Mycological infections represent important public health disorders, and their incidence has increased in recent years to the extent that they are now a frequent cause of visits to dermatology outpatient departments13. Studies in this area have shown that the increase in the incidence of these infections may be due to a number of reasons, such as the increase in a susceptible population, which includes the elderly and immunodeficient individuals, social and cultural exchanges associated with sports, the use of shoes with poor ventilation, the use of swimming pools, sharing of manicure instruments, and an increasing awareness that these mycoses need to be correctly diagnosed and treated5. In immunodeficient individuals the lesions become more intense, and what are initially superficial lesions can result in disseminated and fatal forms6,19. It has thus become more urgent to raise awareness of these infections and their etiology in hospitals so that the magnitude of this increasingly common problem in Brazil can be determined14 and efficient measures established to avoid transmission and spread of this disease. Therefore, the aim of this study was to determine the incidence of dermatophytosis and their causative agents in patients of a public hospital of São Bernardo do Campo, a municipality of São Paulo State, Brazil.
Patients and methods
From February 2005 to May 2006, 273 samples were collected from 191 patients being seen in a public dermatology outpatient department suspected of having a fungal infection. Before the samples were collected, the patients completed a form with information regarding gender, age, location of the lesion, clinical features (such as color and aspect), and predisposing factors (such as contact with animals and handling soil). The use of antifungal therapy was considered as an exclusion criterion. Materials were collected and a direct microscopic examination using 40% potassium hydroxide was performed. The specimens were then inoculated on Sabouraud Agar Medium (Difco) with chloramphenicol alone and with both chloramphenicol and cycloheximide and incubated for 4 weeks at 37 °C and room temperature, respectively. Cultures were examined every 5 days. After this period, negative and inconclusive samples were inoculated on Sabouraud Agar Medium again for confirmation and second samples were collected. Positive samples were identified according to the macroscopic and microscopic characteristics of their colonies using a fungi identification key2,9. For this purpose, a subculture was made onto Potato Agar Medium (Difco) for filamentous fungi or onto Cornmeal Agar supplemented with Tween 80 for yeasts. Identification of both moulds and yeasts was based on two successive collections of samples and examination of the clinical features of the lesions. Additionally, carbohydrate assimilation tests, based on traditional methodologies16. were used to identify yeasts. The data obtained were encoded in a database and submitted to analysis with Epi Info version 3.3.2; p-values with Yate's correction were used.
Results
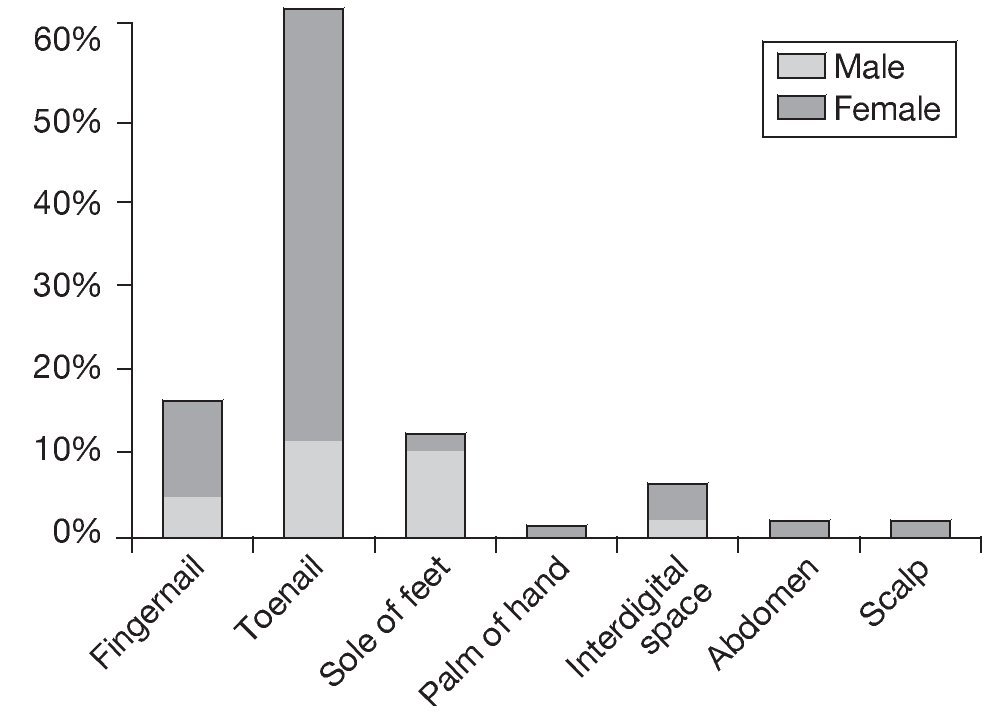
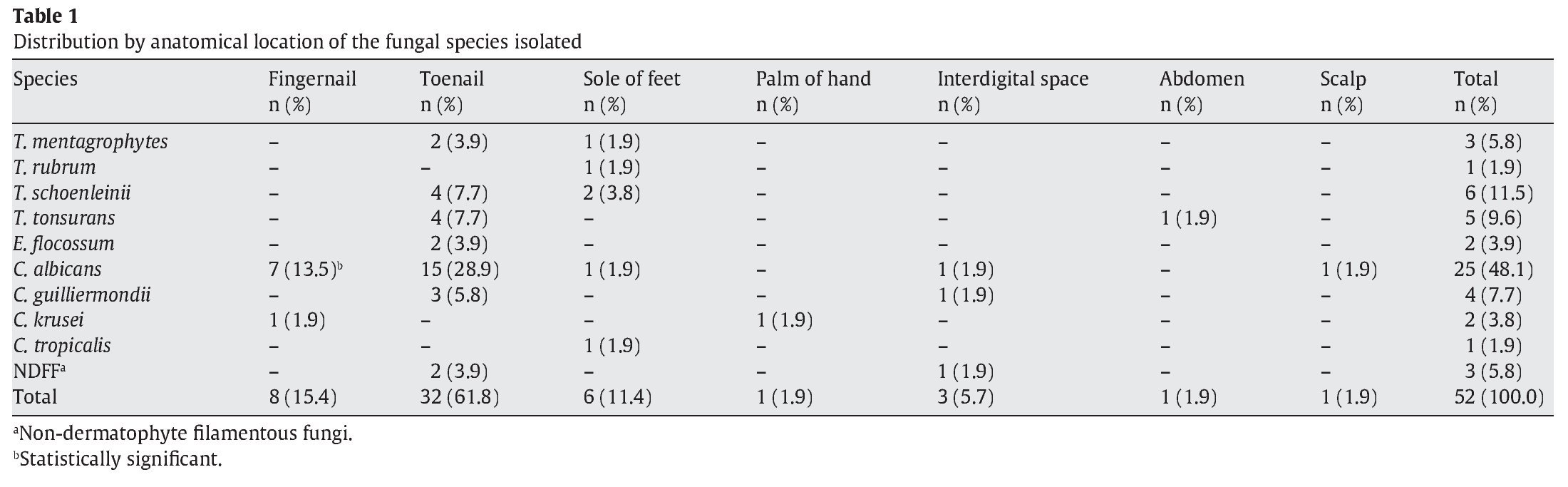
Fifty-two samples (19%) produced positive cultures, while 221 (81%) were negative. Forty patients had positive samples, 29 (72%) females and 11 (28%) males, and as 12 patients had the infection in more than one anatomical location, the total number of samples was 52. Infections were the most frequent in females between 31 and 40 years old and males between 61 and 70 years old. Direct examination of the samples showed that 37 (71%) were positive and 15 (29%) negative. In terms of the anatomical location of the lesions, the most common infection sites were the toenails, fingernails, and soles of the feet, as shown in Figure 1. The presence of lesions on the soles of the feet in males was statistically significant (p = 0.00797). Finally, the laboratory findings revealed that yeasts were the most frequent cause of infection (61.6%), followed by dermatophytes (32.7%) and non-dermatophytic filamentous fungi (NDFF; 5.7%), as shown in Table 1. Distribution of the species according to the age of the population revealed that infection by Candidaalbicans was most common in adults between 31 and 40 years old (11.5%) and between 71 and 80 years old (13.6%). However, only infection by Epidermophyton floccosum in adults between 71 and 80 years old was found to be statistically significant (p = 0.0413). Distribution of the species according to anatomical location showed that infection by C. albicans was the most frequent in toenails (28.9%), but only infection by C. albicans in fingernails was statistically significant (p = 0.0412). Of the samples whose cultures were positive for Candida spp., 60% were positive by direct examination (50.5% positive for C. albicans and 9.5% for Candida guilliermondii) and 40% negative by direct examination in two samples collected consecutively (of these 27.6% were positive for C. albicans, 6.2% for Candida krusei, 3.1% for C. guilliermondii, and 3.1% for Candida tropicalis). With regard to predisposing factors for infection, 20 patients (50%) cited contact with animals, and 5 (12.5%) had handled soil. However, no correlation was found between predisposing factors and the occurrence of infection.
Figure 1. Distribution by gender of the anatomical location of the infection.
Discussion
Based on the IBGE (Brazilian Institute for Geography and Statistics) estimate for the total population of São Bernardo in 2005 of 788,560, our population (191 patients) represents just 0.02% of this figure. We believe that this rate of fungal infection must be below the real rate because some patients do not seek medical assistance and others use different public or private health services13 (the present study was carried out in just one location). The finding of 52 positive samples (19%) correlates with other studies, in which incidences of 16.5%13, 20.8%12, and 22.8%3 of the study populations were reported. However, the frequency of exposure to predisposing factors and variations in population distribution over the years should be taken into account before establishing a relationship between gender and susceptibility to infection. It should be noted that 12 patients were infected in more than one anatomical location, a finding that can be accounted for by dissemination of the infection to different sites by self-inoculation or by the appearance of new lesions by repeated infections because of a permanent source of contamination10.
Of the samples that yielded positive cultures, 37 (71%) revealed fungal elements on direct examination. In a recent review of the literature, Siqueira et al17 found rates of 35.6%, 66.7%, and 70.4% cited for both a positive direct examination and a positive culture. It should be stressed that diagnosis based on direct microscopic observation of samples alone has a number of limitations, such as low sensitivity, the fact that it is nearly always impossible to identify the fungal species, and the inability to perform antifungal sensitivity testing15.
With regard to the anatomical localization of the lesions, the results agree with the results of studies by Crocco et al4, who considered the higher prevalence of onychomycosis in females to be the result of greater trauma, continued exposure to water, or irritation as a result of contact with chemical substances. In our study we also observed a higher rate of infection in toenails, a finding that can be explained by the slow growth of these nails compared to that of fingernails (fingernails: 3-4 mm/month vs. toenails: 1.5-2 mm/month) and their greater exposure to trauma1. Toenail onychomycosis can cause pain and discomfort, making it difficult to practice sports, walk, or even stand. This infection can also result in significant damage to general health, physical appearance, and social performance. In males, the higher prevalence of infection in the soles of the feet can also be attributed to the greater frequency with which males practice sports, greater occlusion by shoes, and a lack of attention to foot hygiene.
The predominance of yeasts (61.6%) followed by dermatophytes (32.7%) and NDFF (5.7%) in this study agrees with studies in which these three types of fungi were identified as the causative agents of many cutaneous mycoses in humans, particularly onychomycosis. However, there are striking geographic differences in the epidemiology and etiology of onychomycosis, especially the prevalence of the infections caused by each group of fungi7. Yeasts are often secondary pathogens4 and cause infections when they find conditions that favor growth, such as constant humidity, contamination that can occur when women are performing personal hygiene11 or the immunodeficiency16 that can follow hormonal variations in female patients. NDFF other than Scytalidium species are variously said to cause between 0% and 50% of all toenail onychomycoses, although most estimates are in the 2-5% range18.
Several studies on the efficacy of antifungal agents in onychomycosis have been published. These, together with the fact that different agents have different response rates to treatment, have led to increasing recognition of the importance of rigorous study design and reporting and the subsequent improvement in the quality of studies in this field7.
In conclusion, our findings showed that yeasts were the predominant cause of infection in our study population, and are in agreement with other studies that reported varying frequencies and prevalence according to the country or the regions in a particular country. Many studies on this subject have been carried out to date. The present study shows, however, that there is an increasing prevalence of yeasts as etiologic agents of dermatomycosis.
Acknowledgments
To Dr. Luiz G. Filho, Dra. Fátima T. Reis, Dra. Amélia R.M. Akabane, Dra. Lúcia Morilhara, Dr. Sebastião N. Travassos, Dr. Marcus V.G. Buissa, Dr. Willian Buissa, and the staff of the Clínica Municipal de Especialidades Médicas and the Universidade Metodista de São Paulo.
* Corresponding author.
E-mail address:marta.souza@metodista.br (M.C. Souza).
ARTICLE INFO
Article history:
Received November 6, 2007
Accepted December 2, 2008