Vacunas COVID-19: desarrollo y práctica - COVID-19 vaccines: development and practice
Más datosIn December 2020, vaccination against SARS-CoV-2 started in Spain. Until March 2022, 91.1% of the target population was vaccinated. The objective of the study is to describe the characteristics of patients with SARS-CoV-2 infection, the risk of serious outcomes and their vaccination status.
Material and methodsRetrospective longitudinal analytical observational study. Demographic characteristics and outcomes of COVID-19 infections and vaccination history from 01/01/2021–08/10/2021 were collected from electronic medical records and the Unified Vaccination Registry. Data analysis was performed with Excel and Stata 16.
Results4161 COVID-19 cases were detected; of which 185 (4.5%) had received a complete vaccination schedule. The most affected age group was 80–89 years (34.1%). 1697 patients were hospitalized, of whom 78 (4.6%) had been vaccinated against SARS-CoV-2. No patient admitted to the ICU had a history of vaccination. The mean hospitalization time in unvaccinated patients was 11 days (95% CI -41.54–63.54) compared to 8.5 days (95% CI 7.04–9.96) in vaccinated patients. The relative risk of hospitalization in vaccinated patients compared to unvaccinated patients for the age group 40 to 59 years was 0.29 (95% CI 0.11–0.72) and 0.77 (95% CI 0.67–0). 0.90) for people over 60 years of age.
ConclusionsThe risk of hospitalization and death was lower in vaccinated patients compared to unvaccinated patients in the age groups 40–59 and older than 60 years. This finding supports current clinical evidence.
En diciembre 2020 inició la vacunación frente al SARS-CoV-2 en España. Hasta principios de marzo 2022 el 91,1% de la población diana ha sido vacunada. El objetivo del estudio es describir las características de los pacientes con infección por SARS-CoV-2, el riesgo de desenlaces graves y el estado de vacunación.
Material y métodosEstudio observacional analítico longitudinal retrospectivo. Se recogieron características demográficas y desenlace de infecciones COVID-19 de casos confirmados y sus antecedentes de vacunación desde 01/01/2021–10/08/2021 de las historias clínicas electrónicas y del Registro Unificado de Vacunación. El análisis de datos se realizó con Excel y Stata 16.
ResultadosSe detectaron 4161 casos COVID-19; 185 (4,5%) recibieron pauta de vacunación completa. El grupo de edad más afectado fue 80–89 años (34,1%). 1697 pacientes hospitalizaron, de los cuales 78 (4,6%) recibieron pauta de vacunación completa. Ningún paciente ingresado en UCI tenía antecedentes de vacunación. El tiempo medio de hospitalización en no vacunados fue de 11 días (IC95% -41,54-63,54) frente a 8,5 días (IC95% 7,04-9,96) en vacunados. El riesgo relativo de hospitalización en vacunados respecto a no vacunados para el grupo de edad de 40 a 59 años fue de 0,29 (IC95% 0,11-0,72) y de 0,77 (IC95% 0,67-0,90) para las personas de más de 60 años.
ConclusionesEl riesgo de hospitalización y muerte fue menor en los pacientes vacunados en comparación con los no vacunados en los grupos de edad 40–59 y mayores de 60 años. Este hallazgo está de acuerdo con los datos de la evidencia clínica actual.
Commercialized and approved COVID-19 vaccines in Spain are Comirnaty (Pfizer/BioNTech) and Moderna mRNA vaccines, and AstraZeneca and Janssen adenovirus vector vaccines. Efficacy in reduction of symptomatic cases ranges from 60% for AstraZeneca and 67% for Janssen to 94% for Moderna and 95% for Comirnaty vaccines.1 Vaccine failures have been described mainly in immunocompromised patients with underlying conditions and on immunomodulating therapy with different outcomes in different countries.2–5 Data from the US CDC shows that 27% of vaccine breakthrough infections are asymptomatic.6 We aim at describing the demographic characteristics of patients with COVID-19 infection, risks of adverse outcomes and their vaccination status at a secondary hospital in Madrid, Spain.
COVID-19 vaccination started in December 2020. The national vaccination strategy is comprised of three phases: the first phase targeted nursing home residents and personnel, healthcare workers and non-institutionalized major dependants; followed by phase 2 in February 2021 (people aged up to 59 years in descending 10-year intervals and unvaccinated essential personnel) and phase 3 in June 2021 (people aged 49–12 years). Additional booster vaccinations have started.1
By the end of August 2021, 64.714.501 vaccines have been administered in Spain. Comirnaty/Pfizer vaccines account for 70%, AstraZeneca for 15%, Moderna for 12% and Janssen for 3% of all administered doses. 36.111.205 people had received one dose and 32.239.782 two doses, which makes up for 67.9% of the Spanish population.1 Full protection is reached 7 days after the administration of the second dose for Pfizer and 14 days for AstraZeneca, Moderna and Janssen vaccines.7
Material and methodsPositive PCR and antigen testing for SARS-CoV-2 of patients who were attended at Infanta Sofía University Hospital are reported daily to the Preventive Medicine Department, which reports incident cases to the local public health authorities. Patients were tested either at the Emergency Department (ED), during hospitalization or prior to certain diagnostic or therapeutic interventions. Administered COVID-19 vaccines are registered at a centralized regional vaccination database. We reviewed vaccination history of positive COVID-19 cases from Jan 1, 2021 to Aug 10, 2021 at our centre using the centralized regional Madrid vaccination database. We retrieved demographic characteristics and COVID-19 infection outcomes from hospital clinical records. Five patients who remained hospitalized at the time of data analysis were excluded from median days of hospitalization. Data analysis was performed using Microsoft Office Excel 2016 and Stata version 16.
ResultsDuring the study timeframe, 4161 COVID-19 cases were detected, of which 185 (4.5%) had received full COVID-19 vaccination prior to COVID-19 infection. Of these, 161 patients (87.0%) had been vaccinated with Pfizer vaccines, 15 (8.1%) with AstraZeneca and 9 (4.9%) with Moderna. Ninety-nine patients (53.5%) were female. Median time from second dose to positive COVID-19 testing was of 82 days.
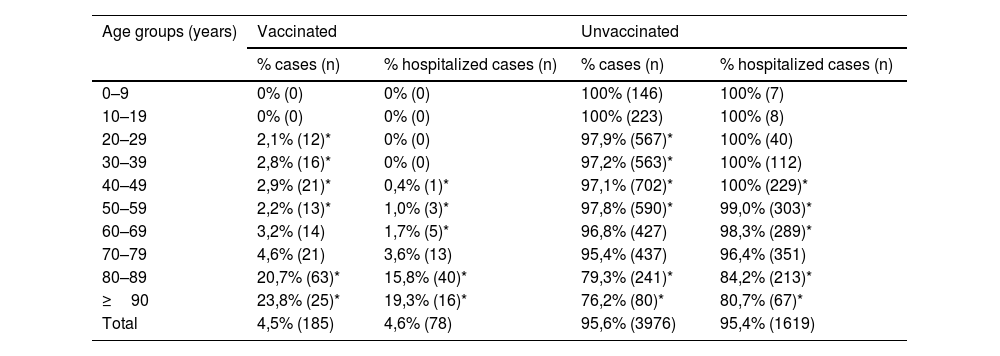
Median age was 78 years. However, the most affected age group was 80 to 89 years (n = 63, 20.7%). Fully vaccinated COVID-19 cases ranged from zero in younger age groups to 20.7% in people aged 80–89 and 23.8% in people aged 90 or older (Table 1). Most of the study patients attended the emergency department and only a minority was routinely screened before diagnostic or therapeutic procedures.
Percentage of COVID-19 cases and hospitalization by age groups according to their vaccination situation at our centre, Jan 01-Aug 10, 2021. *p < 0.05.
| Age groups (years) | Vaccinated | Unvaccinated | ||
|---|---|---|---|---|
| % cases (n) | % hospitalized cases (n) | % cases (n) | % hospitalized cases (n) | |
| 0–9 | 0% (0) | 0% (0) | 100% (146) | 100% (7) |
| 10–19 | 0% (0) | 0% (0) | 100% (223) | 100% (8) |
| 20–29 | 2,1% (12)* | 0% (0) | 97,9% (567)* | 100% (40) |
| 30–39 | 2,8% (16)* | 0% (0) | 97,2% (563)* | 100% (112) |
| 40–49 | 2,9% (21)* | 0,4% (1)* | 97,1% (702)* | 100% (229)* |
| 50–59 | 2,2% (13)* | 1,0% (3)* | 97,8% (590)* | 99,0% (303)* |
| 60–69 | 3,2% (14) | 1,7% (5)* | 96,8% (427) | 98,3% (289)* |
| 70–79 | 4,6% (21) | 3,6% (13) | 95,4% (437) | 96,4% (351) |
| 80–89 | 20,7% (63)* | 15,8% (40)* | 79,3% (241)* | 84,2% (213)* |
| ≥90 | 23,8% (25)* | 19,3% (16)* | 76,2% (80)* | 80,7% (67)* |
| Total | 4,5% (185) | 4,6% (78) | 95,6% (3976) | 95,4% (1619) |
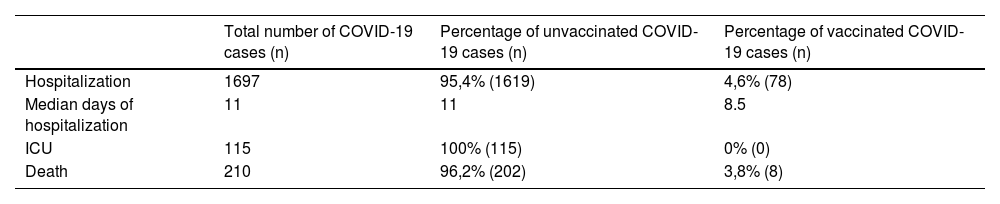
Of 4161 COVID-19 cases that were detected at our centre during the study period, 1697 required hospital admission. Among these, 1619 (95.4%) had not received any vaccine in comparison to 78 (4.6%) who were vaccinated (Table 2). All 115 patients that were admitted to the Intensive Care Unit (ICU) were unvaccinated. Median hospitalization time for fully vaccinated patients was significantly shorter (8.5 days; CI95% 7.04–9.96) than for unvaccinated patients (11 days; CI95% -41.54–63.54). The relative risk of dying was 0.82 (CI95% 0.42–1.60) for vaccinated patients (Table 3).
Percentage of admitted patients' COVID-19 infection outcomes according to their vaccination situation at our centre, Jan 01-Aug 10, 2021.
| Total number of COVID-19 cases (n) | Percentage of unvaccinated COVID-19 cases (n) | Percentage of vaccinated COVID-19 cases (n) | |
|---|---|---|---|
| Hospitalization | 1697 | 95,4% (1619) | 4,6% (78) |
| Median days of hospitalization | 11 | 11 | 8.5 |
| ICU | 115 | 100% (115) | 0% (0) |
| Death | 210 | 96,2% (202) | 3,8% (8) |
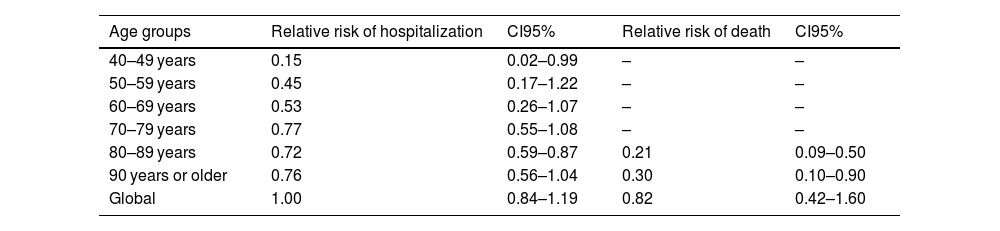
Relative risk of hospitalization and death among different age groups between vaccinated and unvaccinated cases at our centre, Jan 01-Aug 10, 2021.
| Age groups | Relative risk of hospitalization | CI95% | Relative risk of death | CI95% |
|---|---|---|---|---|
| 40–49 years | 0.15 | 0.02–0.99 | – | – |
| 50–59 years | 0.45 | 0.17–1.22 | – | – |
| 60–69 years | 0.53 | 0.26–1.07 | – | – |
| 70–79 years | 0.77 | 0.55–1.08 | – | – |
| 80–89 years | 0.72 | 0.59–0.87 | 0.21 | 0.09–0.50 |
| 90 years or older | 0.76 | 0.56–1.04 | 0.30 | 0.10–0.90 |
| Global | 1.00 | 0.84–1.19 | 0.82 | 0.42–1.60 |
We observed statistically significant differences in hospitalization between vaccinated and unvaccinated patients among different age groups. Grouped by age categories the relative risk of hospitalization was 0.29 (CI95% 0.11–0.72) for age group 40 to 59 years, 0.77 (CI95% 0.67–0.90) for people aged 60 or more and 0.73 (CI95% 0.62–0.86) for people aged 80 or more comparing vaccinated and unvaccinated patients. Relative risks of hospitalization and death are shown in Table 3.
DiscussionMost patients were vaccinated with Pfizer, which was the first vaccine to be approved in Spain and has been so far the most frequently administered one.1 This is mainly due to the vaccination strategy adopted in Spain, which prescribes the types of vaccines to be administered to the general population and to specific risk groups.7
The risk of hospitalization was statistically significantly lower in vaccinated versus unvaccinated patients for age groups 40 to 59 years and older than 60 years. Therefore, our study reinforces existing evidence that a COVID-19 vaccine is highly effective in preventing hospitalization.1,8,9 Immunosenescence has been reported as a major factor that reduces immunogenicity of COVID-19 vaccines and COVID-19 outcomes.8–13 However, we also observed that patients aged 80–89 years or older presented a lower risk of hospitalization and dying as a result of a COVID-19 infection than unvaccinated patients of the same age. In previous studies these age groups accounted for the majority of deaths during the first COVID-19 wave.14
Our study has some shortcomings since we only retrieved data from patients that were attended at our centre. Therefore, the protective effect of vaccination could be greater in the general population. Moreover, this study analyses vaccination status at a specific time of the pandemic. Since then, new variants and new pandemic waves have been appearing. In addition, other factors like social distancing, circulating variants or the use of facemasks may influence COVID dynamics. Finally, asymptomatic patients who did not undergo any testing or were diagnosed at Primary Care have not been included in this study.
ConclusionThe risk of hospitalization and death was lower in vaccinated patients compared to unvaccinated patients for age groups 40 to 59 years and older than 60 years. This finding is in accordance with the data of current clinical evidence.
Authors' contributionsStudy design: Christine Giesen, Angelica Ortega-Torres, Carmen Saa-Requejo, Inmaculada López-Carrillo, Cristina García-Fernández, COVID-19 Vaccination Team.
Data collection, management and quality control: Christine Giesen, Angelica Ortega-Torres, Carmen Saa-Requejo, COVID-19 Vaccination Team.
Data analysis: Christine Giesen, Carmen Saa-Requejo, Angelica Ortega-Torres, Inmaculada López-Carrillo, Cristina García-Fernández.
Interpretation of the data: Christine Giesen, Carmen Saa-Requejo, Angelica Ortega-Torres, Inmaculada López-Carrillo, Cristina García-Fernández, COVID-19 Vaccination Team.
Manuscript draft: Christine Giesen.
All authors critically revised the manuscript and approved the final draft.
Conflict of interestNone.
Funding statementThe authors received no funding.
The authors would like to thank all vaccination and source and contact tracing personnel who have been invaluable for control and surveillance of the COVID-19 pandemic in our country.
We would like to thank the Madrid Dirección General de Salud Pública for sharing data regarding vaccination status with us.
COVID-19 Vaccination Team: Greta Amat-Baeza, Víctor Manuel Irigoyen-Mansilla, Ana Gomez-Santana, Blanca Pedroche-Calleja, Carmen Alejandra Lopez-Vilela, Laura Lopez-Vazquez.