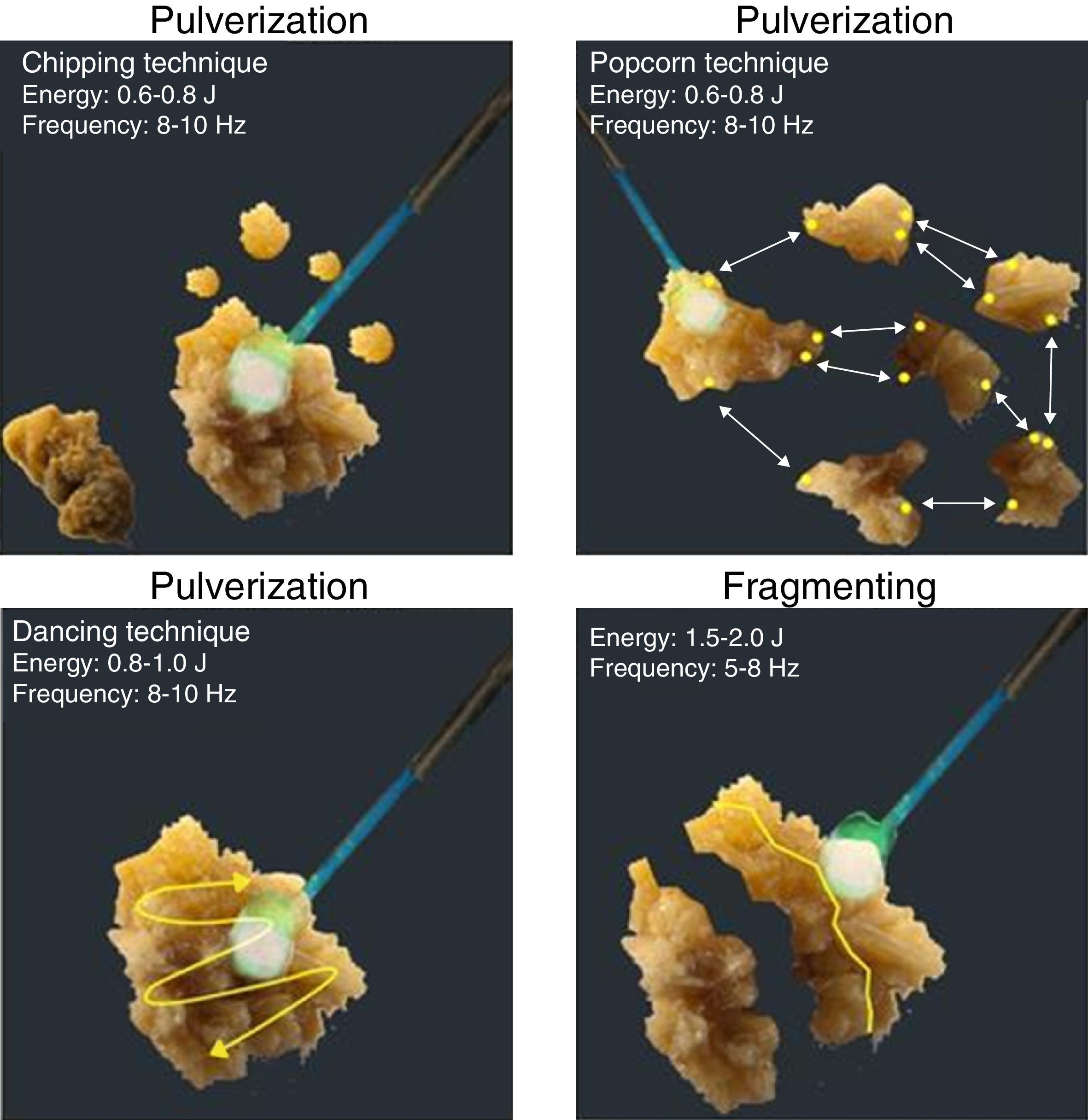
These two techniques can be subdivided into four techniques known as dancing, chipping, fragmenting, and popcorn technique.
The dancing technique is used to fragment soft calculi; the tip of the laser fiber is brushed back and forth across the stone surface, so that the stone is fragmented layer by layer. Recommended settings are: 0.8–1.0J energy and 08–10Hz frequency. The chipping technique is used for hard calculi. The laser fiber is directed at the periphery of the calculus and held steady; thus energy is focused at an eccentric point causing the stone to rotate, presenting a new surface for subsequent chipping. Recommended parameters are similar to the dancing technique. The fragmenting technique is used for very hard calculi. Here the laser fiber is aimed at the stone's center and fired continuously; this weakens the calculus along natural cleavage planes until it splits into portions up to 1mm that can pass spontaneously. Recommended parameters for this technique are: 1.5–2.0J energy and 05–08Hz frequency. The popcorn technique is used for collections of stones in a renal calyx. The fiber must be positioned close to the stone and fired continuously at high frequency, which induces a “whirlpool-like phenomenon”. This causes collisions between stone fragments, which, in addition to laser energy, allows their rapid fragmentation. Recommended parameters for this technique are: 0.5–0.8J energy and 16–20Hz frequency18 (Fig. 1).
The use of fragmentation and extraction with basket has been discussed, because it increases the risk of ureteral injury. However, it also increases stone-free rate. It is important to know these principles for stone fragmentation in order to obtain better surgical results.
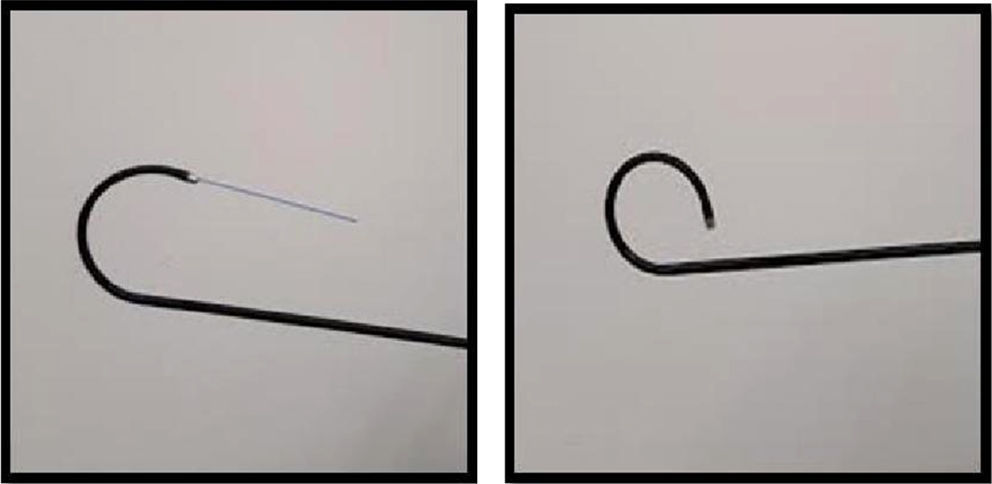
The passage of the laser fiber through the working channel decreases the ability of deflection of the tip of equipment from 10° to 15° on average (Fig. 2), which makes it difficult to approach the stone at sharp angles.36,37 This is why stones in difficult locations must be repositioned so that they can be approached more favorably, either by using a basket or with hydro-washing.
Tips and tricks for the surgical procedure of flexible ureterorenoscopy and device usageUrologists must know the current concepts of urinary lithiasis when performing fURS, stone-free rate and methods for its evaluation, comparison tools for follow-up, as well as working instruments; the optimal functioning of the equipment must be verified too. We will describe the procedure step by step, as well as the elements used during each step, along with tips and tricks that we consider practical when performing the surgery.
The first step is to place the patient correctly; fURS is usually performed in dorsal lithotomy position under general anesthesia. Antibiotic prophylaxis should be administered prior to surgery, according to the guidelines on urological infections of the European Association of Urology, only in ureteroscopy for the management of proximal or impacted stones or percutaneous access to the collecting system, using trimethoprim±sulfamethoxazole or 2nd or 3rd-generation cephalosporin.38,39 It is recommended to partially empty the bladder before entering the urinary tract to minimize this way the constricting effect of the intramural portion of the ureter; air should be prevented from entering the bladder, since it may cause the stricture of urethral meatus and hinder the access to the ureter.40,41
Advancement of the guidewireThe most important characteristics for the use of ureteral guidewires are: the guidewire should have a flexible tip, which should be between 3 and 15cm, low friction (coated in polytetrafluoroethylene), while the rest of its length must be rigid. The guidewire should always be inserted under fluoroscopic guidance to avoid complications; it is recommended to use two guidewires, a safety wire and a working guidewire. The ureteral access sheath must pass through the working guidewire, which will subsequently allow the passage of fURS (Fig. 3).
The tips and tricks in this step are:
- 1.
Moisten the guidewire prior to its insertion.
- 2.
Hold the guidewire with fingernails or a dry gauze, not with fingers, to prevent it from slipping.
- 3.
The safety wire must be fixed and secured, so in case of losing access to the collecting system, there is another access ready in order to continue the procedure.
- 4.
In men, the penis should be held straight to stretch the urethra and facilitate the passage of the ureteral access sheath.
- 5.
When the passage of the guidewire is difficult through the ureteral meatus, it is possible to advance through a 6-Fr ureteral catheter, or to pass the ureteroscope through the ureter under direct vision and to treat the underlying problem that complicates the access (stricture or calculus in the ureterovesical junction). Smaller guidewires with a diameter of 0.025in. can also be used, allowing easier access to the collecting system.
- 6.
When the passage of the hydrophilic guidewire is not achieved, it should be considered the option of using a guidewire with nitinol core, platinum distal tip, and polyurethane outer sleeve.
- 7.
If resistance to guidewire advancement is encountered, attempts to pass the wire should be restricted in order to avoid creating a false passage and injuring the ureter.
- 8.
It is advisable to perform a diagnostic ureteroscopy before the passage of the ureteral access sheath, in order to identify important anatomical alterations, strictures, or extrinsic compressions.
The next step is to advance the ureteral access sheath through the working guidewire under fluoroscopic guidance to avoid injury to the ureter. The ureteral access sheath consists of two hydrophilic parts that are the ureteral sheath itself and an inner dilator which is removed along with the working guidewire once the ureteral access sheath is in the desired position. Ureteral access sheaths are measured by inner or outer diameters; inner diameters range from 9.5Fr to 14Fr, and outer diameters range from 11.5Fr to 17.5Fr. Their length ranges from 20 to 55cm. Important tricks in this step are:
- 1.
Avoid forced insertion of the ureteral access sheath. Perform again a diagnostic ureteroscopy with semirigid equipment in case of resistance.
- 2.
When ureteral dilatation is required, sequential ureteral dilators, ranging from 4Fr to 14Fr, or a balloon dilation catheter can be used, to inject the same contrast medium and, under fluoroscopic guidance, to dilate the ureter always below the stone.
- 3.
The appropriate ureteral access sheath must be selected based on the anatomy of the ureter. For this effect, there are several access sheaths available in different lengths and diameters. The use of short access sheaths is recommended in short patients to avoid perforation of the collecting system with the tip due to excessive passage of the access sheath.
- 4.
We recommend using the smallest possible ureteral access sheath because we believe that this measure reduces trauma to the ureter. We make this recommendation despite clear evidence in the literature on this measure.
fURS have different diameters ranging from 8.5Fr to 18Fr. The surgeon must hold the ureteroscope with the dominant hand, with the thumb on the deflection control; this allows to have 180° of deflection in conventional fURS, but there are fURS that allow a maximum deflection of up to 300°. This is possible as long as the working channel remains free. The non-dominant hand must control the advancement and removal of the equipment through the ureteral access sheath. Light source, irrigation, and camera must be connected to fURS prior to accessing the collecting system.39,42
Important tips are:
- 1.
Perform leak test before and after each procedure.
- 2.
Always advance with the tip completely straight without any degree of deflection in order to avoid damage to the equipment.
- 3.
Calibrate the equipment prior to use (target balance, definition of panel vision intensity).
- 4.
Avoid reaching the maximum degree of deflection with tools in the working channel.
- 5.
Avoid excessive bending in the proximal end of the equipment (where the handle joins the flexible part) as it affects the longevity of the equipment.
For the management of renal stones smaller than 2cm that are not susceptible to treatment with extracorporeal lithotripsy (>10mm, >1000HU, >10cm skin-stone distance), the best treatment option is FURSL. After completing the above-described steps and having accessed the renal collecting system, the stone must be located and the laser fiber moved forward in “off” mode. The laser fiber is advanced between 1 and 3mm outside the ureteral access sheath, it is positioned in contact with the calculus, and laser configuration mode is changed to “on” to start stone fragmenting. It is recommended to start with the following settings: 5–10Hz frequency and 0.6–1.5J energy.28,33
The shaft must always be straight (0° of deflection) when laser fiber is in the working channel; deflection of the equipment can only be made when the fiber is at the tip of working channel.28
To prevent accumulation of residual stone fragments in the lower calyceal system, this can be sealed with an autologous blood clot. 5–10ml of blood is extracted from a peripheral vein; the location of the equipment in the lower calyx must be verified under fluoroscopic guidance; the autologous blood is injected, completely obscuring the visual field; fURS is removed, and after a 3–5min wait the clot is formed, sealing the lower calyx.43–45
The use of self-retaining ureteral catheters (JJ stents) are recommended in patients who are at high risk for complications such as residual fragments, bleeding, ureteral perforation, urinary tract infections, or pregnancy. It has been demonstrated that routine catheterization with JJ catheters is unnecessary after an uncomplicated fURS.41
It is recommended to leave the JJ catheter between 1 and 2 weeks after a complicated fURS.36
ConclusionsThe management of renal and proximal ureteral lithiasis has undergone great changes with the advent of new technologies such as flexible equipment for retrograde approach, in some cases displacing traditional treatments such as extracorporeal lithotripsy. Lower calyceal calculi of intermediate size, among others, justify the use of fURS, considering that stone-free rates are close to 83 vs. 48% with ESWL. In addition, improvements in retrograde flexible techniques increasingly support its indication for the treatment of larger stones; it is even considered for a combined approach or for cases of difficult percutaneous approach.
Therefore, the limits and clinical scenarios for the use of flexible endoscopic equipment should be gradually expanded for the management of kidney stones.
Until now, the use of ureteral access sheaths remains a major topic of discussion among urologists. Among the arguments in favor we can highlight that it allows multiple accesses with flexible equipment, decreased intrarenal pressures, extended longevity of equipment, and it can also improve the elimination of fragments. Some authors argue that the use of access sheaths can reduce morbidity associated with the procedure; nevertheless, it is well-known that about half of the patients may have ureteral injury associated with the passage of the ureteral access sheath. Therefore, we recommend to avoid its use as long as the conditions of the case permit it, such as size and location.
Additionally, we suggest to quantify the time of use of the ureteral access sheath and to reduce it to the maximum in order to avoid possible strictures associated with ischemia of the ureteral wall.
The use of preoperative self-retaining ureteral catheter may cause increased cost per procedure. However, it is clearly related to decreased morbidity associated with the procedure, mainly with ureteral injuries associated with the passage of the ureteral access sheath. Moreover, catheters can considerably facilitate the surgical procedure, and even avoid the use of the ureteral access sheath. In our experience, whenever a patient requires a retrograde surgical treatment and he or she is admitted to the emergency room due to severe pain prior to the date of the scheduled surgery, we invariably proceed to the placement a self-retaining ureteral catheter. The average pre-surgical time of use is approximately 3.6 weeks.
Regarding the use of post-surgical self-retaining ureteral catheter, there are data that confirm reduced postoperative morbidity, which results in fewer emergency room visits. However, in our experience, emergency room visit rate for intolerance of the self-retaining catheter is around 5%, with outpatient analgesic treatment in all cases. The average time for the use of post-surgical catheter is 3 weeks. So far we have not reported strictures secondary to the procedure.
Recently, and based on our experience and technological resources we have at our disposal, we take as a reference and starting point the stone-free rate understood as a complete absence of post-procedure calculi. We evaluate cases in the first postoperative month using plain abdominal radiography and, in most cases, when conditions permit it, in the third month. We intend to evaluate all cases with uro-CT, but we acknowledge the financial burden this can cause to our health care system.
We invite groups that are routinely engaged in flexible ureterorenoscopy and are reference centers for this procedure to objectively evaluate stone-free rate and to accept, following the experience of high-volume centers, that SFR should be 100% in a maximum of two procedures.
It is important to take into account that technological advances, inputs and experiences obtained from frequent performance of FURSL do not imply changing the management of larger stones as recommended in the literature.
We recommend that the groups engaged in this technique compose a document, and use it as institutional guidelines, where all the steps are described in an orderly manner, in order to standardize processes and to report in all cases possible injuries associated with the procedure with the objective of identifying risk factors and making this procedure a safe and reproducible surgery.
Conflict of interestThe authors have no conflicts of interest to declare.