The emergence of the strain of coronavirus SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) that causes corona virus disease 2019 (COVID-19) and its impact on in the world have made imperative progress to develop an effective and safe vaccine. Despite several measures undertaken, the spread of this virus is ongoing. So far, more than 1,560,000 cases and 1000,000 deaths occurred in the world. Efforts have been made to develop vaccines against human coronavirus (CoV) infections such as MERS and SARS. However, currently, no approved vaccine exists for these coronavirus strains.
Such Previous research efforts to develop a coronavirus vaccine in the years following the 2003 pandemic have opened the door for the scientist to design a new vaccine for the COVID-19. Both SARS-CoV and SARS-CoV-2 has a high degree of genetic similarity and bind to the same host cell ACE2 receptor.
By using different vaccine development platforms including whole virus vaccines, recombinant protein subunit vaccines, and nucleic acid vaccines several candidates displayed efficacy in vitro studies but few progressed to clinical trials. This review provides a brief introduction of the general features of SARS-CoV-2 and discusses the current progress of ongoing advances in designing vaccine development efforts to counter COVID-19.
La aparición de la cepa de coronavirus SARS-CoV-2 (síndrome respiratorio agudo severo por coronavirus 2), que causa la enfermedad por coronavirus 2019 (COVID-19), y su impacto a nivel mundial, ha urgido el avance hacia el desarrollo de una vacuna efectiva y segura. A pesar de las diversas medidas adoptadas, la diseminación de este virus es continua. Hasta la fecha, se han producido más de 1.560.000 casos y 1.000.000 de muertes en todo el mundo. Se han realizado esfuerzos para desarrollar vacunas frente a las infecciones por coronavirus humano (CoV), tales como MERS y SARS. Sin embargo, actualmente no existe ninguna vacuna autorizada para estas cepas de coronavirus.
Los esfuerzos previos sobre investigación, para desarrollar una vacuna frente a coronavirus en los años posteriores a la pandemia de 2003, han abierto la puerta a los científicos para diseñar una nueva vacuna para el COVID-19. Tanto SARS-CoV como SARS-CoV-2 poseen un alto grado de similitud genética y capacidad de adherirse al mismo receptor ACE2 de la célula huésped.
Utilizando diferentes plataformas para el desarrollo de vacunas, incluyendo vacunas de virus completos, vacunas de subunidades de proteína recombinante y vacunas de ácido nucleico, algunas de estas han mostrado su eficacia en estudios in vitro, pero pocas de ellas han progresado hacia ensayos clínicos. Esta revisión aporta una breve introducción a las características generales del SARS-CoV-2, y trata el progreso actual de los avances en curso para diseñar el desarrollo de vacunas frente al COVID-19.
Viruses have a great potential to become a dangerous life-threatening and cause irreparable loss to human beings. Hardly the world learns to cope with one strain of virus when another emerges and poses a threat to the future of humanity. A similar situation has emerged when a new strain of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that has not been previously known in human history. Coronaviruses (CoVs) are nonsegmented, enveloped, single-strand, positive-sense, ribonucleic acid viruses belonging to the Coronaviridae family. CoVs named due to their Sun's corona or crown-like surface projections. CoVs are the largest group of host-specific RNA viruses infecting birds, snakes, bats, and mammals including humans. CoVs grouped into Alphacoronaviruses and Betacoronaviruses which mostly mammals like bats, civets, rodents, and humans and Gammacoronaviruses and Deltacoronaviruses which are mostly found in birds.1 In the 21st century, three CoVs outbreaks emerged from their reservoirs to cause disease in human. Several members of the Coronaviridae family infect humans. Humans are infected and developed upper respiratory tract diseases with different CoVs strains including 229E, OC43, NL63 and HKU1. OC43 and 229E causes common cold mainly during winter with 4%-15% prevalence. NL63 causes mild respiratory tract disease, especially in children with a prevalence of 10% of all respiratory diseases. HKU1was isolated from a 71-year-old man with pneumonia.2,3
The three CoVs that cause serious respiratory diseases are severe acute respiratory disease (SARS-COV) which emerged in late 2002, Middle East respiratory syndrome (MERS-COV), which emerged in 2012, and SARS-CoV-2 that causes COVID-19 disease emerged in China as a threat to the human lives and declared as a global health emergency by the World Health Organization (WHO).4 According to Johns Hopkins’ COVID-19 global case dashboard, almost all countries are now facing the Corona Virus Disease 2019 (COVID-19) wave with 5,518,905 cases and 346,700 total deaths reported on May 26th, 2020. So, there is an urgent need for an effective drug and vaccine for COVID-19 disease.5
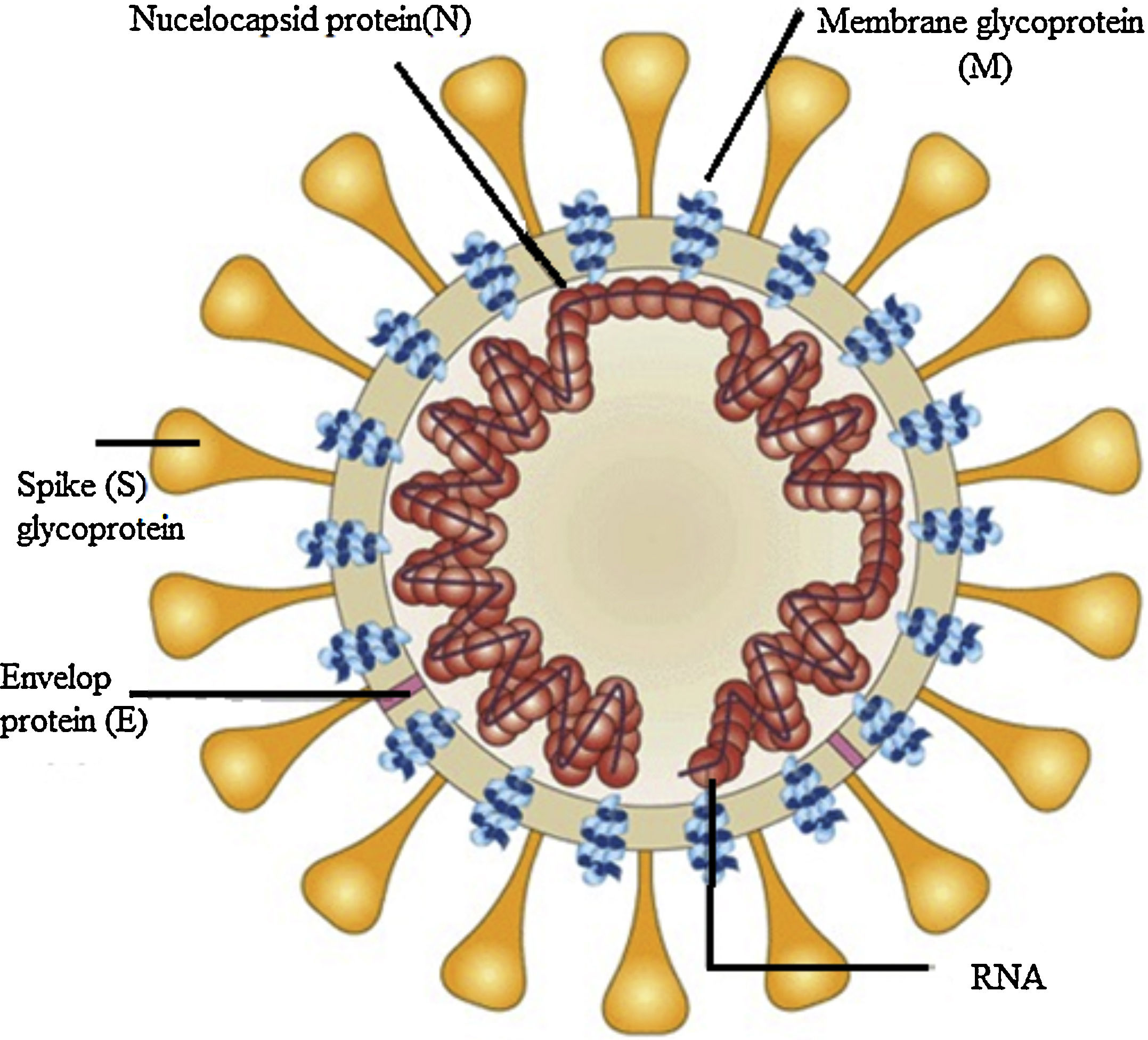
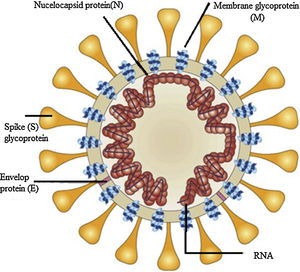
Structure component of SARS-CoV-2 virusThe SARS-CoV-2 virus has relatively a large genome structure about 30 (26-32) kb pairs. The virus has several non-structural proteins (NSP) like, NSP12, NSP13, NSP3 and NSP5 that are vital for their life cycle and pathogenesis. Like other CoVs, the SARS-CoV-2 virus has four structural proteins naming the S-spike Protein (outer spiky glycoprotein), envelope protein (E), Membrane glycoprotein (M), and nucleocapsid protein (N), that enhance the attachment, transport and interferes with host immune response and other five accessory proteins (ORF3a, ORF6, ORF8, ORF7, and ORF9). These proteins help the corona virus-host cell interactions to create an optimal condition for viral replication, modify host gene induction, and neutralization of the host's antiviral defenses system. These coronavirus–host interactions have a vital role in the viral disease pathogenesis.6
The S protein is a transmembrane glycoprotein that promotes the entrance of the virus into host cells. S protein is a major target for design of vaccines and inhibitors of viral entry. The S protein has two domains, S1 and S2. The S1 domains contain the receptor binding domain (RBD) that mediates attachment to the host receptor cell, whereas the S2 domain facilitates the fusion of the virus to the host cell. The Entry of SARS-CoV-2 into host cells initiated by binding of the RBD to angiotensin-converting enzyme 2 (ACE2), the main receptor for SARS-CoV-2 on the host cells surface and CD209L is another receptor with lower affinity.7 ACE2 receptors expressed in type II alveolar cells, airway epithelial cells, fibroblasts, endothelial cells, and in several immune cells. S2 subunit has a domain that facilitates the fusion of the viral envelope with the host cell membrane. These domains are internal membrane fusion peptide (FP), a membrane proximal external region (MPER), two 7-peptide repeats, and a transmembrane domain (TM). The S glycoprotein has a key role in the induction of immunity during infection with SARS-CoV-2 by eliciting neutralizing-antibodies and T-cell responses. Studies showed that the S protein has several immunodominant parts that do not induce neutralizing antibodies, but the RBD in the S1 region is a potent inducer of neutralizing antibodies. Thus, full-length S glycoprotein, the S1 subunit, the RBD domain, NTD, and FP are proposed as the promising candidate to develop an effective vaccine against SARS-CoV-2.7,8 The M- membrane protein is a transmembrane glycoprotein that gives a definite shape to the virus structure. It binds to nucleocapsid and organizes the virus assembly. The M protein contains T cell epitope clusters that elicit a neutralizing antibody in SARS patients. The E protein has a key role in the pathogenesis, assembly, and release of the virus. The E protein Inactivation modifies the virulence of CoVs due to changes in morphology and tropism. The N-nucelocapsid protein (which is within the phospholipid bilayer) has a key role in complex formation with viral genome, enhances M protein interaction during assembly, and increases replication of the virus. The E, S, and M proteins form the viral envelope.8–10
Current treatment strategies for COVID-19Currently, no corona virus-specific therapeutic agent, monoclonal antibodies, or vaccine has been approved. Thus, there is an urgent need for effective medication and vaccine for COVID-19 disease to limit the transmission in the community. Most treatment options for COVID-19 taken from previous experiences in treating SARS-CoV, MERS CoV, and other viral diseases.11 Currently, mechanical ventilation, ICU admission, and supportive care are the most important management strategy for severe cases. According to WHO guidelines infected patients treated with supportive care like bed rest, oxygen saturation, adequate nutrition, prevention of dehydration, keeping electrolyte and acid-base balance, antibiotics, and isolation of patients suspected or confirmed for COVID-19.12 Several pre-existing and potential drug candidates, including remdesivir, chloroquine, and others are considered. the Therapeutic options that could be evaluated and used for COVID-19 include molecules binding to the virus, inhibiting enzymes involved in viral replication and transcription, inhibitors targeting helicase and proteases, host cell protease inhibitors, host cell endocytosis inhibitors, anti-sense RNA and ribozyme, neutralizing antibodies, mAbs targeting host cell receptor or interfere with S1 RBD, antiviral peptide targeting S2, and natural products.13
Current Sars-Cov-2 Vaccines PlatformsEffective SARS-CoV-2 vaccines are crucial for controlling the CoVs pandemic. Vaccines decrease disease severity, viral shedding, and person to person transmission. Currently, no vaccine has been licensed to prevent SARS-CoV-2 infection.14 There are different vaccine development platforms against SARS-CoV-2, including live attenuated virus, viral vectors, inactivated virus, subunit vaccines, recombinant DNA, and protein vaccines. Several studies are in progress, but requires months to years to develop the vaccines for SARS-CoV-2. Currently, there may be many promising targets for SARS-CoV-2. COVID-19 candidate vaccines under development include S protein or RBD subunit vaccines and replicating or non-replicating vector vaccines expressing mainly S protein or the RBD. Several studies showed that viral S protein subunit vaccines displayed higher neutralizing antibody titers and protection than live-attenuated SARS-CoV, full, and DNA-based S protein vaccines. Collectively, S protein/gene is the preferred target site in SARS vaccine development, and the same strategy can be potentially useful in developing SARS-CoV-2 vaccine.14,15
Live-Attenuated VaccinesLive attenuated vaccine (LAV) is the most immunogenic vaccines that do not require adjuvant to gain optimal response due to its effectiveness to provoke immunity mimic to the natural infection. The LAV contain viable but attenuated live virus with low virulence property that does not cause disease in a person with normal immune systems. They reproduce slowly, and thus remain a continuous source of antigen for a long period after Single immunization, reducing the need for booster dose.16 Several LAVs are found in the market to protect various disease including mumps, rubella, measles and varicella vaccines. LAV produced by passing virus in cell cultures, in animals or at suboptimal temperatures, allowing less virulent strains selection or by mutagenesis, or deletion of the viral genes that give virulence. LAV is not suitable for infants, immune-compromised patients, and elderly people due to the risk of reversion to virulent strain.17 Currently, several pharmaceutical companies isolated the virus strains of SARS-CoV-2 and started relevant vaccine development. Codagenix Biotec Inc collaboration with the Serum Institute of India Ltd developing a live-attenuated SARS-CoV-2 vaccine in which the sequence of the target gene of interest has been changed by swapping its optimized codons with non-optimized ones.18
Inactivated Whole-Virus VaccineInactivated whole-virus comprises the entire disease-causing virion which is inactivated physically (heat) or chemically. It has several antigenic parts to the host and can induce diverse immunologic responses against the pathogen. Inactivated whole-virus has several advantages, including low production cost, safe, and does not involve genetic manipulation. IWV is conventional vaccines with mature technology and may become the first SARS-CoV-2 vaccine put into clinical use.19 There are three inactivated whole-virus vaccines against SARS-CoV-2 reached phase 1/2 clinical trial. These phase 1/2 clinical trials are done by the Beijing Institute of Biological Products (ChiCTR2000032459), Sinovac (NCT04352608) and Wuhan Institute of Biological Products (ChiCTR2000031809).20
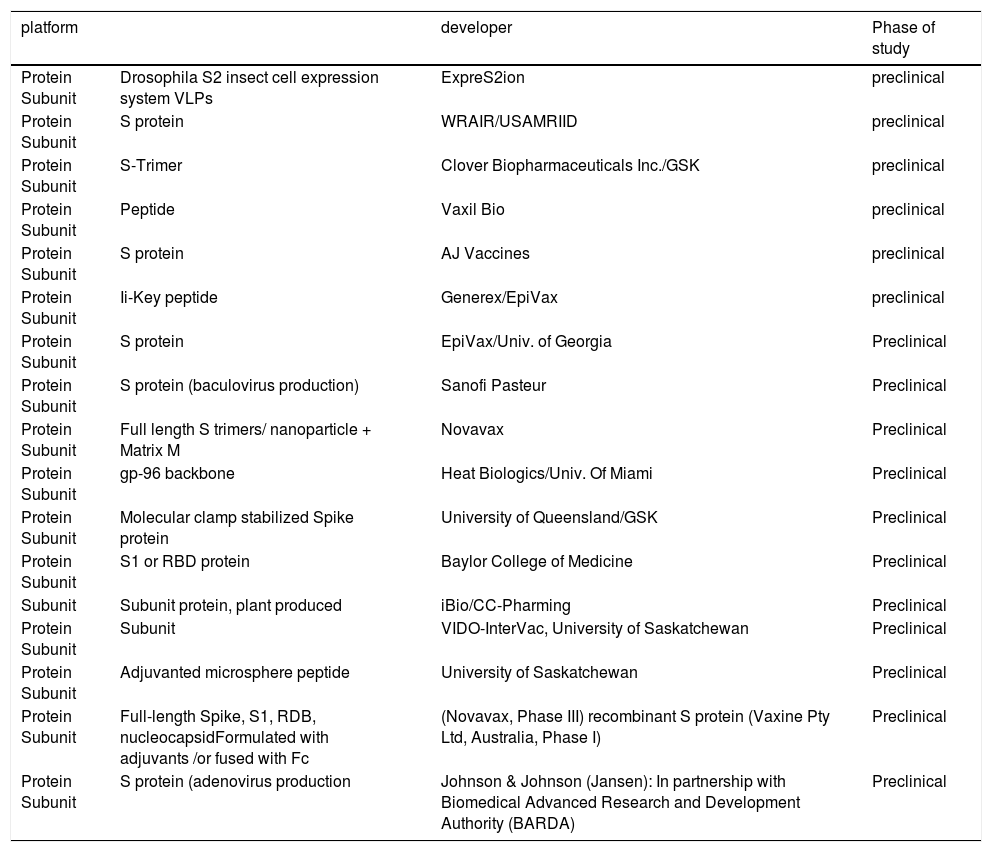
Subunit VaccinesSubunit vaccines contain pathogen-derived proteins (antigens) with immunogenicity that can elicit the host immune system. Subunit vaccine is safe and easily manufactures by recombinant DNA techniques but requires adjuvant to enhance a immune response. So far, many research institutions initiated the SARS-CoV-2 subunit vaccine, and use the spike glycoprotein S, and its fragments, such as S1, S2, RBD, and nucleocapsid protein as a prime target antigens as shown in Table 1.21 Novavax, Inc. developed a candidate vaccine based on S protein. So far, Clover Biopharmaceuticals constructed a SARS-CoV-2 S protein trimer vaccine (S-Trimer) by using its patented Trimer-Tag© technology.22 Since the RBD of S protein directly bind with the ACE2 receptor on host cells, RBD immunization induces specific antibodies that may block this recognition and effectively prevent the invasion of the virus. Most of SARS-CoV-2 subunit vaccines currently under development use RBD as the antigen. In a study in monkeys, recombinant RBD protein was used to successfully reduce viral loads in the lungs and oropharynx and to prevent MERS-CoV pneumonia.23 Similar to RBD, the N-terminal domains (NTD) of the S protein, E, M, N, and NSPs proposed carbohydrate receptor-binding activity. For example, the carbohydrate-binding properties of IBV M41 strain related to the NTD of the S protein. Thus, this domain is a candidate antigen for the development of the vaccine.24
Vaccines under development based on Protein Subunit for COVID-19 according to the WHO as of April 20, 2020 (adapted from (20).
| platform | developer | Phase of study | |
|---|---|---|---|
| Protein Subunit | Drosophila S2 insect cell expression system VLPs | ExpreS2ion | preclinical |
| Protein Subunit | S protein | WRAIR/USAMRIID | preclinical |
| Protein Subunit | S-Trimer | Clover Biopharmaceuticals Inc./GSK | preclinical |
| Protein Subunit | Peptide | Vaxil Bio | preclinical |
| Protein Subunit | S protein | AJ Vaccines | preclinical |
| Protein Subunit | Ii-Key peptide | Generex/EpiVax | preclinical |
| Protein Subunit | S protein | EpiVax/Univ. of Georgia | Preclinical |
| Protein Subunit | S protein (baculovirus production) | Sanofi Pasteur | Preclinical |
| Protein Subunit | Full length S trimers/ nanoparticle + Matrix M | Novavax | Preclinical |
| Protein Subunit | gp-96 backbone | Heat Biologics/Univ. Of Miami | Preclinical |
| Protein Subunit | Molecular clamp stabilized Spike protein | University of Queensland/GSK | Preclinical |
| Protein Subunit | S1 or RBD protein | Baylor College of Medicine | Preclinical |
| Subunit | Subunit protein, plant produced | iBio/CC-Pharming | Preclinical |
| Protein Subunit | Subunit | VIDO-InterVac, University of Saskatchewan | Preclinical |
| Protein Subunit | Adjuvanted microsphere peptide | University of Saskatchewan | Preclinical |
| Protein Subunit | Full-length Spike, S1, RDB, nucleocapsidFormulated with adjuvants /or fused with Fc | (Novavax, Phase III) recombinant S protein (Vaxine Pty Ltd, Australia, Phase I) | Preclinical |
| Protein Subunit | S protein (adenovirus production | Johnson & Johnson (Jansen): In partnership with Biomedical Advanced Research and Development Authority (BARDA) | Preclinical |
mRNA carries instruction from the protein-encoding DNA to the proteins translating ribosomes. There are two types of mRNA vaccines platform: non- replicating mRNA and self-amplifying mRNA that encodes not only the required antigen but also the viral replication machinery.
mRNA vaccines mimic the natural infection of the virus, but they retain only a short synthetic viral mRNA which encodes only the required antigen.25 mRNA vaccine is a promising alternative to traditional vaccine approaches due to their safety, potency, quick vaccine-development time, and low-cost production. The procedures to develop the mRNA vaccine include the screening of antigens, the optimization of sequences, modified nucleotides screening, delivery systems optimization, evaluation safety, and immune response.26 mRNA vaccines strongly induce both cellular and humoral immune responses. It is relatively safe and effective because it is only a transient carrier of message that does not interact with the host genome and it also does not need the whole virus.27 Currently, no mRNA based vaccine entered the market. So far, a SARS-CoV-2 mRNA vaccine (mRNA-1273, encoding S protein) developed by Moderna/NIAID, launched Phase 1 (NCT04283461) clinical trial as shown in Table 2.28 Bluebird Biopharmaceutical Company is developing mRNA vaccine candidates using two different approaches. The first is to use mRNA to express the virus S protein and RBD domain and the second is to express virus-like particles in vivo. Duke-NUS Medical School and Arcturus Therapeutics partnered to develop a self-replicating mRNA vaccine candidate, currently in a preclinical trial. BioNTech and Pfizer are collaborating to co-develop mRNA-based vaccine candidate BNT162.28,29
vaccine candidates based on RNA platform for COVID-19 according to the WHO as of April 20, 2020 (adapted from (20).
| Type of candidate vaccine | developer | stage of clinical evaluation |
|---|---|---|
| LNP-encapsulated mRNA(mRNA-1273) | USA (Moderna/NIAI) | Phase 2(NCT04283461) |
| LNP-encapsulated mRNA cocktail encoding VLP | Fudan University/ Shanghai JiaoTong University/RNACure Biopharma | preclinical |
| mRNA | China CDC/Tongji University/Stermina | preclinical |
| mRNA | Arcturus/Duke-NU | preclinical |
| mRNA | BioNTech/Fosun Pharma/Pfizer | preclinical |
| saRNA | Imperial College London | preclinical |
| mRNA | Curevac | preclinical |
DNA vaccines (DVs) have a plasmid into which a particular gene incorporated that encodes the antigens that identified from the pathogenic microorganism. The bacterial plasmid carrying the desired gene delivered into the host and translated the antigenic protein that stimulates the immune system normally activated by the pathogenic microorganism. DVs elicit both the cell-mediated and humoral immune system. DVs induce long-lasting immunity that defends the diseases effectively in the future. DVs are very stable, can be produced within weeks because they does not need culture or fermentation; instead used synthetic processes and began clinical trial within monthes. Currently, DNA vaccine not approved for the market.30 Several pharmaceutical companies have advanced DNA platforms for COVID-19 vaccine discovery. Most DVs candidates encode the S protein or the S1 domain to prevent attachment to the human ACE2 receptor, a receptor for viral entry. The DNA translating S protein stimulates antibodies and elicits cell-mediated immune response in mice, macaques, and camels. When the immunized macaques were challenged with MERS-CoV, characteristic clinical symptoms including pneumonia were mitigated. So far, two SARS-CoV-2 DVs are under development.31 Inovio Pharmaceuticals developed a DVs candidate termed INO-4800, which is in phase 1 (NCT04336410) clinical trial. Takis/Applied DNA Sciences/Evvivax and Zydus Cadila are developing a DVs candidate for COVID-19 disease which is now in preclinical studies.32
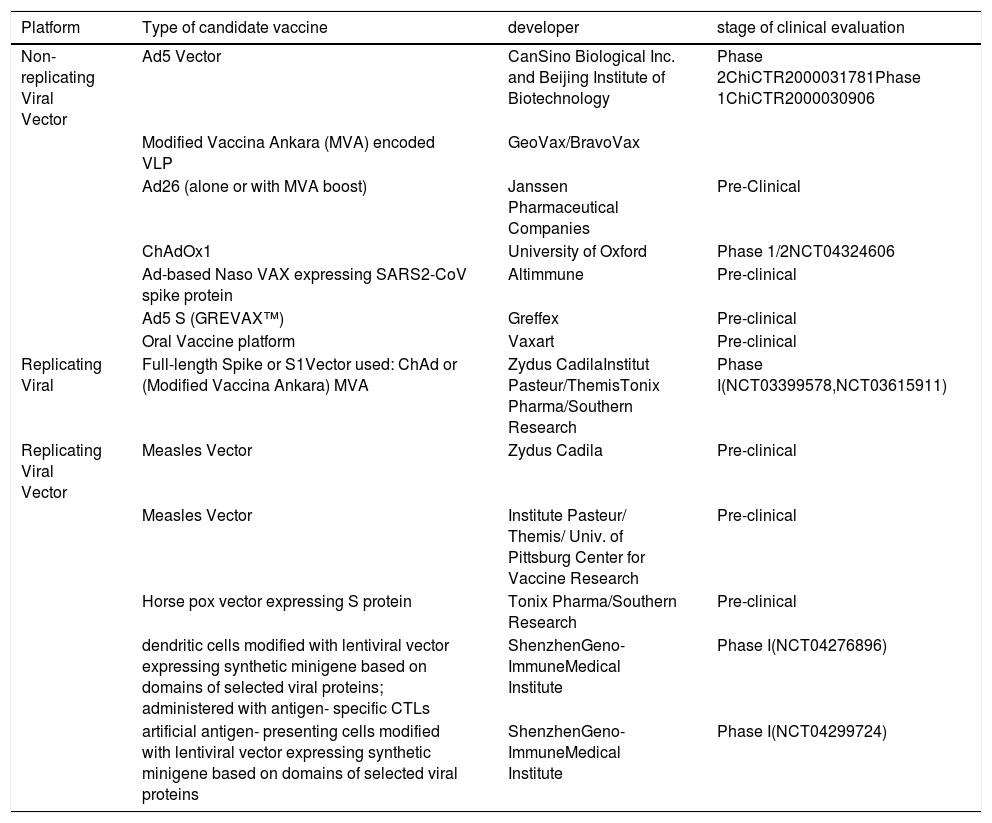
Viral Vector-Based VaccineViral vector vaccine works by carrying a DNA express or antigen(s) into host cells, thereby eliciting cell-mediated immunity in addition to the humoral immune responses. Viral Vectors Vaccines characterized by strong immunogenicity and safety.33 Several viral vectors are available for vaccine development including vaccinia, modified vaccinia virus Ankara (MVA), adenovirus (Ad), adeno-associated virus (AAV), retrovirus/lentivirus, alphavirus, herpes virus, Newcastle disease virus, poxvirus, and others. Viral vectors can be replicating or non-replicating viruses.34 Adenovirus and modified vaccinia virus Ankara (MVA) are the most common viral vectors used in vaccine developments as shown in Table 3. There are over 50 subtypes of human Ad, with Ad serotype 5 (Ad5) is a stable, non-replicating virus that used in several vaccine development. This virus allows the insertion of large segments of foreign DNA (∼8kb) into its genome. But, pre-existing immunity against human Ad5 is widespread, hampering its use as a vector for vaccine development.35 Chimpanzee adenovirus (ChAdOx1) represents an alternative to the human Ad vector due to its safety and lack of preexisting immunity in humans.36 Mice immunized with the Ad5 vector encoding the S protein-induced systemic neutralizing antibodies and mucosal T-cells immunity. Ad5 vectors encoding the S1 domain of the S protein displayed stronger neutralizing antibody responses than that encoding the full length, suggesting the effect of immune focusing.37 Currently, Can Sino Biological Inc. and the Beijing Institute of Biotechnology are developing Ad5- vector COVID-19 vaccine candidate in Phase 1(ChiCTR2000030906). Another adenovirus vectored vaccine developed by Chen Wei group entered in phase 1 clinical trial (NCT04313127). Johnson & Johnson is developing an adenovirus vectored vaccine using AdVac®/PER.C6® vaccine platforms. Shenzhen Geno-Immune Medical Institute also developing two lentivirus vector based vaccine candidates named COVID-19/aAPC and LVSMENP- DC. Recombinant MVA, Newcastle disease virus (NDV), and poxvirus used as a viral-vector vaccine candidate due to their safety, efficacy, and decent immunogenicity.38
Vaccine developments by using Non-Replicating Viral Vector platform for COVID-19 according to the WHO as of April 20, 2020 (adapted from (20).
| Platform | Type of candidate vaccine | developer | stage of clinical evaluation |
|---|---|---|---|
| Non-replicating Viral Vector | Ad5 Vector | CanSino Biological Inc. and Beijing Institute of Biotechnology | Phase 2ChiCTR2000031781Phase 1ChiCTR2000030906 |
| Modified Vaccina Ankara (MVA) encoded VLP | GeoVax/BravoVax | ||
| Ad26 (alone or with MVA boost) | Janssen Pharmaceutical Companies | Pre-Clinical | |
| ChAdOx1 | University of Oxford | Phase 1/2NCT04324606 | |
| Ad-based Naso VAX expressing SARS2-CoV spike protein | Altimmune | Pre-clinical | |
| Ad5 S (GREVAX™) | Greffex | Pre-clinical | |
| Oral Vaccine platform | Vaxart | Pre-clinical | |
| Replicating Viral | Full-length Spike or S1Vector used: ChAd or (Modified Vaccina Ankara) MVA | Zydus CadilaInstitut Pasteur/ThemisTonix Pharma/Southern Research | Phase I(NCT03399578,NCT03615911) |
| Replicating Viral Vector | Measles Vector | Zydus Cadila | Pre-clinical |
| Measles Vector | Institute Pasteur/ Themis/ Univ. of Pittsburg Center for Vaccine Research | Pre-clinical | |
| Horse pox vector expressing S protein | Tonix Pharma/Southern Research | Pre-clinical | |
| dendritic cells modified with lentiviral vector expressing synthetic minigene based on domains of selected viral proteins; administered with antigen- specific CTLs | ShenzhenGeno- ImmuneMedical Institute | Phase I(NCT04276896) | |
| artificial antigen- presenting cells modified with lentiviral vector expressing synthetic minigene based on domains of selected viral proteins | ShenzhenGeno- ImmuneMedical Institute | Phase I(NCT04299724) |
Synthetic Peptide vaccines have chemically produced from fragments of antigens that elicites the immune response. These vaccines are inexpensive, easy for preparation, and quality control. But display low immunogenicity, thus antigen modification and adjuvant required during formulation. Fragments of antigen has B_ and/or Tepitope activity, which affect the specificity of the immune response. Currently, a set of B and T cell epitopes isolated from S and N proteins of SARS-Cov, these epitopes are highly conserved in SARS-CoV-2 and may assist efforts to develop vaccine for covid-19 disease.39 Several pharmaceutical companies like Generex Biotechnology developing peptide based vaccines against SARS-CoV-2 viruses by producing synthetic peptides that mimic crucial antigens from a virus that is chemically bonded to the 4-amino acid Ii-Key to ensure robust immune system activation.40
Virus-Like Particles Based VaccineVirus-like particles (VLPs) are“hollow-core” virus particles formed by the viral structural component with self-assembly character into nanostructure. VLP mimic the structure of the whole virus. VLPs can elicit innate immunity response via pathogen recognition receptors. VLPs represent advanced subunit vaccine with increased immunogenicity because they contain the structural protein of the virus. VLPs have a noninfectious and non-replicative property due to lose of genomic component. VLPs help to develop safer and cheaper vaccine candidates.41 VLPs can be synthesized by encoding the viral structural proteins. In general, the VLPs-based vaccine is similar to the whole inactivated virus vaccine, but it does not require the viral inactivation step which may affect the immunogenicity of a viral protein. Because no live virus is involved in the production process, VLPs can be easily generated in a low-containment production environment.41,42 So far, VLP-based vaccines produced for greater than 30 different viruses including GlaxoSmithKline's Engerix® (hepatitis B virus) and Cervarix® (human papillomavirus), and Merck and Co., Inc.’s Recombivax HB® (hepatitis B virus) and Gardasil® (human papillomavirus).43,44 Currently, several VLP expression systems available like recombinant vaccinia virus, mammalian cells (293T, CHO), baculovirus and yeast expression systems. The RBD domain based VLP vaccine candidate (RBD-CuMVTT) derived from cucumber mosaic virus induced significant levels of antibodies in mice which can inhibit the binding of S protein to ACE2 receptor and effectively neutralized the SARS-CoV-2 virus in vitro.45
The most promising vaccinesThe COVID-19 outbreak is rapidly increasing in the number of cases, deaths, and countries affected. Pharmaceutical companies are searching to develop an effective vaccine for controlling the COVID-19. Currently, several countries and Research institutions are developing vaccine against SARS-CoV-2. However, vaccine development could not be done overnight. After vaccine design and preparation, it will undergo safety and efficacy evaluation before entering clinical trials. Generally, three phases of clinical trials undertaken to evaluate the safety, efficacy and immunogenicity of the vaccine. Phase 1 done for studing safety and immunogenicity of the vaccine, and later, phase 2 and phase 3 studies done for both safety and efficacy. Typically, a novel vaccines development takes 10–20 years and less than 10% success rate even for a vaccine that enters clinical trials. In the past 30 years, FDA approved around 3,000 clinical trials applications for vaccine development but less than 20 vaccines approved. Figure 1
Currently, there are no effective vaccines for the prevention of COVID-19. But, few candidates moved into clinical trial.
CanSino Biologicals started Phase 2 clinical trial in China (NCT04341389) for Ad5- nCoV which showed safe, tolerable and immunogenic in phase 1 clinical trial. Moderna Announces Positive Interim Phase 1 Data for mRNA-1273 vaccine. The vaccine elicited virus- neutralizing antibodies at the levels displayed in convalescent sera and showed full protection against viral replication in the lungs in a mouse model. The vaccine candidate mRNA-1273 also showed safe and well tolerated.
Inovio Pharmaceutical developed INO-4800 which is in phase 1 trials showed safe and protective immunity. LV- SMENP- DC from Shenzhen Geno-Immune Medical Institute (phase1) may offer promising vaccines against COVID-19.
University of Oxford developed a vaccine based on Chimpanzee Adenovirus Vector (ChAdOx1) which is immunogenic in mice. The vaccine candidate entered phase I/II (NCT04324606) clinical trial in April 2020 to study its safety, tolerability and immunogenicity in 510 volunteer.
AbbreviationsAd5, Ad serotype 5, AAV, adeno-associated virus, ACE2; angiotensin-converting enzyme 2, CoVs; Coronaviruses, ChAdOx1; Chimpanzee adenovirus, DVs; DNA vaccines, MVA, modified vaccinia virus Ankara, SARS-CoV-2; severe acute respiratory syndrome coronavirus 2, WHO; World Health Organization, S protein;Spike Protein, RBD; receptor binding domain, VLPs;Virus-like particles, NDV; Newcastle disease virus
FundingNo funded
Conflict of interestsThe authors declare no conflict of interests.
I would like to acknowledge the School of Pharmacy (University of Gondar) for providing the resources.