Vacunas COVID-19: desarrollo y práctica - COVID-19 vaccines: development and practice
Más datosThe SARS-CoV-2 omicron variant is recent member of variant of concerns that confer neutralizing antibodies and escape immune system due to harboring more than 40 mutations. Current evidences suggest two dosages SARS-CoV-2 vaccine dose not efficient protects against new variants of SARS-CoV-2; however, recent studies declare that the third booster vaccination can elicit higher antibodies concentration as well as cross-reaction between neutralizing antibodies and new SARS-CoV-2 variants. On the other hand, although a third booster vaccination seems to be benefit in some immunocompromised patients such as recipients of solid-organ transplants or hemodialysis patients, but in other immunosuppressed patients, for instance patients with B cell lymphoproliferative disease partially respond to SARS-CoV-2. Herein, we evaluate the effectiveness of the third booster vaccination against Omicron variant using comprehensive literature review.
La variante ómicron de SARS-CoV-2 es un miembro reciente de variantes preocupantes, que confiere anticuerpos neutralizantes y escapa al sistema inmune debido a que alberga más de 40 mutaciones. Las evidencias actuales sugieren que dos dosis de la vacuna contra la SARS-CoV-2 no protegen eficientemente frente a las nuevas variantes de SARS-CoV-2. Sin embargo, los estudios recientes afirman que la tercera vacuna de refuerzo puede suscitar una mayor concentración de anticuerpos, así como una reacción cruzada entre los anticuerpos neutralizantes y las nuevas variantes de SARS-CoV-2. Por otro lado, aunque la tercera vacuna de refuerzo parece ser beneficiosa para algunos pacientes inmunocomprometidos, tales como los receptores de trasplantes de órganos sólidos, o los pacientes de hemodiálisis, otros pacientes inmunosuprimidos, como por ejemplo los pacientes con enfermedad linfoproliferativa de células B, responden parcialmente a la SARS-CoV-2. Por tanto, evaluamos la efectividad de la tercera vacuna de refuerzo frente a la variante ómicron, utilizando una revisión amplia de la literatura.
The coronavirus disease 2019 (COVID-19) is atypical pneumonia that first discovered from Wuhan China in December 2019.1 To date, there were more than 440 million reported cases as well as 5.97 million deaths throughout the worldwide (https://covid19.who.int/). Unfortunately, there is no effective therapeutic agents various SARS-CoV-2 variants; however, strict traveling bans, physical distancing, mask wearing, convalescent plasma therapy, and mass vaccination are still main strategy to fighting with the SARS-CoV-2 pandemics.2–5 According to the literatures, the efficacy of two coronavirus vaccination doses not complete protects against the SARS-CoV-2 omicron variants.6–8 The aim of this study was review of the literature regarding the efficacy of the third booster vaccination against Omicron variant.
The emergence of the VOC OmicronOne and a half years have passed since the emergence of SARS-CoV-2 (severe acute respiratory syndrome Coronavirus 2), while according to WHO, more than 418 million infected cases and 5.85 million deaths have been recorded globally due to different variants of this virus.9 Omicron is the latest variant of concern (VOC, Pango lineage B.1.1.529, Nextstrain clade identifier 21K) that was first characterized and reported on November 2, 2021 from Botswana, South Africa (GISAID sequence accession ID: EPI_ISL_8182767).10 According to the GISAID database, the VOC Omicron was first characterized simultaneously in contiguous geographical areas of Botswana (hCoV-19/Botswana/R42B90_BHP_000842207/2021), Hong Kong (hCoV-19/Hong Kong/VM21045145/2021) and South Africa (hCoV-19/South Africa/CERI-KRISP-K032250/2021). The Omicron variant seems to have evolved in the African population due to the poor vaccination rates in the African population and their weakened immune system due to HIV-infection. Gao et al. stated that the unvaccinated African HIV-infected population has become a reservoir for the evolution, multiplication and emergence of Omicron variants.11 Sequence analysis of the Omicron variant showed that this variant has only 30 mutations in its spike protein and genomic substitutions have caused it to have greater doubling time, infectivity, persistence and escape from immune system than previous variants.12
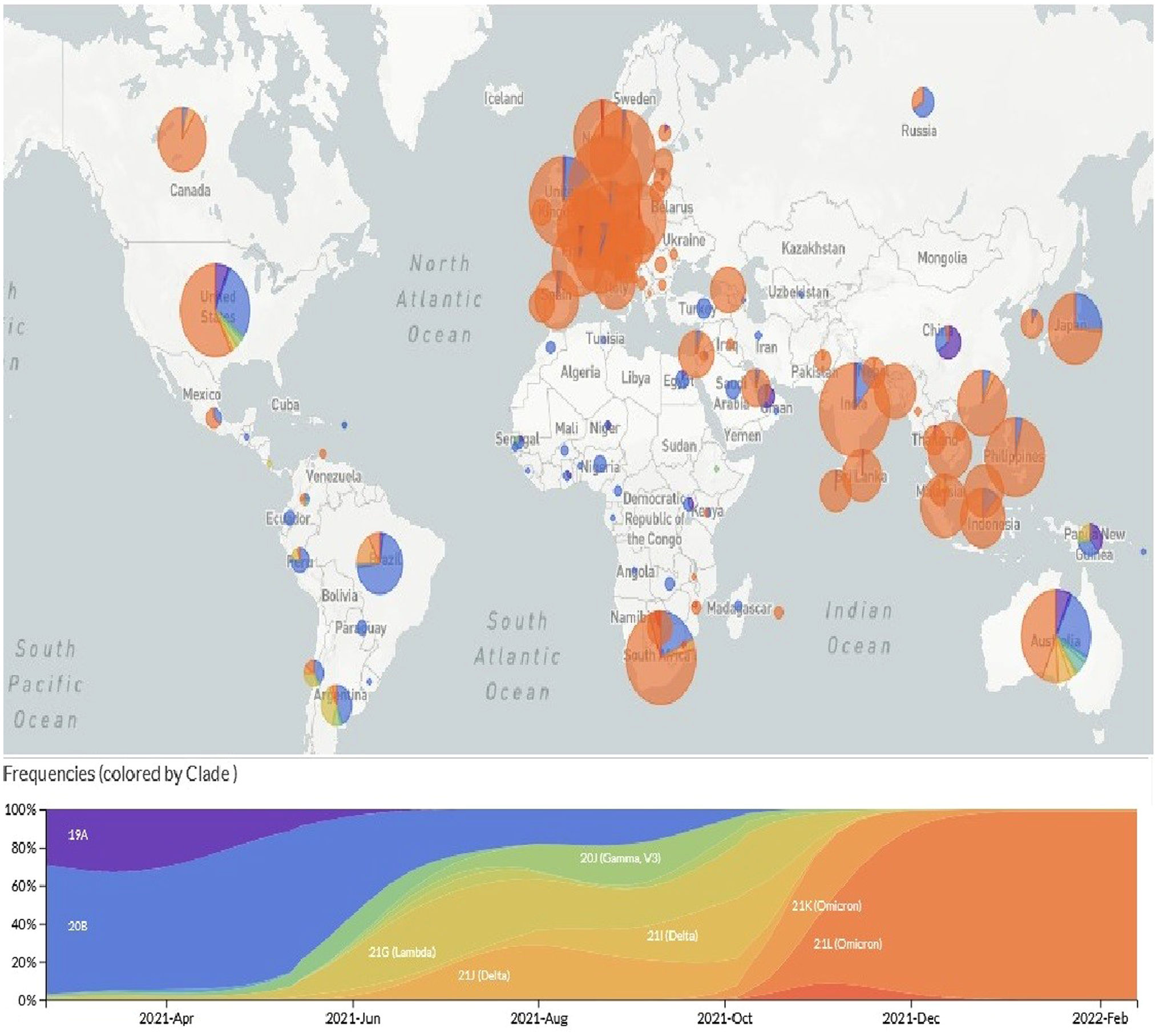
Global dissemination of Omicron variant and current statusDue to the dramatic surge of the Omicron variant, WHO has intensified its efforts to prioritize the global dissemination and monitoring of the novel SARS-CoV-2 variant to prevent the spread of Omicron variant. However, based on the sequences uploaded to GISAID databases, Omicron has been reported in 151 different countries (https://www.gisaid.org/hcov19-variants/). As many as 511 Omicron genome sequences have been recorded till February 17, 2022, 1,362, mostly being from UK, USA, Denmark, Germany and Canada. Although many countries now have banned travel from South Africa, continuous monitoring of Nextstrain databases reflects the unbridled expansion of the VOC Omicron around the world so that it has replaced the earlier Delta variant (Fig. 1).
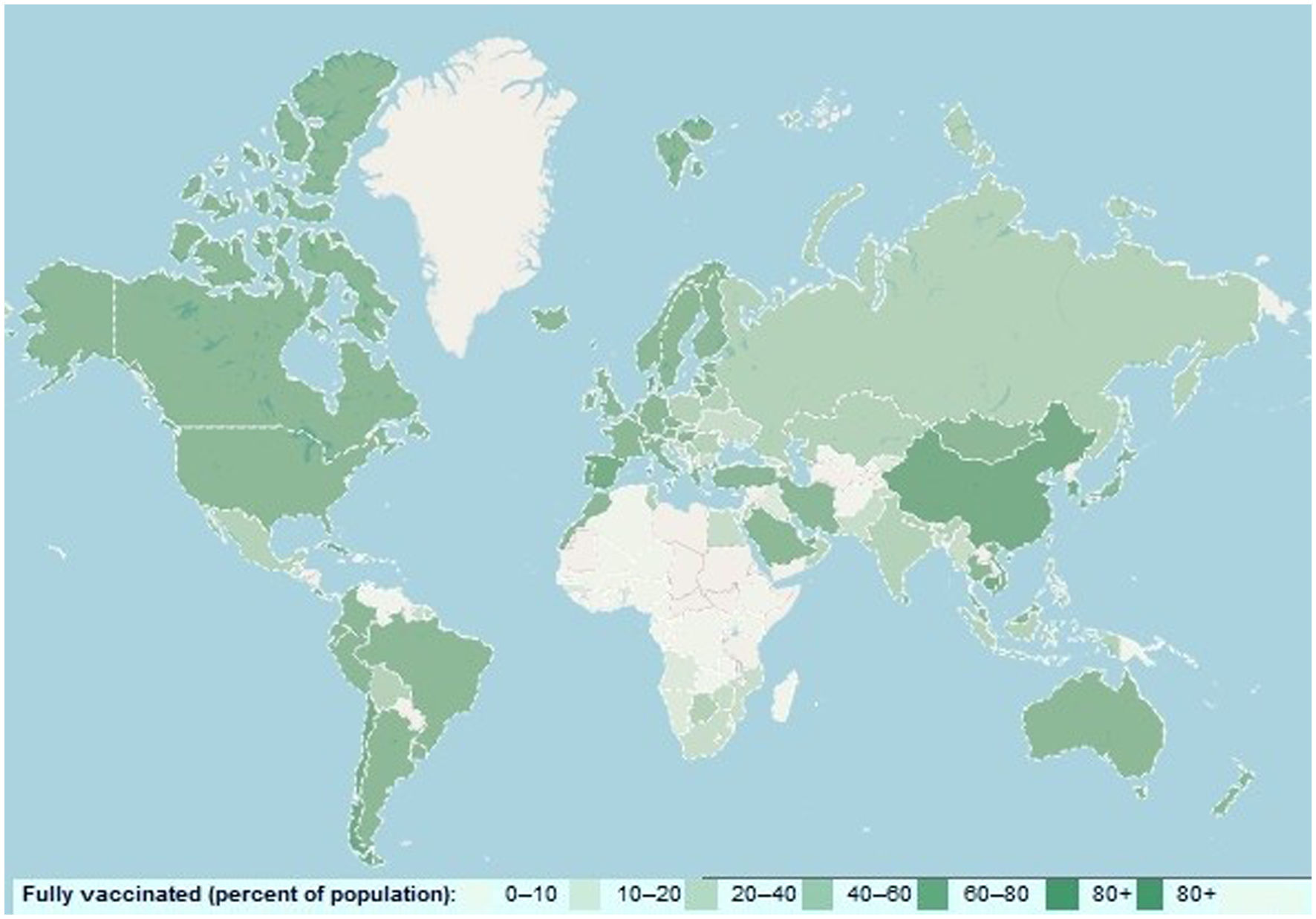
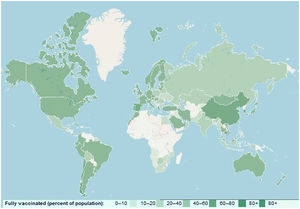
Although disease severity has been shown to be lower in the Omicron than in the delta variant, ICU admission and mortality rates are increasing in most countries, especially considering that this variant is more prevalent among younger age groups.13 To cope with the uncontrollable surge of Omicron, the U.S. Food and Drug Administration (FDA) has recently approved seven spike protein-targeted monoclonal antibodies, including Tixagevimab (COV2-2196), Cilgavimab (COV2-2130), Sotrovimab (S309), Bamlanivimab (LY-CoV555), Etesevimab (CB6), Casirivimab (REG) and Imdevimab (REGN10987) for clinical use.14 It has also been suggested that double-dose vaccination with BNT162b2 (Pfizer – BioNTech) and ChAdOx1 nCoV-19 (Oxford – AstraZeneca), mRNA-1273 (Moderna), and Ad26.COV2S (Johnson & Johnson) vaccines may also be useful in reducing and controlling the surge of Omicron as well as novel SARS-CoV-2. However, global vaccination coverage is poor and the need for mass vaccination intensifies with the emergence of the novel COVID-19 variant (Fig. 2).
The rapid spread of Omicron in Western European countries with a high proportion of vaccinated population shows that the current vaccination is not a sufficient and effective strategy to stop the spread of Omicron globally. The increasing trend of Omicron re-infection in fully vaccinated individuals and inadequate neutralizing antibodies against this variant in fully vaccinated or previous natural infection by SARS-CoV-2 indicate its high ability to escape from immune system.15,16 While we need new formulations of prophylactic vaccine to control Omicron, three important challenges are exacerbating global concern: 1) The emergence and evolution of new SARS-CoV-2 carrying novel mutations confer substantial resistance against vaccines. 2) Neutralizing antibodies titer waned months after administration, and 3) We need a span of 6-9 months to produce an effective vaccine against the new variant.
Overview of Pfizer vaccineAccording to the literature, RNA viruses are more dangerous to human life than DNA viruses, so that the continuous mutations in RNA molecules create new variants of RNA viruses such as SARS-CoV-2.17,18 Pfizer vaccine is an mRNA-based vaccine that upon injection and uptake, its S protein is expressed by local cells, and this phenomenon eventually led to the generation of immune system response; the vaccine is now available to people over the age of 16 in several countries including the United Kingdom, the United States, the European Union and Canada.19 The effectiveness of mRNA-BNT162b2 vaccine after the second dose is 95% and it should be noted that the Pfizer vaccine can be stored for up to 10 days at -70 °C.20
Overview of Moderna vaccineThe Moderna mRNA-1273 vaccine is also an mRNA-based vaccine encapsulated in nanoparticle liposome.21 Like Pfizer vaccine, this vaccine is usable in individuals 16 years and older, as well as the effectiveness of this vaccine is 94.1% after the second dose.22 Unlike the Pfizer vaccine, the Moderna vaccine is less sensitive to temperature changes; this vaccine requires -20 °C for shipping, and potentially is stable for 6 months in this temperature; it can be stored at 2-8 °C for 30 days.23
Overview of AstraZeneca vaccineThe AZD1222 (ChAdOx1 nCoV-19) vaccine from Oxford-AstraZeneca is based on a recombinant adenovirus expressing the full-length spike protein of SARS-CoV-2 virus.24 The interval between the first and second doses of this vaccine can be changed from four to 12 weeks, which in turn affects the flexibility of vaccination in European countries.25,26 Thrombotic events are considered as the serious side effects of this vaccine, so that a cerebral venous sinus thrombosis may occur 4-16 days after vaccination.27 AstraZeneca vaccine is authorized for active immunization to prevent COVID-19 in individuals ≥18 years old.28 Considering the four-week interval between two doses, the effectiveness of this vaccine is 76% two weeks after receiving the second dose; the AstraZeneca vaccine remains stable at normal refrigerator temperatures between 2-8 °C.29
Advantages of heterologous prime-boost strategyAccording to the literature, the administration of a heterologous of vaccination regimen is better than homologous regimen.30 In a study by Barros-Martins et al., they found that a heterologous regimen of vaccination with AstraZeneca’s ChAdOx1-nCov-19 vaccine (first dose) followed by BNT162b2 vaccine (second dose) could significantly induce higher levels of cellular and humoral immunities against the Alpha (B.1.1.7), Beta (B.1.351) and Gamma (P.1) variants of concern of SARS-CoV-2 than only homologous vaccination.31 In a study conducted by Schmidt et al., they observed that heterologous priming with the ChAdOx1-nCoV-19 vector vaccine followed by boosting with the mRNA vaccines (BNT162b2 or mRNA-1273) could induce a high level of immune responses e.g. spike-specific IgG, spike-specific CD4+ Tcells, as well as neutralizing antibodies compared to homologous vector vaccine boost.32 The findings of a study by Liu et al. showed that anti-spike IgG titer was higher in the ChAd/BNT schedule compared with ChAd/ChAd.33 Pozzetto et al. also demonstrated that the heterologous vaccination of ChAd/BNT regimen could induce a stronger protective immunity than the homologous BNT/BNT schedule.34
Interval between doses of COVID-19 vaccinesStudies show that about AstraZeneca vaccine, a 12-week interval between two doses can be more effective in inducing the immune system compared with a 4- to 8-week interval.35 On the other hand, for Moderna and Pfizer vaccines, the best time interval between two doses is at least 9 weeks, so that a 9-week interval significantly reduces hospitalization and death compared with a 3- or 4-week interval.36
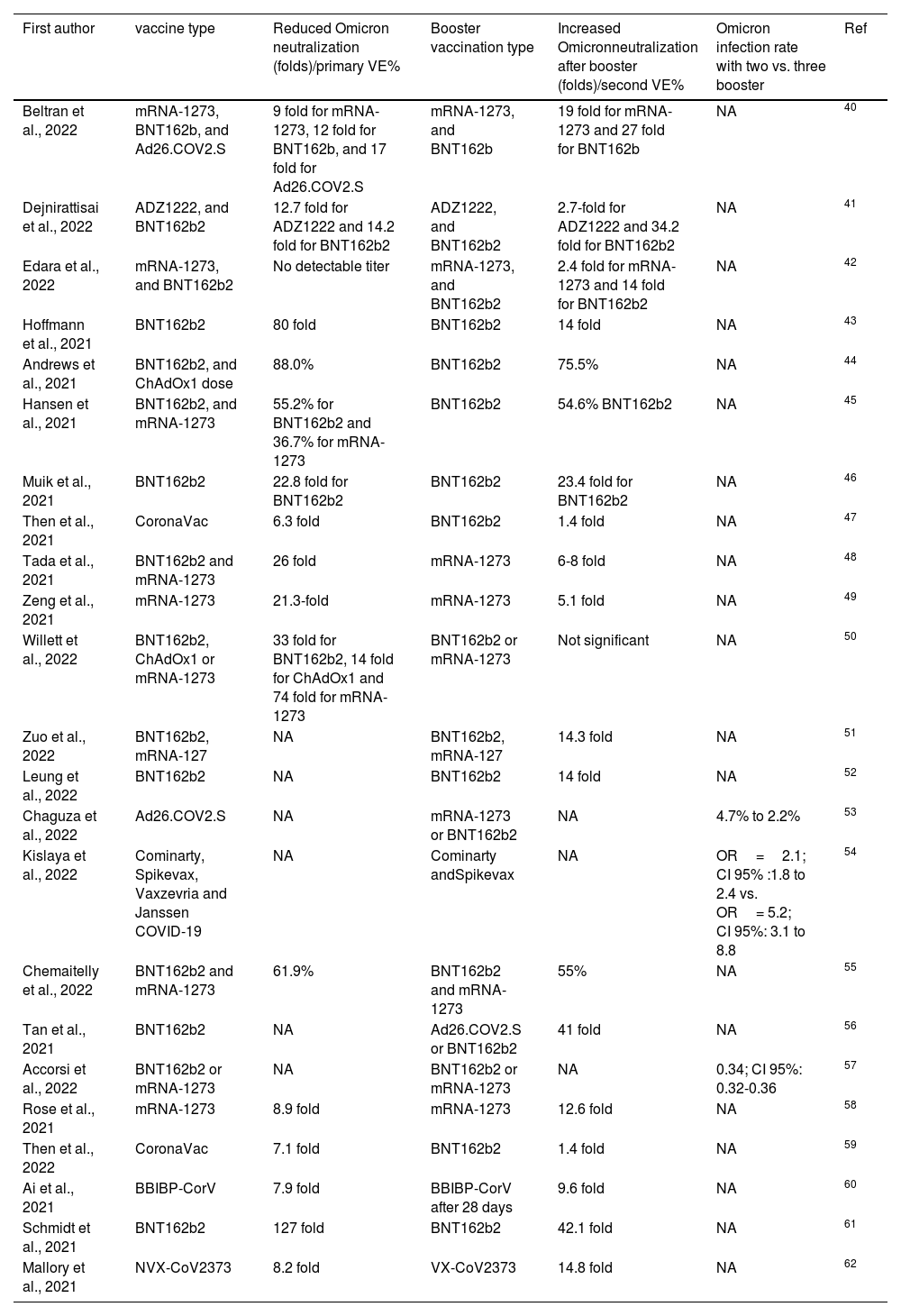
Is the third booster vaccination an effective option against SARS-CoV-2 Omicron variant?During the immune-pathogenesis of SARS-CoV-2 infection, humoral immunity blocks viral attachment and hampers entry of virus to host cell via neutralizing specific antibodies.37 In addition, T cells play an important role in diminishing viral spread to other susceptible host cells by producing interferon gamma.38 Neutralizing antibodies titers are typically weaned after a few months of vaccination, and Omicron mutations cause neutralizing titers to escape and confer, while mutations in immunodominant epitopes are less likely to affect T cell responses, which leads to superiority of mRNA vaccine against SARS. -CoV-2 variants.39 Some recent reports have suggested that heterologous vaccination or the third dose may stimulate the immune system or cross-reactivity of neutralizing antibodies against new SARS-CoV-2 variants; thus, the potential effect of the third booster heterologous vaccination for effective immunity against the VOC Omicron has been investigated by numerous scientists. We conducted a systematic search using the ISI Web of Science, PubMed, Scopus, and Google scholar databases to retrieve all articles related to the third booster vaccination dose against SARS-CoV-2 Omicron using the keywords "Omicron", "B.1.1.529", "Vaccine", "Booster", "Neutralization" and "Antibodies". Then conclusive remarks of each study were extracted in Table 1.40–62
Characteristics of included studies.
| First author | vaccine type | Reduced Omicron neutralization (folds)/primary VE% | Booster vaccination type | Increased Omicronneutralization after booster (folds)/second VE% | Omicron infection rate with two vs. three booster | Ref |
|---|---|---|---|---|---|---|
| Beltran et al., 2022 | mRNA-1273, BNT162b, and Ad26.COV2.S | 9 fold for mRNA-1273, 12 fold for BNT162b, and 17 fold for Ad26.COV2.S | mRNA-1273, and BNT162b | 19 fold for mRNA-1273 and 27 fold for BNT162b | NA | 40 |
| Dejnirattisai et al., 2022 | ADZ1222, and BNT162b2 | 12.7 fold for ADZ1222 and 14.2 fold for BNT162b2 | ADZ1222, and BNT162b2 | 2.7-fold for ADZ1222 and 34.2 fold for BNT162b2 | NA | 41 |
| Edara et al., 2022 | mRNA-1273, and BNT162b2 | No detectable titer | mRNA-1273, and BNT162b2 | 2.4 fold for mRNA-1273 and 14 fold for BNT162b2 | NA | 42 |
| Hoffmann et al., 2021 | BNT162b2 | 80 fold | BNT162b2 | 14 fold | NA | 43 |
| Andrews et al., 2021 | BNT162b2, and ChAdOx1 dose | 88.0% | BNT162b2 | 75.5% | NA | 44 |
| Hansen et al., 2021 | BNT162b2, and mRNA-1273 | 55.2% for BNT162b2 and 36.7% for mRNA-1273 | BNT162b2 | 54.6% BNT162b2 | NA | 45 |
| Muik et al., 2021 | BNT162b2 | 22.8 fold for BNT162b2 | BNT162b2 | 23.4 fold for BNT162b2 | NA | 46 |
| Then et al., 2021 | CoronaVac | 6.3 fold | BNT162b2 | 1.4 fold | NA | 47 |
| Tada et al., 2021 | BNT162b2 and mRNA-1273 | 26 fold | mRNA-1273 | 6-8 fold | NA | 48 |
| Zeng et al., 2021 | mRNA-1273 | 21.3-fold | mRNA-1273 | 5.1 fold | NA | 49 |
| Willett et al., 2022 | BNT162b2, ChAdOx1 or mRNA-1273 | 33 fold for BNT162b2, 14 fold for ChAdOx1 and 74 fold for mRNA-1273 | BNT162b2 or mRNA-1273 | Not significant | NA | 50 |
| Zuo et al., 2022 | BNT162b2, mRNA-127 | NA | BNT162b2, mRNA-127 | 14.3 fold | NA | 51 |
| Leung et al., 2022 | BNT162b2 | NA | BNT162b2 | 14 fold | NA | 52 |
| Chaguza et al., 2022 | Ad26.COV2.S | NA | mRNA-1273 or BNT162b2 | NA | 4.7% to 2.2% | 53 |
| Kislaya et al., 2022 | Cominarty, Spikevax, Vaxzevria and Janssen COVID-19 | NA | Cominarty andSpikevax | NA | OR=2.1; CI 95% :1.8 to 2.4 vs. OR= 5.2; CI 95%: 3.1 to 8.8 | 54 |
| Chemaitelly et al., 2022 | BNT162b2 and mRNA-1273 | 61.9% | BNT162b2 and mRNA-1273 | 55% | NA | 55 |
| Tan et al., 2021 | BNT162b2 | NA | Ad26.COV2.S or BNT162b2 | 41 fold | NA | 56 |
| Accorsi et al., 2022 | BNT162b2 or mRNA-1273 | NA | BNT162b2 or mRNA-1273 | NA | 0.34; CI 95%: 0.32-0.36 | 57 |
| Rose et al., 2021 | mRNA-1273 | 8.9 fold | mRNA-1273 | 12.6 fold | NA | 58 |
| Then et al., 2022 | CoronaVac | 7.1 fold | BNT162b2 | 1.4 fold | NA | 59 |
| Ai et al., 2021 | BBIBP-CorV | 7.9 fold | BBIBP-CorV after 28 days | 9.6 fold | NA | 60 |
| Schmidt et al., 2021 | BNT162b2 | 127 fold | BNT162b2 | 42.1 fold | NA | 61 |
| Mallory et al., 2021 | NVX-CoV2373 | 8.2 fold | VX-CoV2373 | 14.8 fold | NA | 62 |
Planas et al. (2021) revealed that Sera collected from previous COVID-19 convalescent patients had little or no neutralizing activity against Omicron, while a booster with BNT162b2 can induce the production of neutralizing antibodies against the Omicron variant.63 Lee et al. (2022) have also recently found that heterologous vaccination with ChAdOx1 followed by BNT162b2 provides an immune response equivalent to homologous BNT162b2 vaccination.64 As shown in Table 1, we summarized 24 eligible studies in which the mRNA-1273, BNT162b2, ADZ1222, Spikevax, Ad26.COV2S, BBIBP-CorV, and VX-CoV2373 vaccines had been used as boosters. Various studies have attempted to stimulate the immune system with heterologous or heterologous vaccination to produce an efficient neutralizing antibody titer. All included studies have reported a decreased neutralization of SARS-CoV-2 Omicron variant from 122 folds to absence of neutralization. However, the third booster vaccination induced the increased Omicron neutralization titers in most studies, but the absence of neutralization was also investigated in some studies. According to Table 1, the third booster using mRNA vaccine can induce neutralizing antibodies titer more effectively. In addition, some studies have shown that booster vaccination reduces vaccine effectiveness and the incidence of Omicron (B.1.1.529) variant. It should, however, be noted that the neutralizing antibodies titer was significantly lower against the delta variant in most studies. In a recent study in the Israeli population, On et al. (2022) showed that the 4 dose with BNT162b2 reduces COVID-19 cases and disease severity.65 Thus, a review of similar studies suggests that a third dose of vaccination can be advisable for reducing the risk of VOC Omicron breakthrough infection particularly in vulnerable populations.
Conclusion and future perspectiveThe VOC Omicron variant has a shorter doubling time and its mutations have caused it to spread faster and confer neutralizing antibodies, so that it has become the predominant variant in most countries/territories. Although disease severity is lower in Omicron, ICU admission and mortality rates are rising in most countries. In addition, re-infection with the Omicron variant in the fully vaccinated population has exacerbated global concern. Recent studies have shown that the third dose of vaccination leads to the cross-reactivity of antibodies and an increase in the neutralizing antibody titer. Thus, the third booster vaccination can provide better protection in vulnerable populations e.g. immunocompromised individuals. Based on studies, while administration of a third dose of vaccine can be benefit in some immunocompromised patients such as recipients of solid-organ transplants or hemodialysis patients, a partial response of immune system to SARS-CoV-2 is seen in patients with B cell lymphoproliferative disease.66 According to randomized clinical trial studies, administration of monoclonal antibodies significantly reduces (70-80%) hospitalizations and mortality in immunosuppressed patients.67 Recent studies have shown that the third booster was safe and well tolerated. The booster administration after the double-dose vaccination is an advisable strategy to reduce the risk of infection and spread of the Omicron variant. However, the efficacy of the third booster vaccination in fully vaccinated subjects has not been confirmed yet, nor has the role of heterologous or homologous vaccination in increasing neutralizing antibodies against Omicron been determined yet. We have no complete detailed information on the vaccine type, booster dosing, optimal dosing intervals and vaccination timeline yet. The third booster vaccination particularly in vulnerable subjects as well as the world mass vaccination to efficient vaccine production can be a critical step in regressing the surge of the new SARS-CoV-2 variant and controlling the Omicron pandemic.
Conflict of interest statementThe authors have no conflict of interest.