Magnetic resonance imaging is a diagnostic tool used for obtaining an image through the combination of electromagnetic fields and radiofrequency. Given its properties and safety, it is the imaging modality of choice in pregnant women. However, little is known about the effects of MRI on the developing foetus.
ObjectivesTo identify the effects of the use of magnetic resonance imaging on the foetus when used as a diagnostic tool during pregnancy.
Materials and methodsA literature search was performed in PubMed, Embase, and LILACS. Clinical guidelines and the grey literature were also reviewed. An analysis was made based on the findings.
ResultsFour potentially adverse effects where found: (1) The impact on the auditory development due to the acoustic sound made by the resonator. (2) Teratogenic effects on DNA. (3) Physical deformities secondary to temperature increase. (4) Teratogenic effects due to the use of gadolinium as a contrast agent.
ConclusionThe risk assessment on the use of magnetic resonance imaging on the foetus is complex, owing to the multiple differences in field strength, force gradients, and radiofrequency pulses used. Although the adverse effects of using this method are not very clear, there are studies that describe the possible outcomes that can result from the use of this imaging modality. It is recommended to use MRI with caution, as long as the benefits outweigh the risk in pregnant patients.
La resonancia magnética nuclear es una herramienta diagnóstica que actúa mediante la obtención de imágenes por campos electromagnéticos en combinación con radiofrecuencia. Dadas sus propiedades y seguridad es el estudio de elección en mujeres en estado de embarazo. Sin embargo, poco se sabe acerca de las consecuencias que la exposición a la resonancia magnética nuclear tiene sobre los fetos en gestación.
ObjetivosIdentificar los efectos que tiene la resonancia magnética sobre el feto cuando se utiliza como método diagnóstico durante el embarazo.
Materiales y métodosSe realizó una búsqueda de la literatura en PubMed, Embase y LILACS. Se revisaron además guías de práctica clínica, literatura gris y se hizo un análisis en función de los hallazgos.
ResultadosSe encontraron 4 efectos potencialmente adversos: 1) El impacto en el desarrollo auditivo por el sonido acústico que genera el resonador, 2) Efectos teratogénicos sobre el ADN, 3) Deformaciones físicas por aumento de temperatura, y 4) Efectos teratogénicos por el uso de gadolinio como medio de contraste.
ConclusiónLa evaluación del riesgo que genera el uso de resonancia magnética nuclear sobre el feto es compleja debido a las múltiples diferencias entre las fuerzas de los campos, gradientes de fuerza y pulsos de radiofrecuencia utilizados. Consecuentemente los efectos adversos del uso de este método no son muy claros, sin embargo sí hay estudios que describen los posibles desenlaces que puede tener su utilización por lo que se sugiere usar este método con cautela, siempre y cuando los beneficios sobrepasen los riesgos en las pacientes embarazadas.
All living things are constantly exposed to natural electromagnetic fields; these are, however, weak and non-ionizing fields, so most people are unaware of their existence.1 Magnetic resonance imaging (MRI) has been established as an essential tool in the study of various diseases. Its use during pregnancy has increased and it is even used to diagnose alterations of prenatal development that cannot be properly studied with ultrasound.2–7 Little is known, however, about the consequences of exposure to magnetic fields on the developing fetus.8 It is difficult to assess the risks due to the many variables involved.3,9 Nevertheless, there are guidelines that aim to limit the use of this method despite the lack of information. The main objective of this systematic review is to find basic information about the effects of MRI on the fetus.
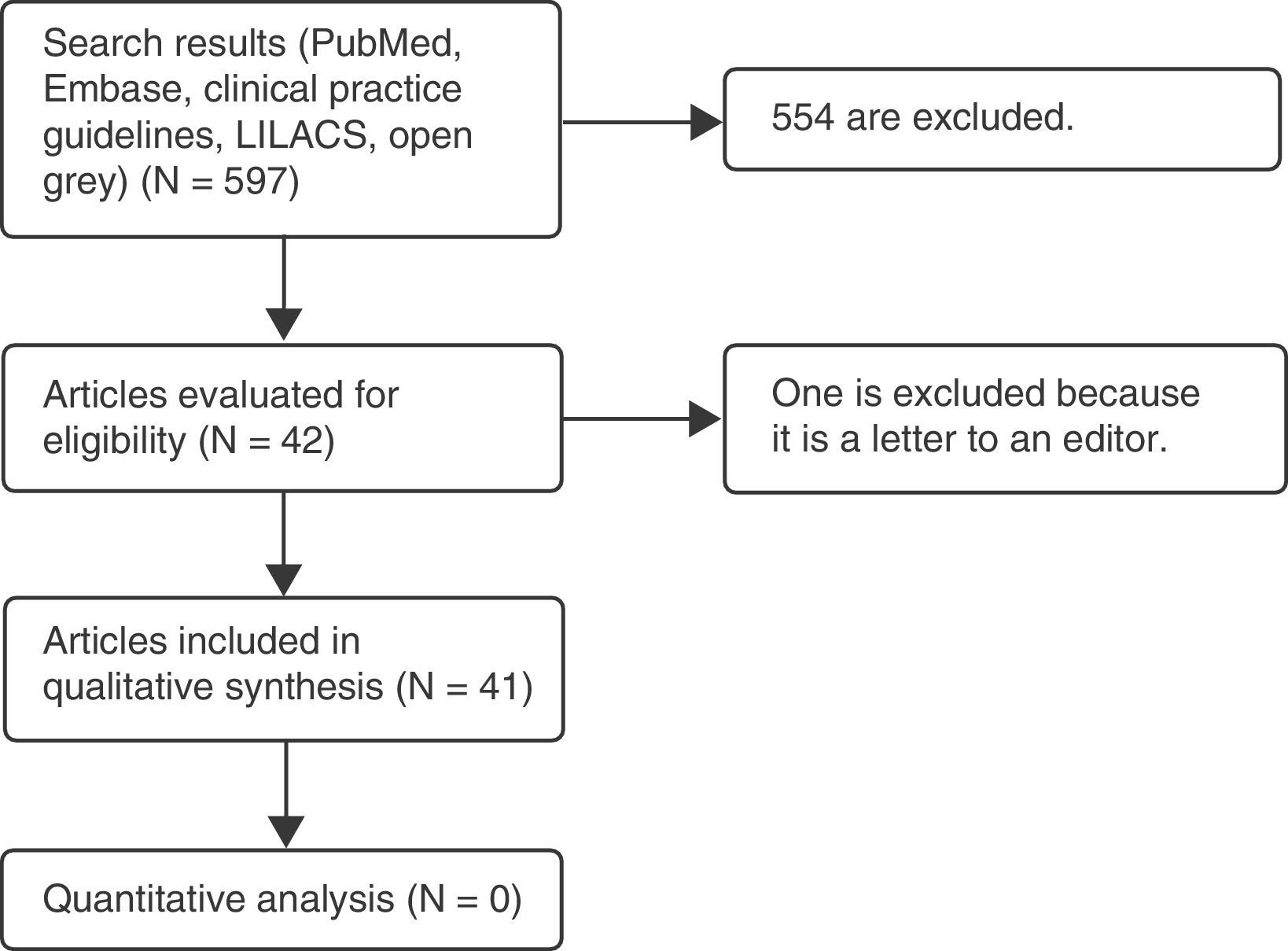
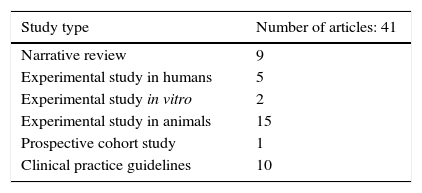
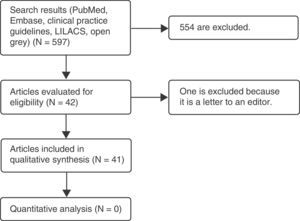
Materials and methodsA systematic literature review was carried out on the teratogenic effects of magnetic resonance imaging in the Embase and PubMed databases, as well as in clinical practice guidelines. The following MeSH terms and their respective cross-references were used: “magnetic resonance imaging,” “pregnancy,” “teratogen,” “DNA damage,” and “congenital abnormalities.” The filters used were: observational study, clinical study, systematic reviews, humans, other animals. No language restriction was established. A total of 597 articles were found, 42 of which were selected: one prospective cohort study, 9 narrative reviews, 15 experimental studies in animals, 2 experimental studies in vitro, 5 experimental studies in humans, and 10 clinical practice guidelines.
Gray literature was searched using the “open grey” portal, looking for papers presented at important international conferences; this search gave only one result, which was not useful for our study, since magnetic resonance was not used.
For Latin American studies, the LILACS database was searched without obtaining any results.
Only those studies were included that related magnetic resonance imaging, either positively or negatively, with teratogenic effects; these were narrative-type, observational, and clinical studies, as well as systematic literature reviews. Articles were discarded if they did not evaluate the outcomes of interest, comparing the implementation of the proposed interventions. After an initial selection based on a review of titles and abstracts, the remaining articles were read in their totality and an analysis of the findings was carried out. This process was completed by multiple investigators (Fig. 1 and Table 1, Appendix 1).
Magnetic resonance imaging as a diagnostic methodGeneralitiesMagnetic resonance images are generated by manipulating the polarity of the protons in the tissues, using electromagnetic fields and radio frequency.1,9,10 The image is projected after the body is exposed to a static magnetic field stimulated by an oscillating field, which results in the emission of the energy absorbed by the nuclei (also called echo) in the form of radio frequency waves. Its quantification generates an image in two sequences: T1 and T2, where both depend on the relaxation time of tissues after a 180-degree radio frequency pulse, with T1 being the longitudinal relaxation time and T2 the transverse relaxation time.10–12
Magnets are the main element for the generation of images, since they provide the “external” magnetic field to which the patient is subjected. The force generated by the magnet will determine the strength of the field, which is measured in Tesla (T) or Gauss (G); a Tesla unit is equivalent to 10,000 Gauss units. Magnetic field strength may vary between mild (0.1–0.5T), medium (0.5–1.2T), high (1.5T), and ultrahigh (3 and more).10,11 In daily clinical practice, frequencies between 0.2T and 2T are more commonly used; however, there are places where up to 3T is used and in research centers there are resonators that use up to 8T.3 Each exposure type has its risks for both the mother and the fetus.
Gadolinium as a contrast agentGadolinium belongs to a series of elements called lanthanides, which are chemically unpredictable and usually found in oxidation state +3 (Gd3+). Given that its ionic radius is very similar to that of calcium, gadolinium can compete with calcium in all the systems that require it for its operation. Since gadolinium is so similar to calcium, it can alter the kinetics of the biological processes of which calcium form part.13,14 Nevertheless, Gd3+ can form stable complexes with a variety of organic ligands, preventing the cell consumption of free gadolinium.15
Gadolinium-based contrast agents act as extracellular space markers, since they are highly paramagnetic due to their 7 unpaired electrons; thus, they can reduce T1 and T2 relaxation times, which leads to an improved signal in T1.13 There are several types: (1) Extracellular: They are the most used ones due to their safety profile, complete renal elimination of up to 98% in 24h, and usefulness in a variety of tumor, inflammatory and angiographic studies.10,13,15 (2) Hepatobiliary: They are widely used to study liver injuries due to their biliary excretion. (3) Intravascular: They are used almost exclusively in angiography due to their longer intravascular half-life, which allows the extension of the study beyond the arterial phase.10
Safety profile in pregnant womenMany international guidelines present MRI as a relatively safe diagnostic method during pregnancy, which must meet the following criteria: (1) Images must be taken immediately; they cannot wait until after pregnancy is over. (2) It must provide key information that cannot be obtained by any other diagnostic method. (3) The benefits must outweigh the possible risks.8,16–20
Gadolinium is able to cross the placental barrier where it is filtered by the fetal kidney and later is excreted in the amniotic fluid, where it remains for indeterminate time.5,8,16,17,19,21 Since the fetus may be at risk for developing conditions such as nephrogenic systemic fibrosis, it is recommended not to use it routinely.9 If needed, it should be administered in the lowest possible doses to achieve the desired effect and its use should be avoided during breastfeeding (although only a very small quantity will pass into milk).22,24
If it is necessary to use MRI, several recommendations must be followed to improve safety profile, such as reducing the sound exposure of the fetus,17,18 using the scanner in normal mode when possible, and using the controlled mode only if the benefits outweigh the risks.9,18,22
Regarding when to perform the procedure, according to the UK radiation protection group, it is “prudent” to exclude patients during the first trimester of pregnancy. However, the American College of Radiologists considers it to be a safe method in any quarter.16,23,24 In general, the use of MRI up to 3T is considered safe during the second and third trimesters.5,24
There are few studies on the exposure of pregnant workers to MRI scanners; it is recommended, however, that they remain outside the area at the time of imaging.9,16
ResultsAcoustic damageWhen performing an MRI, it is necessary for both the staff and patients to wear hearing protection devices when the sound range is more than 85dB in 8h or 100dB in 15min, since magnetic resonators produce high noise levels.25 The sound is due to the vibration produced by magnetic field combination and the rapid change of current in the coil; sound intensity, however, changes according to the intensity used for the scanner.3,9,21,25
There are studies that suggest a possible risk of exposing the fetus to MRI scanners. One of the studies followed a group of children who underwent magnetic resonance imaging in echo-planar sequence while in the uterus. Two of the 18 patients did not get satisfactory hearing tests at 8 months after birth.26 Nevertheless, the sample is considered too small to draw reliable conclusions. In another study, a microphone was placed in a fluid-filled stomach, which intended to simulate the acoustic environment of the gravid uterus, and sound intensity was measured while performing an MRI, evidencing sound attenuation greater than 30dB.27 Although the experimental conditions were not exactly the same as the environment of the fetus, this study indirectly shows that the risk of acoustic damage is lower in the fetus than in the mother. To date, reports of acoustic damage when using magnetic resonance imaging during pregnancy are scarce and inconclusive. However, it is not possible to rule out its potential harmfulness.
Teratogenic effectsIn general, the effects of electromagnetic fields depend on several factors, such as cell type, efficiency of the DNA repair, mode of exposure, radio frequency pulses, intensity and duration of exposure, and the use of contrast agents.28 There have been found multiple effects of MRI on different cell lines. For example, a study using a 4.7T static magnetic field in rabbits found that this possibly stimulated fetal endochondral ossification by boosting cell differentiation, not at the level of DNA synthesis, but by increasing vascular endothelial growth factor.29 Another possible effect may be the activation of cascades associated with cellular differentiation and proliferation of second messengers.30
Most studies of teratogenic effects are based on animal models and are difficult to extrapolate to humans; however, there have been reports of malformations secondary to MRI exposure. For example, one study described a reduction in crown-rump length when exposing pregnant mice to magnetic fields for 16h on day 9 of gestation.31
Another study found a higher rate of ocular malformations in litters of mice exposed to magnetic fields of 1.5T.32 Finally, a study exposed chick embryos at different stages of development to a static magnetic field of 1.5T for 6h and found that the group in the period of organogenesis (6th day of incubation) showed more physical abnormalities.33
When talking about tissue exposure to the effects of an MRI scanner the main problem is not cell death per se, but the process of DNA repair; given its complexity, it may result in mutations and carcinogenesis, especially in the long term due to a cumulative effect on the cells.34 A group of researchers found that prolonged exposure to magnetic fields causes DNA strand breaks, leading to apoptosis and cell necrosis in the brain of mice. They proposed a possible explanation for this phenomenon, which happens in two steps. First, exposure to magnetic fields alters cellular homeostasis, leading to increased free cellular iron in cytoplasm and nucleus, which increases the number of free radicals and induces DNA, lipid and protein damage, by inducing calcium escape from intracellular storage sites. The second step consists of an increased synthesis of nitric oxide due to an increase in calcium levels, which results in the exponential formation of free radicals by directly influencing iron metabolism.35
Another study attempted to replicate the conditions of the previous study and found no evidence that exposure to magnetic fields would cause damage to the adult or immature brain cells of rats.36 However, a group of researchers, after studying human blood cells following a 1.5T exposure, found an increase in DNA double-strand breaks in lymphocytes; although the damage was minimal and did not lead to lymphocyte apoptosis or activation, the effect lasted up to one month after exposure, even though some level of DNA repair appeared to happen in 24h.7,9 These results on DNA damage are shared by multiple studies.34,37,38 They are also discussed by many others that did not find DNA alterations when studying blood cells39,40 or other cell types.41
Physical deformations due to temperature increaseThe most frequently reported accident due to MRI exposure in the general population are burns.3 Most of the radio frequency energy transmitted by the scanner is absorbed by the tissue and transformed into heat; the term “specific absorption rate” refers to the amount of heat absorbed by the tissue, which is influenced by different environmental factors.2,5,10,42 Manufacturers of MRI scanners must comply with an international standard (IEC 60601-2-33:2010), according to which none of the sequences can cause a temperature increase of more than 0.5°C for normal mode, 1°C for controlled mode, or more than 1°C for experimental modes. To achieve this, explicit limits are established for specific absorption rate at a room temperature of <24°C with a humidity of <60%.3
Fetal temperature is linked to maternal temperature, since the fetus has almost no ability to control heat43; in mammals, prenatal growth is the result of highly organized sequences of proliferation, differentiation, migration and apoptosis that are sensitive to changes in temperature. Several windows of vulnerability to temperature have been discovered, particularly during organogenesis, when the central nervous system is more vulnerable to an increase in temperature. In humans, an increase of up to 2°C in 24h has been showed to cause a range of neural tube and craniofacial defects.3,43 In other mammals, an increase in temperature may result in microencephaly, reduced cerebral cortex thickness, and learning deficiencies.43 However, there is not enough information on exactly what happens in shorter periods of exposure that more closely resemble MRI exposure. In addition, it is believed that heat is concentrated on the maternal surface, so no heat damage occurs in the fetus.6,44 However, a study in anesthetized dogs reported a significant increase in the temperature of their internal organs; although the study does not correspond completely to the scenario proposed by this article, it does raise concerns.45
Risks associated with the use of gadolinium as a contrast agentAs previously mentioned, gadolinium can be detected in the fetus as early as 60min after being administered intravenously to the mother. In its free form, gadolinium can interfere with calcium-dependent physiological processes; it can also inhibit some enzymes, depress the reticuloendothelial system, increase the expression of hepatic cytokines, induce neuronal apoptosis secondary to mitochondrial dysfunction even at low concentrations, and induce diseases such as nephrogenic systemic fibrosis in patients with advanced kidney disease.13,46,47 Multiple studies have attempted to test the safety of gadolinium during pregnancy; for example, a study of 26 pregnant women exposed to gadopentetate in the periconceptional period and first trimester of gestation reported 2 miscarriages and one baby was born with genetic disorder. However, these numbers were not representative of the general population and therefore the results were not attributed to the use of this contrast agent.48 Another study exposed rabbits to gadobenate dimeglumine at doses of 0.3, 0.9 and 2mmol/kg, and reported anorexia and slight weight loss at a dose of 0.9mmol/kg/day, and weight loss and marked anorexia, irregularities in the retina, microphthalmia, endochondral ossification of segments of the sternum, and/or thoracolumbar vertebrae at a dose of 2mmol/kg/day.49 Another study in mice that used anaphase bridges as markers of chromosomal defects failed to demonstrate an increase in morphological changes, early abortions, fetal deaths or unstable chromosomal changes when using gadopentetate dimeglumine in magnetic resonance imaging.50
Much information is needed regarding the effects and safety of gadolinium during pregnancy due to the lack of conclusive studies in this regard; however, it is advisable to avoid the use of gadolinium during the first trimester.13
DiscussionMagnetic resonance imaging is widely used for the study of various diseases, since it has a good safety profile compared to other diagnostic methods, which expose the patient to risks associated with radiation. To date, there is little information about the real risks that MRI resonators might have on the general population and pregnant women, with most studies being done on animal models that are difficult to extrapolate to the human species.
Magnetic fields may exert influence on biological systems by generating electric fields, changing their properties and catalyzing reactions that involve free radicals. This review examined the four main possible effects of magnetic resonance imaging on the fetus, with the first one being acoustic damage, which, due to the lack of recent studies, is considered more a theoretical risk than a practical one, a problem which warrants further study. The second effect explored in the article was the risk of physical deformities in the fetus due to temperature increase, which seems to be one of the most real dangers, since heat damage is evidenced in both animal and human models.3,43 Consequently, MRI resonators have to comply with quality standards. It is assumed that if manufacturer recommendations and safety guidelines are followed, the risk of this type of complications, although real, is low.3 The third aspect was the effects of gadolinium as a contrast agent, and this study found that despite the considerable number of studies that aim to demonstrate the harmful effects of this compound in animals, especially in the period of organogenesis, there is no conclusive information on this subject. Nevertheless, as a precautionary measure, it is advisable to avoid the use of gadolinium during the first trimester.13 The fourth and last point explored in the article was teratogenesis and DNA damage. It was found that the use of magnetic resonance imaging causes single- and double-strand DNA damage and these effects can last up to one month.1,28
It is pertinent to clarify that there are parameters able to influence the results of these studies, such as temperature, field strength, and time of exposure.8,9 Before arriving at any conclusions, it is important to carry out further studies to examine the interaction among the three types of electromagnetic fields generated by scanners, taking into account that MRI exposure can enhance the damage produced by other genotoxic agents, such as epigenetic factors.1
ConclusionsThe greatest limitation found in this study was the lack of information on the effects of magnetic resonance imaging on the fetus; in addition, it was found that most studies use animal models that are difficult to extrapolate to humans.
The main finding and what causes more concern is DNA damage, which would require further studies that review the long-term implications of genetic mutations and carcinogenesis.
Regarding recommendations, it is advised that the benefits always outweigh the risks when performing this type of interventions and even more when studies do not completely endorse its safety. Professionals must make the decision they consider appropriate after consulting with the patient, using the informed consent as a measure of mutual protection.
FinancingNo funding was received from any private or public institution.
Conflict of interestsAuthors have no conflict of interests to declare.
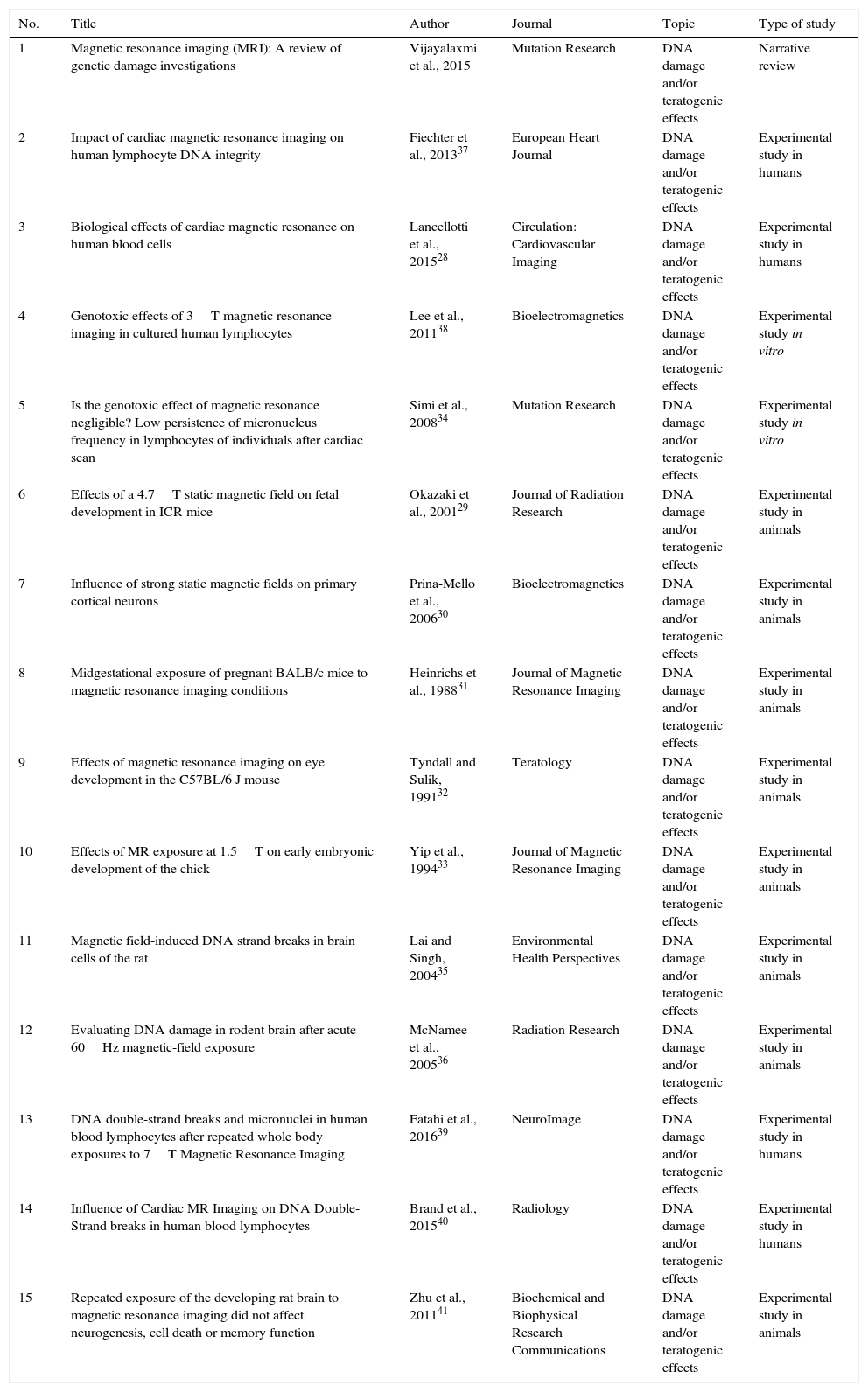
Descriptive table of articles on DNA damage and/or teratogenic effects.
Descriptive table of articles on general effects and patient safety.
Descriptive table of articles on the effects of gadolinium.
Descriptive table of articles on effects on the hearing system.
Descriptive table of articles on heat-induced effects.
| No. | Title | Author | Journal | Topic | Type of study |
|---|---|---|---|---|---|
| 1 | Magnetic resonance imaging (MRI): A review of genetic damage investigations | Vijayalaxmi et al., 2015 | Mutation Research | DNA damage and/or teratogenic effects | Narrative review |
| 2 | Impact of cardiac magnetic resonance imaging on human lymphocyte DNA integrity | Fiechter et al., 201337 | European Heart Journal | DNA damage and/or teratogenic effects | Experimental study in humans |
| 3 | Biological effects of cardiac magnetic resonance on human blood cells | Lancellotti et al., 201528 | Circulation: Cardiovascular Imaging | DNA damage and/or teratogenic effects | Experimental study in humans |
| 4 | Genotoxic effects of 3T magnetic resonance imaging in cultured human lymphocytes | Lee et al., 201138 | Bioelectromagnetics | DNA damage and/or teratogenic effects | Experimental study in vitro |
| 5 | Is the genotoxic effect of magnetic resonance negligible? Low persistence of micronucleus frequency in lymphocytes of individuals after cardiac scan | Simi et al., 200834 | Mutation Research | DNA damage and/or teratogenic effects | Experimental study in vitro |
| 6 | Effects of a 4.7T static magnetic field on fetal development in ICR mice | Okazaki et al., 200129 | Journal of Radiation Research | DNA damage and/or teratogenic effects | Experimental study in animals |
| 7 | Influence of strong static magnetic fields on primary cortical neurons | Prina-Mello et al., 200630 | Bioelectromagnetics | DNA damage and/or teratogenic effects | Experimental study in animals |
| 8 | Midgestational exposure of pregnant BALB/c mice to magnetic resonance imaging conditions | Heinrichs et al., 198831 | Journal of Magnetic Resonance Imaging | DNA damage and/or teratogenic effects | Experimental study in animals |
| 9 | Effects of magnetic resonance imaging on eye development in the C57BL/6 J mouse | Tyndall and Sulik, 199132 | Teratology | DNA damage and/or teratogenic effects | Experimental study in animals |
| 10 | Effects of MR exposure at 1.5T on early embryonic development of the chick | Yip et al., 199433 | Journal of Magnetic Resonance Imaging | DNA damage and/or teratogenic effects | Experimental study in animals |
| 11 | Magnetic field-induced DNA strand breaks in brain cells of the rat | Lai and Singh, 200435 | Environmental Health Perspectives | DNA damage and/or teratogenic effects | Experimental study in animals |
| 12 | Evaluating DNA damage in rodent brain after acute 60Hz magnetic-field exposure | McNamee et al., 200536 | Radiation Research | DNA damage and/or teratogenic effects | Experimental study in animals |
| 13 | DNA double-strand breaks and micronuclei in human blood lymphocytes after repeated whole body exposures to 7T Magnetic Resonance Imaging | Fatahi et al., 201639 | NeuroImage | DNA damage and/or teratogenic effects | Experimental study in humans |
| 14 | Influence of Cardiac MR Imaging on DNA Double-Strand breaks in human blood lymphocytes | Brand et al., 201540 | Radiology | DNA damage and/or teratogenic effects | Experimental study in humans |
| 15 | Repeated exposure of the developing rat brain to magnetic resonance imaging did not affect neurogenesis, cell death or memory function | Zhu et al., 201141 | Biochemical and Biophysical Research Communications | DNA damage and/or teratogenic effects | Experimental study in animals |
| 16 | Dealing with pregnancy in radiology: a thin line between science, social and regulatory aspects | Buls et al., 20099 | Journal of the Belgian Society of Radiology | General effects and patient safety | Narrative review |
| 17 | ACR Practice Guideline for Imaging Pregnant or Potentially Pregnant Adolescents and Women With Ionizing Radiation | Guenin et al., 200851 | The American College of Radiology | General effects and patient safety | Clinical practice guidelines |
| 18 | SOGC clinical practice guideline for the use of magnetic resonance imaging in the obstetric patient | Patenaude et al., 20145 | Journal of the Society of Obstetricians and Gynecologists of Canada | General effects and patient safety | Clinical practice guidelines |
| 19 | What are the risks of ultrasound and MRI to the fetus? | Wozniak, 20096 | Future Science Group | General effects and patient safety | Narrative review |
| 20 | Magnetic resonance imaging in fetal medicine: a pictorial review of current and developing indications | Weston, 20124 | British Medical Journal | General effects and patient safety | Clinical practice guidelines |
| 21 | ACR Guidance Document on MR Safe Practices: 2013 | Kanal et al., 201316 | Journal of Magnetic Resonance Imaging | General effects and patient safety | Clinical practice guideline |
| 22 | RANZCR MRI Safety Guidelines | The Royal Australian and New Zealand College of Radiologists, 200717 | The Royal Australian and New Zealand College of Radiologists | General effects and patient safety | Clinical practice guidelines |
| 23 | Safety in Magnetic Resonance Imaging | Lipton, 201318 | Society of Radiographers | General effects and patient safety | Clinical practice guidelines |
| 24 | Guidelines for Computed Tomography and Magnetic Resonance Imaging Use During Pregnancy and Lactation | Chen et al., 200821 | Obstetrics & Gynecology | General effects and patient safety | Clinical practice guidelines |
| 25 | Safety guidelines for magnetic resonance imaging equipment in clinical use | Medicines and Healthcare Products Regulatory Agency, 201422 | MHRA Guidelines | General effects and patient safety | Clinical practice guidelines |
| 26 | MRI: a developing technique for the developing patient | Coackley et al., 200424 | Journal of Magnetic Resonance Imaging | General effects and patient safety | Narrative review |
| 27 | Safety considerations in MR imaging | Kanal et al., 199044 | Radiology | General effects and patient safety | Narrative review |
| 28 | MR procedures: biologic effects, safety, and patient care | Shellock and Crues, 200420 | Radiology | General effects and patient safety | Clinical practice guidelines |
| 29 | Extracellular gadolinium-based contrast media: an overview | Bellin et al., 200813 | European Journal of Radiology | Effects of gadolinium | Narrative review |
| 30 | Contrast-enhanced peripheral MRA: technique and contrast agents | Nielsen and Thomsen, 201215 | Acta Radiologica | Effects of gadolinium | Narrative review |
| 31 | ACR Manual on Contrast Media Version 10.2 | ACR Committee on Drugs and Contrast Media, 201619 | American College of Radiology | Effects of gadolinium | Clinical practice guidelines |
| 32 | Effect of magnetic resonance exposure combined with gadopentetate dimeglumine on chromosomes in animal specimens | Rofsky et al., 199550 | Academic Radiology | Effects of gadolinium and patient security | Experimental study in animals |
| 33 | Reproductive and developmental toxicity study of gadobenate dimeglumine formulation (E7155). Study of embryo-fetal toxicity in rabbits by intravenous administration | Okuda et al., 199949 | Journal of Toxicological Sciences | Effects of gadolinium | Experimental study in animals |
| 34 | Impaired mitochondrial function and oxidative stress in rat cortical neurons: Implications for gadolinium-induced neurotoxicity | Feng et al., 201046 | NeuroToxicology | Effects of gadolinium | Experimental study in animals |
| 35 | Gadolinio y fibrosis sistémica nefrogénica [Gadolinium and nephrogenic systemic fibrosis] | Cejas andAcuna, 201247 | Imagen Diagnóstica | Effects of gadolinium | Narrative review |
| 36 | Gadolinium periconceptional exposure: pregnancy and neonatal outcome | De Santis et al., 200748 | Acta Obstetricia et Gynecologica Scandinavica | Effects of gadolinium | Experimental study in humans |
| 37 | An assessment of the intrauterine sound intensity level during obstetric echo-planar magnetic resonance imaging | Glover et al., 199527 | The British Journal of Radiology | Effects on the hearing system | Experimental study in humans |
| 38 | A three year follow-up of children imaged in utero with echo-planar magnetic resonance | Baker et al., 199426 | American Journal of Obstetrics and Gynecology | Effects on the hearing system | Prospective cohort study |
| 39 | Radiofrequency energy-induced heating during MR procedures: a review | Shellock, 200052 | Journal of Magnetic Resonance Imaging | Heat-induced effects | Narrative review |
| 40 | Effects of heat in embryos and fetuses | Edwards et al., 200353 | International Journal of Hyperthermia | Heat-induced effects | Experimental study in animals |
| 41 | Superficial and deep-tissue increases in anesthetized dogs during exposure to high specific absorption rates in a 1.5T MR imager | Shuman et al., 198845 | Radiology | Heat-induced effects | Experimental study in animals |