Herpes zoster (HZ) chiefly affects older individuals and the immunocompromised and may lead to persistent pain, postherpetic neuralgia (PHN), and other complications.
MethodsFrom 2012–2015, we conducted a prospective observational cohort study of adults ≥50 years with HZ presenting in primary care in Spain (NCT01521286). We estimated HZ incidence rates (IR) per 1,000 person-years (PY) over a 12-month period, and prospectively followed up a broader patient cohort; assessing pain severity using the ‘Zoster Brief Pain Inventory (ZBPI) and PHN development (defined as ZBPI ‘worst pain’ ≥3 at Day 90).
ResultsHZ IR in adults ≥50 years was 4.88/1,000 PY (95% confidence interval [CI]: 4.44–5.35), similar for both genders. Incidence increased with age, from 2.54 (95% CI: 1.84–3.41) in 50–54-year-olds to 6.40/1,000 PY (95% CI: 4.86–8.28) in 75–79-year-olds. In 545 HZ patients followed prospectively, the proportion of HZ patients with PHN was 4.59% (95% CI: 2.99–6.70); and 11.79% (95% CI: 7.78–16.91) in those 212 patients with available ZBPI scores at Day 90. The proportion of patients developing PHN increased with increasing age. Mean ZBPI ‘worst pain’ scores in patients not developing PHN showed a marked decline between Day 0–30 (from 5.4 to 1.6) and none at Day 90; in patients with PHN this score remained high (>5.5) up to Day 150. Other complications were observed in 10.3% of patients.
ConclusionsHZ presents a considerable burden in adults aged ≥50 years in Spain, with an IR of ~5/1,000 PY. HZ and PHN risk increased with age.
El herpes zoster (HZ) afecta principalmente a individuos mayores e inmunocomprometidos, pudiendo originar dolor persistente, neuralgia postherpética (PHN), y otras complicaciones.
MétodosDe 2012 a 2015, realizamos un estudio observacional prospectivo de cohorte de adultos ≥50 con HZ, que acudieron a atención primaria en España (NCT01521286). Calculamos las tasas de incidencia (TI) del HZ por 1.000 personas/año (PA) durante un periodo de 12 meses, y realizamos el seguimiento prospectivo de una cohorte de pacientes más amplia, evaluando la severidad del dolor utilizando la escala ZBPI (Zoster Brief Pain Inventory) y el desarrollo de PHN (definida como puntuación ≥3 de “peor dolor” ZBPI al 90º día).
ResultadosLa TI de HZ en adultos ≥50 años fue de 4,88/1.000 PA (95% de intervalo de confianza [IC]: 4,44 -5,35), similar para ambos sexos. La incidencia incrementó con la edad, de 2,54 (95% IC: 1,84 -3,41) en los individuos de 50 -54 años de edad, a 6,40/1.000 PA (95% IC: 4,86 -8,28) en los individuos de 75 -79 años. En 545 pacientes de HZ seguidos prospectivamente, la proporción de pacientes de HZ con PHN fue del 4,59% (95% IC: 2,99 -6,70), y del 11,79% (95% IC: 7,78 -16,91) en aquellos 212 pacientes con puntuaciones ZBPI disponibles al 90º día. La proporción de pacientes que desarrollaron PHN incrementó al incrementarse la edad. Las puntaciones ZBPI “de peor dolor” medias en los pacientes que no desarrollaron PHN reflejaron una reducción notable entre los días 0 y 30 (de 5,4 a 1,6), y ninguna al 90º día. En los pacientes con PHN esta puntuación siguió siendo alta (>5,5) hasta el día 150º. Se observaron otras complicaciones en el 10,3% de los pacientes.
ConclusionesHZ presenta una carga considerable en los adultos ≥50 años de edad en España, con una TI de ~5/1.000 PA. HZ y el riesgo de PHN se incrementaron con la edad.
Herpes zoster (HZ) is a painful, vesicular rash due to reactivation of latent varicella-zoster virus (VZV) infection.1 The principal risk factor for VZV reactivation and subsequent HZ is increasing age,2,3 with markedly higher incidence in adults ≥ 50 years of age.2,4 Advanced age is associated with more severe disease and with higher risk of postherpetic neuralgia (PHN), a painful neuropathic complication.3,5 HZ risk is greater in females,3,6 and in the immunocompromised.3,7 Although most cases resolve within one month of rash onset without sequelae, about 25% of patients experience various HZ complications during the acute phase, including ophthalmic, neurological or dermatological complications;1,8 while others will develop more prolonged complications, including PHN which may last for months or years.9
Globally, HZ incidence estimates in the overall population range from 3–5 per 1,000 person-years (PY), with little geographical variation.2 In Europe, a recent systematic review reported a mean HZ incidence rate (IR) of 3.4/1,000 PY in the general population, increasing with age; 7–8/1,000 PY in individuals aged ≥50 years and 10/1,000 PY for those aged ≥80 years.4 The proportion of patients with HZ developing PHN also shows some variation, ranging from 5–30%, in part due to differences in terminology and PHN definitions.2
In Spain, epidemiologic data on the incidence and burden of HZ (and PHN) are, until recently, quite limited. Existing data includes retrospective analyses of databases and case records from regional or municipal health systems.6,10–16 A comprehensive retrospective analysis of primary care records for the period 2009–2014 in the Valencia region, evaluating the risk of HZ in patients with/without chronic obstructive pulmonary disease (COPD) reported an IR of 7.2/1,000 PY in patients aged ≥50 years (without COPD).16 A related study across this same period evaluating PHN reported that 15.7% of patients with HZ aged ≥50 years developed PHN.15 However, prospective studies are less common.17,18
At present there are two vaccines against HZ licensed in subjects ≥50 years of age and available for use in Spain; a live-attenuated vaccine (ZVL, Zostavax, Merck and Co., Inc.) and, since 2018, an adjuvanted recombinant zoster vaccine (RZV, Shingrix, GSK).19,20 Understanding local HZ epidemiology is important, in particular as prevention strategies via protective vaccination are available.21 At the time of the present study only the live-attenuated vaccine was available (although uptake in Spain was low). We wished to assess HZ epidemiology prior to wider implementation of HZ preventive strategies.
The primary objective of the present study was to prospectively estimate the incidence of HZ in Spanish adults aged ≥50 years in the primary care setting. Secondary objectives were to estimate the proportion of HZ patients developing PHN. The impact of HZ and PHN on patients’ quality of life, healthcare resource utilization and associated economic costs in the study population has already been reported.22
MethodsThis was a prospective observational study evaluating the incidence of HZ in older adults ≥50 years of age in a primary care cohort. Secondary objectives included pain assessment and PHN development (NCT01521286). Participating physicians were general practitioners (GPs) belonging to one of the four GP networks operating within three regions; Madrid, Catalonia (in Barcelona, the regional capital) and Valencia (two networks: Centro Superior de Investigacion en Salud Pública (CSISP) and Centro de Salud Salvador Pau), caring for an estimated 94,000 persons aged ≥50 years.
Study eligibilityEligible participants were adults aged ≥50 years presenting with an episode of acute HZ within the four participating GP networks. Subjects attending for a secondary GP consultation within 7 days following a HZ diagnosis in an alternative setting (e.g., emergency room or community-care center) were also eligible. Acute HZ was defined as new unilateral pain (broadly defined to include allodynia and pruritus) accompanied by unilateral rash and no alternative diagnosis.
The study and analyses were conducted from two perspectives. We prospectively estimated the incidence of HZ over a one-year period (in three of the GP networks). We also prospectively followed up an according to protocol (ATP) study cohort of HZ patients in a broader patient population from all four GP networks (which included some patients participating in the incidence estimations) and evaluated demographic aspects, clinical features and development of complications (and in particular PHN).
HZ incidence estimationIncidence estimates were based on the number of eligible attending three of the four participating GP networks (Madrid, Barcelona and CSISP Valencia) over a 12-month period; from July 1, 2013 to June 30, 2014. While the initial study aim was to include data from the Pau Valencia network, during data collection it was apparent that a significant proportion (approximately 1/3) of the GPs from the Pau Valencia Network consistently reported fewer cases than would be expected, based on literature17 and our own preliminary estimations. The reasons for this were unclear, but we believed that inclusion may have added uncertainty to the overall incidence data. As such, as an amendment to the planned analyses, the data from the Pau Valencia network were excluded from the HZ incidence analyses. However, as described below, HZ cases within this network were included in the prospective cohort assessing complications.
Participating GPs provided monthly logbooks detailing the number of eligible patients with HZ presenting each week in the respective month; this included those weeks with zero cases. Relevant regional population denominators and regional aggregates for the number of individuals aged ≥50 years (and for all relevant age-strata) were calculated using official registered patient numbers for each participating GP, with adjustment based upon the actual number of patients under their active care during this 12-month period. In this approach, for those weeks where no weekly report was received from a GP, this was considered as a break/ disruption in surveillance; and the population denominator adjusted to account for this corresponding period. From these data HZ incidence was estimated per 1,000 PY (with exact Poisson 95% confidence intervals [CIs]), for all patients and also stratified by gender and age.
Eligibility and data collection in the prospective ATP Study cohort evaluating HZ complicationsThis ATP patient cohort recruited all patients aged ≥50 years with an HZ episode attending any of the four GP networks, with study entry and follow-up between March 2012 and May 2015. Inclusion was based on GP assessment of patient ability to complete study questionnaires and allied study documents. Following enrollment, each GP completed a case report form with demographic data, medical history, and clinical HZ information, including HZ-related complications (also recorded at any subsequent physician consultation during the study period).
Patients rated HZ pain severity using the Zoster Brief Pain Inventory (ZBPI) questionnaire. Participants were asked to complete the ZBPI at Day 0 (defined as the day of HZ onset) and at Days 15, 30, 60 and 90. The ZBPI questionnaire includes 4 pain severity items (‘worst’, ‘least’, ‘average’ over the last 24 hours and ‘now’), each of which is rated on an 11-point Likert scale with 0 = ‘no pain’ and 10 = ‘pain as bad as you can imagine’. The ZBPI is a well-established method for assessing HZ pain and development of PHN,23 used in similar previous studies in HZ patients in Germany and Italy.24,25 The ZBPI questionnaire was supplied in booklet format and assistance in understanding how to complete each questionnaire provided by the GP at the initial visit if necessary. Additional sets of questionnaires were subsequently self-administered and completed by the patient at the days assigned (Days 15, 30, 60 and 90) and returned to the study centers (in person or by mail).
PHN was defined as the presence of HZ-associated ‘worst’ pain rated ≥3 on the ZBPI scale persisting or appearing 90 days or more after HZ onset. Those HZ patients without PHN at Day 90 were considered as having completed the study and not followed up further. Patients with PHN at Day 90 were asked to continue and complete the ZBPI questionnaire for a further 90 days, at Days 120, 150 and 180. At Day 180, those patients with continued PHN remained in the study and asked to complete the ZBPI questionnaire for a final 90-day period, at Days 210, 240 and 270. No patient was followed beyond Day 270.
Data analyses in the prospective ATP study cohort evaluating HZ complicationsDescriptive statistics (mean values, standard deviation [SD] and percentages) are presented for patient demographics, ZBPI pain severity scores and for HZ complications. The proportions of patients with PHN at Days 90, 180 and 270 were calculated with exact 95% CIs; for the overall study cohort ≥50 years (and stratified by gender, age group and severity of HZ at the initial visit). The proportion with PHN at Day 90 was also calculated for those patients that completed the ZBPI at this time point. All statistical analyses were performed using the Statistical Analysis Systems (SAS) version 9.2.
EthicsThis study was conducted in accordance with the ethical principles originating in the Declaration of Helsinki, the principles of "good clinical practice" and all applicable regulatory requirements. Written informed consent was obtained from each enrolled participant prior to study data collection. The study protocol, the informed consent form, and other information that required pre-approval were reviewed and approved by the independent ethics committees for each of the participating networks.
ResultsOverall incidence of HZ (incidence cohort)Between July 1, 2013 and June 30, 2014, a total of 459 HZ cases in patients aged ≥50 years attended one of the three GP networks (Madrid, Barcelona and CSISP Valencia) participating in the incidence estimation part of the study. Of these, 257 (56%) were female. The overall HZ incidence in subjects ≥50 years was estimated at 4.88/1,000 PY (95% CI: 4.44–5.35); increasing with age from 2.54/1,000 PY (95% CI: 1.84–3.41) in those aged 50–54 years to a peak of 6.40/1,000 PY (95% CI: 4.86–8.28) in those aged 75–79 years. Overall HZ incidence was similar in women (4.90/1,000 PY [95% CI: 4.32–5.53]) and men (4.85/1,000 PY [95% CI: 4.21–5.57) with similar trends of greater incidence with increasing age in both genders (Table 1). Some regional variation in the overall incidence was observed across the different GP networks; Barcelona (3.67/1,000 PY; 95% CI: 3.10–4.31); Madrid (5.37/1,000 PY; 95% CI: 4.71–6.10); and CSISP Valencia (7.52/1,000 PY; 95% CI: 5.94–9.40). In all 3 regions a similar pattern of lower incidence in patients aged 50–54 years and higher incidence in older age strata was also observed (Supplementary Table 1).
Estimated incidence of herpes zoster in Spanish adults aged ≥ 50 years by age and gender.
| Age group (years) | Female | Male | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Incidence per 1,000 PY | 95% CI | N | Incidence per 1,000 PY | 95% CI | N | Incidence per 1,000 PY | 95% CI | |
| 50–54 | 22 | 2.45 | 1.53–3.70 | 22 | 2.64 | 1.65–3.99 | 44 | 2.54 | 1.84–3.41 |
| 55–59 | 38 | 4.77 | 3.37–6.54 | 25 | 3.53 | 2.29–5.21 | 63 | 4.19 | 3.22–5.36 |
| 60–64 | 35 | 4.92 | 3.43–6.84 | 23 | 3.72 | 2.36–5.58 | 58 | 4.36 | 3.31–5.64 |
| 65–69 | 43 | 6.15 | 4.45–8.28 | 34 | 5.69 | 3.94–7.95 | 77 | 5.94 | 4.69–7.42 |
| 70–74 | 33 | 5.64 | 3.88–7.92 | 32 | 6.96 | 4.76–9.82 | 65 | 6.22 | 4.80–7.93 |
| 75–79 | 28 | 5.38 | 3.58–7.78 | 30 | 7.78 | 5.25–11.11 | 58 | 6.40 | 4.86–8.28 |
| ≥80 | 58 | 5.60 | 4.26–7.24 | 36 | 6.45 | 4.52–8.93 | 94 | 5.90 | 4.77–7.22 |
| All ≥50 | 257 | 4.90 | 4.32–5.53 | 202 | 4.85 | 4.21–5.57 | 459 | 4.88 | 4.44–5.35 |
CI = Exact Poisson confidence interval; N = number of patients; PY = person-years
A total of 545 HZ patients with an acute HZ episode presenting across the four participating GP networks were included in the prospective study ATP cohort; Madrid (n=229), Barcelona (n=146), CSISP Valencia (n=93) and Centro de Salud Salvador Pau, Valencia (n=77). The mean (SD) age at rash onset was 66.9 (9.7) years (range 50–91 years); 312 patients (57.2%) were female.
Clinical features at the initial visit in the prospective ATP study cohortThe mean (SD) delay between rash onset and the initial GP visit was 6.4 days (18.7). Of the 403/545 (73.9%) patients who reported having experienced one or more symptoms before rash onset, 276/403 (68.5%) reported prodromal pain, 146/403 (36.2%) reported malaise, and 18/403 (4.5%) reported fever (Table 2). Pre-existing co-morbidities were reported in 332/545 patients (60.9%) at the initial visit. Twenty-nine patients (5.3% of the overall ATP cohort) reported receiving immunosuppressive therapy within the previous 30 days, the majority of whom (58.6%) received corticosteroids (Table 2).
Pain severity and clinical characteristics of the herpes zoster patients in the ATP cohort at initial visit.
| Characteristics | n | % |
|---|---|---|
| Severity of HZ pain | N1 = 284 | |
| No or mild | 55 | 19.4 |
| Moderate | 97 | 34.2 |
| Severe | 132 | 46.5 |
| Patients with symptoms before rash onset | N2 = 403 | |
| Prodromal pain | 276 | 68.5 |
| Malaise | 146 | 36.2 |
| Fever | 18 | 4.5 |
| Other symptoms - non-painful | 82 | 20.3 |
| Other symptoms – painful | 36 | 8.9 |
| Patients with comorbidities at initial visita | N3 = 332 | |
| Hypertension | 137 | 41.3 |
| Current emotional problems, stress or depression | 80 | 24.1 |
| Diabetes mellitus | 78 | 23.5 |
| Chronic respiratory disease | 31 | 9.3 |
| Neoplasm | 22 | 6.6 |
| Renal failure, dialysis | 19 | 5.7 |
| Patients under immunological treatment | N4 = 29 | |
| Oral or parenteral corticosteroids | 17 | 58.6 |
ATP = According to protocol; HZ = Herpes zoster; n = Number of patients concerned; N1 = The number of patients with initial pain assessment (for 261 patients, the initial pain assessment was missing); N2 = The number of patients with symptoms before rash onsets; N3 = The number of patients with comorbidities; N4 =The number of patients receiving immunosuppressive/immunomodulatory treatment at initial visit
ZBPI ‘worst pain’ scores at the initial visit were recorded in 284/545 patients (52.1%). Of these, 132/284 (46.5%) indicated severe pain (7–10 score on the ZBPI ‘worst pain’ score); 19.4% of patients with available scores (55/284) indicated having no or mild pain (ZBPI score ≤3) (Table 2). Systemic antivirals were prescribed for 462/545 patients with HZ (84.8%), either prior to the initial patient GP visit (given during their previous medical care before initial GP assessment) or prescribed by their GP; most frequently valaciclovir (28.4%), brivudine (27.0%) and acyclovir (24.4%). A total of 56/545 patients (10.3%) had one or more HZ-related complications (excluding PHN) across the study period; including bacterial superinfection (2.8%), disseminated VZV (2.4%), with 5 patients having ocular/ophthalmic complications.
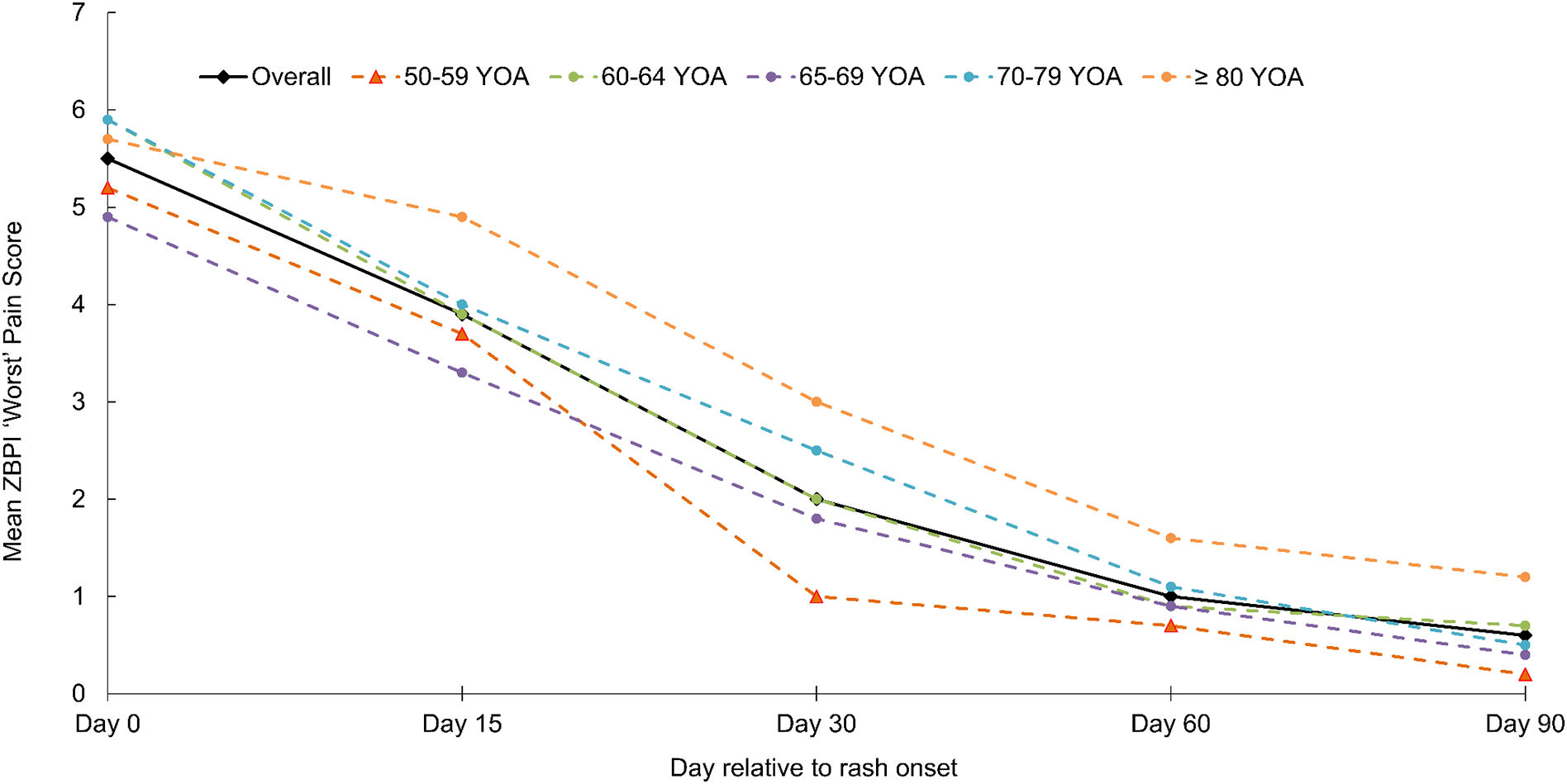
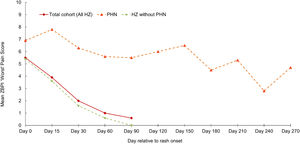
The proportion of HZ patients in the prospective ATP study cohort developing PHN and their characteristicsHZ-related pain, based on ZBPI worst pain scores was assessed throughout the study period. The proportion of patients completing the ZBPI questionnaire ranged across the study from 53.4% (291/545) at Day 0 to 38.9% (212/545) at Day 90; the time point for PHN assessment. Questionnaire completion compliance was greatest in patients aged ≥80 years (Supplementary Fig. 1). At Day 0, the overall mean ZBPI ‘worst pain’ score in the entire cohort was 5.5 at Day 0 and decreased to 2.0 by Day 30. The worst pain score reduced more slowly in the oldest group (Fig. 1).
In the overall ATP cohort, a ZBPI ‘worst pain’ score ≥3 at Day 90 was reported by 25/545 patients; the proportion of all patients ≥50 years of age developing PHN was 4.59% (95% CI: 2.99–6.70). The PHN proportion was greater in older patients, reaching 10.0% (95% CI: 3.76–20.51) in patients aged ≥80 years, and was higher in men (6.01%; 95% CI: 3.32–9.88) than in women (3.53%; 95% CI: 1.77–6.22) (Table 3). Minor regional differences in the proportion of patients who developed PHN were observed; occurring in 4.8% of patients in the Madrid and Barcelona GP networks and 3.9% and 4.3% in the two Valencia networks.
Proportion of HZ patients with PHN.
| Overall ATP cohort(n=545) | Patients with completed ZBPI questionnaire at Day 90(n=212) | |||||||
|---|---|---|---|---|---|---|---|---|
| N1 | n | % | 95% CI | N2 | n | % | 95% CI | |
| Age groups | ||||||||
| 50–54 years | 66 | 2 | 3.03 | 0.37–10.52 | 23 | 2 | 8.70 | 1.07–28.04 |
| 55–59 years | 71 | 1 | 1.41 | 0.04–7.60 | 26 | 1 | 3.85 | 0.10–19.64 |
| 60–64 years | 90 | 6 | 6.67 | 2.49–13.95 | 39 | 6 | 15.38 | 5.86–30.53 |
| 65–69 years | 100 | 4 | 4.00 | 1.10–9.93 | 38 | 4 | 10.53 | 2.94–24.80 |
| 70–74 years | 86 | 6 | 6.98 | 2.60–14.57 | 34 | 6 | 17.65 | 6.76–34.53 |
| 75–79 years | 72 | 0 | 0.00 | 0.00–4.99 | 25 | 0 | 0.00 | 0.00–13.72 |
| ≥80 years | 60 | 6 | 10.00 | 3.76–20.51 | 27 | 6 | 22.22 | 8.62–42.26 |
| All ≥50 years | 545 | 25 | 4.59 | 2.99–6.70 | 212 | 25 | 11.79 | 7.78–16.91 |
| Gender | ||||||||
| Female | 312 | 11 | 3.53 | 1.77–6.22 | 103 | 11 | 10.68 | 5.45–18.31 |
| Male | 233 | 14 | 6.01 | 3.32–9.88 | 109 | 14 | 12.84 | 7.20–20.61 |
| GP networks | ||||||||
| Barcelona | 146 | 7 | 4.79 | 1.95–9.63 | 76 | 7 | 9.21 | 3.78–18.06 |
| Madrid | 229 | 11 | 4.80 | 2.42–8.43 | 70 | 11 | 15.71 | 8.11–26.38 |
| CSISP Valencia | 93 | 4 | 4.30 | 1.18–10.65 | 50 | 4 | 8.00 | 2.22–19.23 |
| Centro de Salud Salvador Pau, Valencia | 77 | 3 | 3.90 | 0.81–10.97 | 16 | 3 | 18.75 | 4.05–45.65 |
ATP = According to protocol; CI = Confidence interval; CSISP = Centro Superior de Investigacion en Salud Pública; GP = General practitioner; HZ = Herpes zoster; PHN = Postherpetic neuralgia; ZBPI = Zoster Brief Pain Inventory
N1 = number of HZ patients in the overall ATP cohort; N2 = number of HZ patients completing the ZBPI questionnaire at Day 90; n = number of HZ patients with PHN (defined as ZBPI ‘worst pain score ≥3 at Day 90); % = percentage of patients with PHN
When considering only those subjects (n=212) with available ZBPI ‘worst pain’ scores at Day 90, then the proportion of patients with PHN was higher across all strata; the overall proportion of patients ≥50 years with PHN was 11.79% (95% CI: 7.78–16.91) and 22.22% (95% CI: 8.62–42.26) in patients aged ≥80 years, and higher in men (12.84%; 95% CI: 7.20–20.61) than in women (10.68%; 95% CI: 5.45–18.31) (Table 3). For these subjects with available ZBPI ‘worst pain’ scores at Day 90, regional differences in the proportion of patients with
PHN were apparent; higher in GP networks in Centro de Salud Salvador Pau, Valencia (18.7%) and Madrid (15.7%) and lower in the Barcelona (9.2%) and CSISP Valencia (8.0%) networks (Table 3).
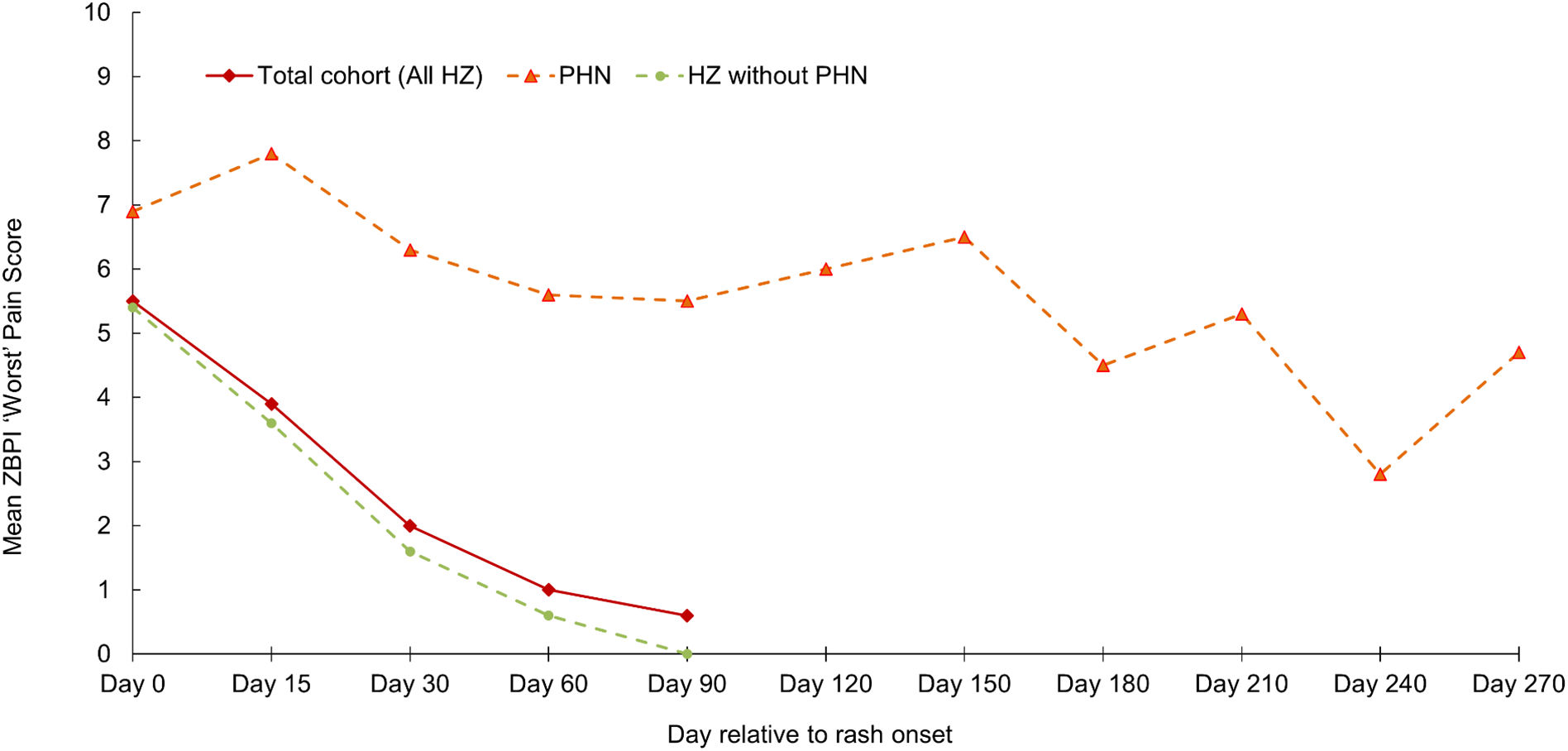
The mean (SD) age of the 25 HZ patients with PHN was 69.5 (10.3) years (range 50–91 years); 44% were women. Most HZ characteristics at the initial visit, and the prevalence of patient comorbidities were broadly comparable in patients with and without PHN. However, differences in pain severity at the initial visit were observed between those patients who developed PHN and those who did not; no/mild pain (0% and 26.9% respectively), moderate pain (42.9% and 31.3%) and severe pain (57.1% and 41.9%). Subsequently, in patients with PHN, the mean ‘worst pain’ score remained above 5 up to and including Day 150. In contrast, those patients not developing PHN showed a marked decline in mean ‘worst pain’ score from Day 0 to Day 30, falling from 5.4 to 1.6 (Fig. 2). Of the 25 patients with PHN, 5 patients had PHN persisting until Day 180; one patient had PHN persisting 270 days after rash onset.
DiscussionWe found an overall HZ IR of 4.88/1,000 PY in Spanish adults aged ≥50 years presenting in primary care over a 12-month period across 3 different regional GP networks. The incidence increased with age, peaking in the 75–79 years age group. We found that HZ incidence was similar in both genders, although some differences were seen in different age-strata.
The HZ incidences we observed are somewhat lower than those reported in other European countries. In the United Kingdom (UK), a retrospective primary care study found an incidence of 5.2/1,000 PY in subjects aged ≥50 years,26 whereas recent prospective studies have reported incidence in ≥50 year-olds of 6.7/1,000 PY in Germany24 and 6.5/1,000 PY in Italy.25 Previous studies in Spain also report somewhat higher incidence in subjects ≥50 years than we observed.11,12,16,17 In a relatively small prospective study from Valencia, the annual incidence of HZ ranged from 6.7 per 1,000 inhabitants in patients 50–59 years of age to 11.1 per 1,000 in those aged ≥70 years,17 while a large retrospective study from this same region reported an IR of 7.2/1,000 PY in those aged ≥50 years.16 Reported incidence in Catalonia ranges from 5.9 per 1,000 aged between 50–54 years to 9.6 per 1,000 in those aged 70–74 years.13 Most studies, including several recent prospective studies in the primary care setting have also consistently reported higher incidence for women across all age groups,24,25,27–29 with higher incidence in females also reported in previous studies from Spain.12,16,17 We have no specific explanation for the relatively lower HZ incidence observed in the present study, nor for our relatively comparable rates in men and women, or for the differences in incidence observed in different regions. In reality, methodological differences may account for some aspects. Previous data from Spain are primarily drawn from retrospective analyses, and it is possible that in our prospective study, not all eligible cases were included. Possible explanations for such underreporting exist; some patients with milder HZ may not have attended for GP care within the relatively narrow inclusion timeframe (within 7 days of rash onset), while others may have been managed in alternative medical care facilities out with the GP network.
We also evaluated a broader group of 545 patients presenting with HZ in primary care and prospectively assessed the proportion developing PHN. We found that 25 patients (4.6%) developed PHN, although the proportion was greater in older patients (10% in those aged ≥80 years), and higher in men than in females (6% and 3.5% respectively). These estimates are lower than those reported in other recent studies (using the same PHN definition we used), where the overall PHN proportion in patients ≥50 years has been reported as 11.9% in Germany,24 9.1% in the UK,29 and 10.2% in Italy.25 They are also lower than reported in previous Spanish studies. A retrospective database analysis of HZ patients ≥50 years of age conducted in the Valencia region reported that 15.7% developed PHN;15 in Catalonia the proportion of HZ patients with PHN has been reported as 12.3% in 50–59 year-olds and 17.5% in those aged ≥70 years.13
A limitation of the present study was the need to have ZBPI pain scores at Day 90 to determine PHN occurrence in the overall ATP cohort, with only 212/545 patients (38.9%) providing this information. When evaluating PHN proportions only in those patients with available ZBPI pain scores at Day 90, the proportion of HZ patients ≥50 years who developed PHN was 11.8%. The corresponding incidences in different age-strata were also higher. These data are more in line with data reported elsewhere. We recognize that failure to return the ZBPI at Day 90 may indicate absence of pain at this timepoint, and so this analytical perspective may be subject to some bias; however, other reasons may have also been responsible for study drop-out. The true frequency of PHN in our cohort remains subject to some uncertainty. We did observe a higher proportion PHN development in males than females, which is contrary to results from similar prospective studies in Italy and Germany,24,25 and also contrary to data from retrospective studies in Spain and elsewhere.5,15,30 This reflect the relatively small number of PHN cases we observed and associated wide and overlapping CIs between the male and female PHN proportions.
Our study has a number of additional limitations. The study population denominators used in incidence estimations were adjusted to account for the proportion of time when GPs were not actively reporting HZ cases during the study period, and the assumptions made in calculating these denominators are subject to some uncertainty. Our study populations (for both the incidence estimations and for the proportion with PHN development) include patients presenting in primary care and also those attending their GP as a secondary consultation after presenting elsewhere (including the emergency department and after hospitalization). Although the majority of HZ cases presented in primary care, as we have reported elsewhere, a small number were hospitalized or attended the emergency department.22 It is possible that differences exist between such cases and those initially presenting to their GP, and this may have influenced the incidence rates and proportions developing PHN we report. Similarly, patient comorbidities would also have an influence. Evaluation of the relative impact of these aspects on incidence and PHN development values was beyond the scope of the present study. In addition, our population comprised subjects with an episode of acute HZ, not necessarily a first episode (and so could include cases of recurrent HZ); again, we did not evaluate differences in incidence or PHN development between these groups. Our study findings should be interpreted in the context of all these limitations.
ConclusionsThis is one of the few prospective studies of the epidemiology of HZ in Spain, conducted in several regions of the country. The HZ incidence in Spanish individuals aged ≥50 years we observed is lower than that previously described in Spain and elsewhere. However, in line with previous studies, we also observed increasing incidence with increasing age. PHN was the most frequent HZ-related complication, especially in patients aged ≥80 years. Fig. 3 presents a plain language summary of the context, outcomes, and impact of this study.
Data statementAnonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Statement on prior presentationSome of the material has been previously presented as an oral presentation at the Asociación Española de Vacunología (AEV) National congress, Valencia, Spain, 2017.
Funding sourceThis study was fully funded by GlaxoSmithKline Biologicals SA (ClinicalTrials.gov: NCT01521286) and all costs related to the development and publication of this manuscript.
Declaration of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
LGC declares having received funds from the GSK group of companies during the conduct of the study. TC declares no financial and non-financial relationships and activities and no conflicts of interest. MDRC, JAGM and KG are employed by the GSK group of companies, and MDRC holds shares in the GSK group of companies. MLS and BS were employed by the GSK group of companies at the time of the study. JDD reports study grant and personal fees from the GSK group of companies. All authors declare no other financial and non-financial relationships and activities.
The authors would like to thank all study participants. They would also like to thank Beatriz Becerro de Bengoa for coordination of data collection, as well as Amit Bhavsar (GSK) for his contributions during manuscript development. The authors would also wish to thank Business & Decision Life Sciences platform for editorial assistance and publications coordination, on behalf of GSK; Matthieu Depuydt, Aurélie Roth, Elena Chaves Rodriguez and Diego Collin coordinated publication development and editorial support. The authors also thank Niels Neymark (of Neymark Scientific Writing SCS) and Iain O’Neill (freelance on behalf of GSK) for providing medical writing support.