Atopic dermatitis (AD) is an inflammatory disease of the skin, which is characterised by a chronic relapsing course.
AimThe aim of the study was to assign the prevalence of clinically active food allergies among a group of children between 3 months and 7 years of age, with AD.
MethodsEighty-eight children with AD were screened for specific IgE antibodies to food proteins. All patients with AD and specific IgE antibodies to food proteins were subjected to Oral Food Challenges (OFCs) with the relevant foods.
ResultsFood-sensitised patients with moderate levels of sIgE had clinically active food allergy to milk (39.28%) and egg (42.34%) on the basis of positive OFCs. High IgE and eosinophilia had a prevalence of almost 80% and 25%, regardless of concomitant food sensitisation and disease severity.
ConclusionsIn this study, clinically active food allergies were recognised in 26.13% of children with AD. Nevertheless, no association was confirmed between food sensitisation and AD severity. High IgE and peripheral eosinophilia have not been found more prevalent among children with severe AD nor among children with food sensitisation. Infants and younger children with AD should be screened for an underlying food allergy, regardless of disease severity.
Atopic dermatitis (AD), also known as atopic eczema, is a pruritic chronic inflammatory skin disease that affects 10–12% of the paediatric population.1 Allergic respiratory disease will develop in 50–80% of patients with AD later in life.2
Food allergy has been strongly correlated with the development and persistence of AD, especially during infancy and early childhood. Skin is the site that is most often involved in food hypersensitivity reactions with pruritus being the most consistent sign. Several studies support a role of food-specific IgE antibodies in the pathogenesis of AD.3–5 Delayed reactions to food seem to play a role in exacerbations of AD in some patients. Oral Food Challenges (OFCs) have been used to examine if food allergens can induce symptoms of a pruritic type of rash, in children with food allergy and AD. In addition, OFC is the gold standard for the diagnosis of food allergy in subjects with AD.6,7 The aim of our study was to assign the prevalence of food allergy among a study group of children with AD and identify causal food allergens that are involved as trigger factors of AD. The prevalence of clinical active food allergy in paediatric populations with AD has not been extensively investigated. There have not been any previous studies investigating the severity of AD in relation to food sensitisation, eosinophilia or total IgE levels.
MethodsChildren between the age of 3 months and 7 years (27.55±19.53 months) referred to the Paediatric Allergy Clinic of the 3rd Paediatric Department, Aristotle University of Thessaloniki were evaluated for AD. The clinical criteria of the American Academy of Dermatology had to be fulfilled for establishing diagnosis.8 All patients who met the criteria were considered eligible for participating in the study.
Disease severity evaluationAll the patients were evaluated for AD severity using the Scoring Atopic Dermatitis (SCORAD) index9 by a Paediatric Allergist (A.M.) at the initial visit. Parents were informed by the Paediatric Allergist that children with AD may have worsening of their symptoms triggered by foods to which they are allergic. None of the patients were previously diagnosed with food allergy. Children with AD and not known or even possible diagnosis of food allergy were offered the opportunity for further investigation regarding food allergy. The parents of these children gave a written consent for participating in the study.
Allergy evaluationTotal and specific IgE antibodies were measured in all patients included in the study with a widely used, commercially available fluorescence enzyme immunoassay (FEIA), (UniCAP™, Phadia, Uppsala, Sweden) in 10 food proteins: α-lactalbumin, ¿-lactoglobulin, casein, whole milk, egg yolk, egg white, wheat, soya, cod fish and beef, which are the most common food allergens in children.10 Microscopy was performed on peripheral blood smear in order to estimate the absolute eosinophil count.
Patients with positive results for specific IgE antibodies to at least one food allergen were contacted by a Paediatric Allergist to return for re-evaluation and counselling. The study was approved by the Ethics Committee of the Aristotle University of Thessaloniki, Greece.
Open Food Challenges (OFCs)All patients with AD and positive levels of specific IgE antibodies, based on the CAP-results, were considered for further evaluation of food allergy by an OFC to the foods to which they were sensitised. Patients with low-moderate levels of specific IgE antibodies to food allergens were considered eligible for an OFC for the relative food products. Patients who had a CAP value more than 95% of the cut-off predictive value for a positive reaction were considered allergic and were not challenged.4,5
All the study subjects were instructed to strictly avoid the relevant foods for a six-week period prior to the OFC and to discontinue antihistamine medications for at least 10 days. They were considered eligible for an OFC only after achieving a good control of their AD and reached the conservative treatment phase with the use of emollients alone or in combination with calcineurin inhibitors. OFC protocols were adopted from Niggerman et al.11 and Sicherer.12 All challenges were performed in the 3rd Paediatric Department of the Aristotle University of Thessaloniki under the supervision of a Paediatric Allergist (A.M.). Intravenous access was obtained and emergency medications were available during the challenge procedure. Cow's milk challenge was always started with a lip challenge followed by cow's milk administration after 20min of patient observation. Cow's milk was administered orally with 20min intervals in four consecutive doses of 5, 10, 30 and 150ml of cow's milk containing 0.15, 0.3, 1 and 6.6g of milk proteins respectively. Regarding the egg challenges, a well-cooked egg was used. Each challenge started with a cutaneous application of egg. A thin layer of cooked egg was placed on the patient's forearm and covered with tegaderm for 20min. Subsequently, the dressings and the egg were removed and the patient was evaluated for local (wheal, erythema) or systemic (respiratory distress, hypotension, tachycardia, oxygen desaturation) symptoms and signs. If no reactions occurred, then well-cooked egg was administered orally with 20min intervals in four consecutive doses of 0.5, 4, 10 and 30g of cooked egg containing 0.12, 0.6, 1.25, and 3.75g of egg proteins respectively. Regarding the wheat challenge, this was started with a cutaneous application of cooked wheat (bread) on the patient's forearm covered with tegaderm for 20min. If no reaction occurred, then oral administration of the bread was completed in five consecutive doses of 1, 2, 4, 8 and 16g containing 0.12, 0.6, 1.2, 2.4 and 4.8g of wheat protein respectively, with 20min intervals. Patients were discharged no earlier than six hours after the OFC had been completed and their parents were asked to report any reactions (exacerbation of eczema, pruritus, cough, wheeze, etc.) that might occur following the discharge and within a period of seven days. In subjects with more than one sensitisation consecutive food challenges were performed with 10 days interval.
Statistical analysisStatistical comparisons of patient proportions were performed by using the Chi-Square test or the Fisher's exact test accordingly. Mean value comparisons were performed using the Mann–Whitney U test which was considered appropriate when there was rejection of normality assumptions and low degrees of freedom. p-Values<0.05 were considered statistically significant. SPSS 22.0 (IBM Corp., Armonk, NY, USA) was used for data analysis.
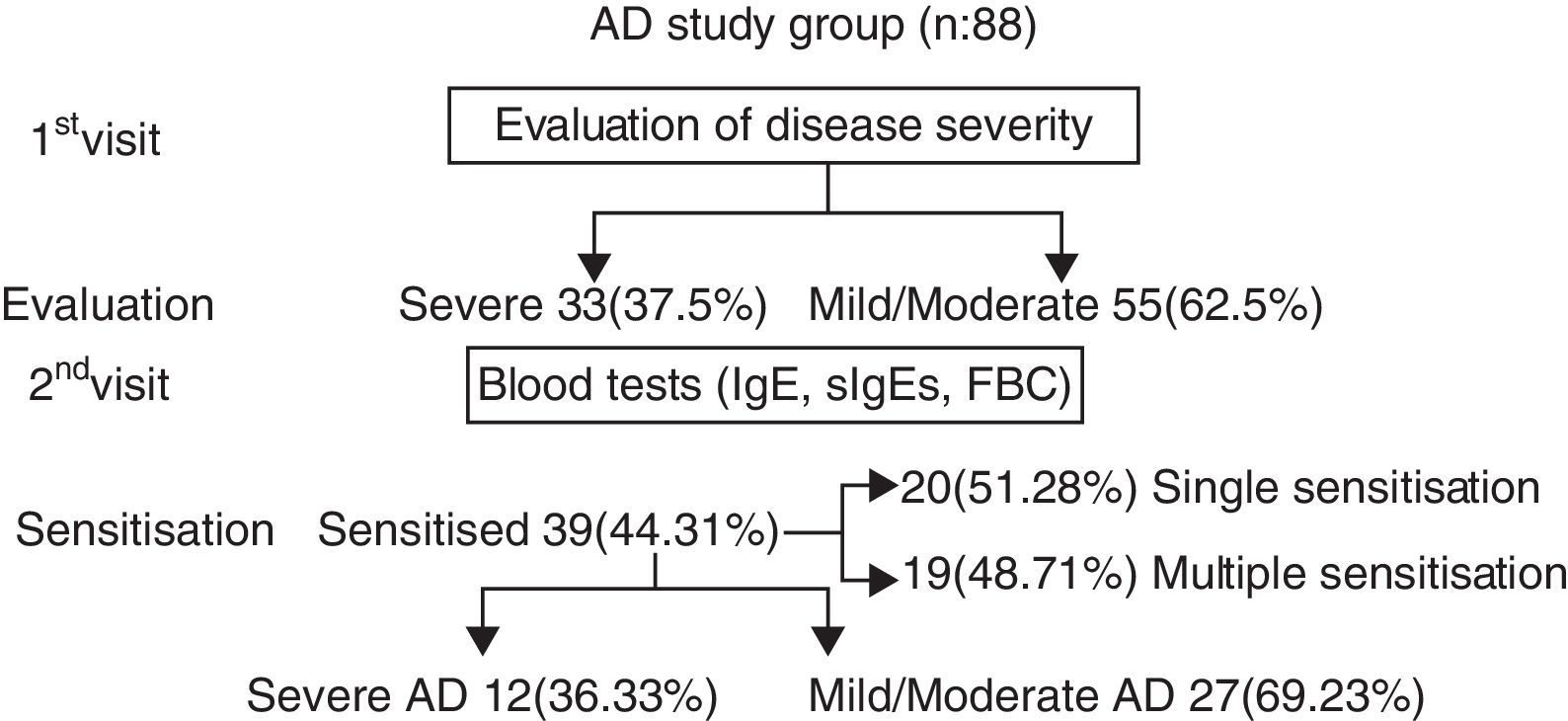
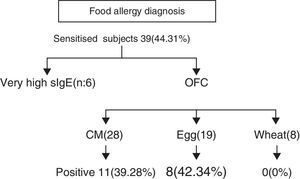
ResultsEighty-eight children with AD (59 boys and 29 girls from 3 months to 7 years of age, mean age 27.55±19.53 months) were subjected to investigation for concomitant food allergies. The study group was divided into two subgroups, on the basis of eczema severity: (a) with mild-moderate eczema (n=55, 62.5%) and (b) with severe eczema (n=33, 37.5%). Thirty-nine out of 88 subjects (44.31%) were sensitised to food allergens. Twenty (51.28%) were sensitised to only one food, while 19 (48.71%) had multiple sensitivities. (Chi-square: 0.017, p=0.895) (Diagram 1). Twenty-six out of 55 (47.27%) with mild-moderate AD and 13 out of 33 (39.39%) with severe AD were sensitised to various food allergens (Chi-square: 0.203, p=0.652).
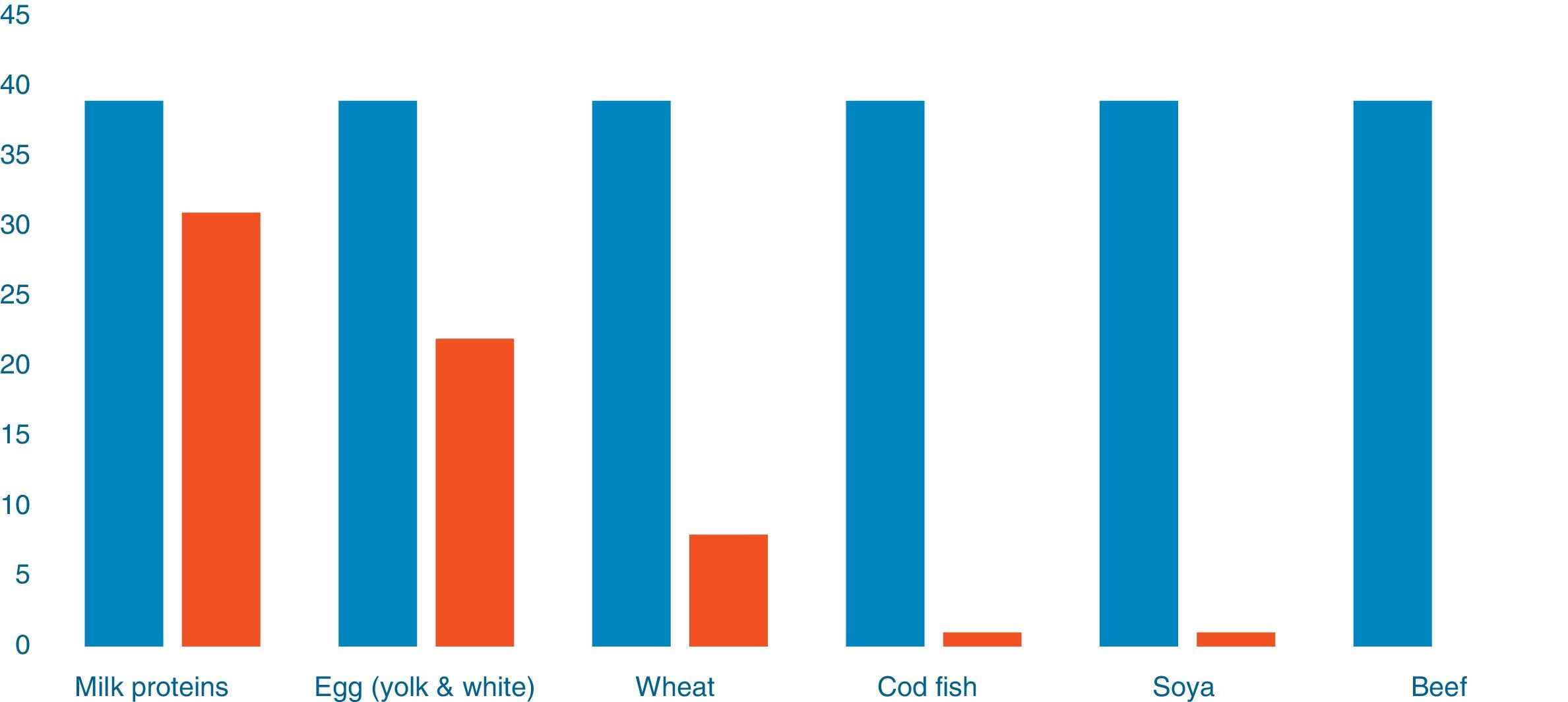
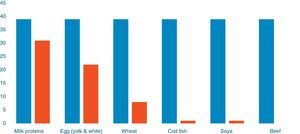
Out of 39 patients sensitised to food allergens, 31 (79.48%) were sensitised to cow's milk, 22 (56.41%) to egg, eight (20.51%) to wheat, one patient was sensitised to cod (2.56%) and one to soya (2.56%) (Fig. 1). Of 20 patients with a single sensitisation seven (35%) had severe AD, while among the 19 patients with multiple food sensitisations six (31.57%) had severe AD (Fisher's Test: 1, p=0.501) (Diagram 2). The percentages of sensitisation to one or more than one food proteins in relation to AD severity did not differ in a statistically significant manner.
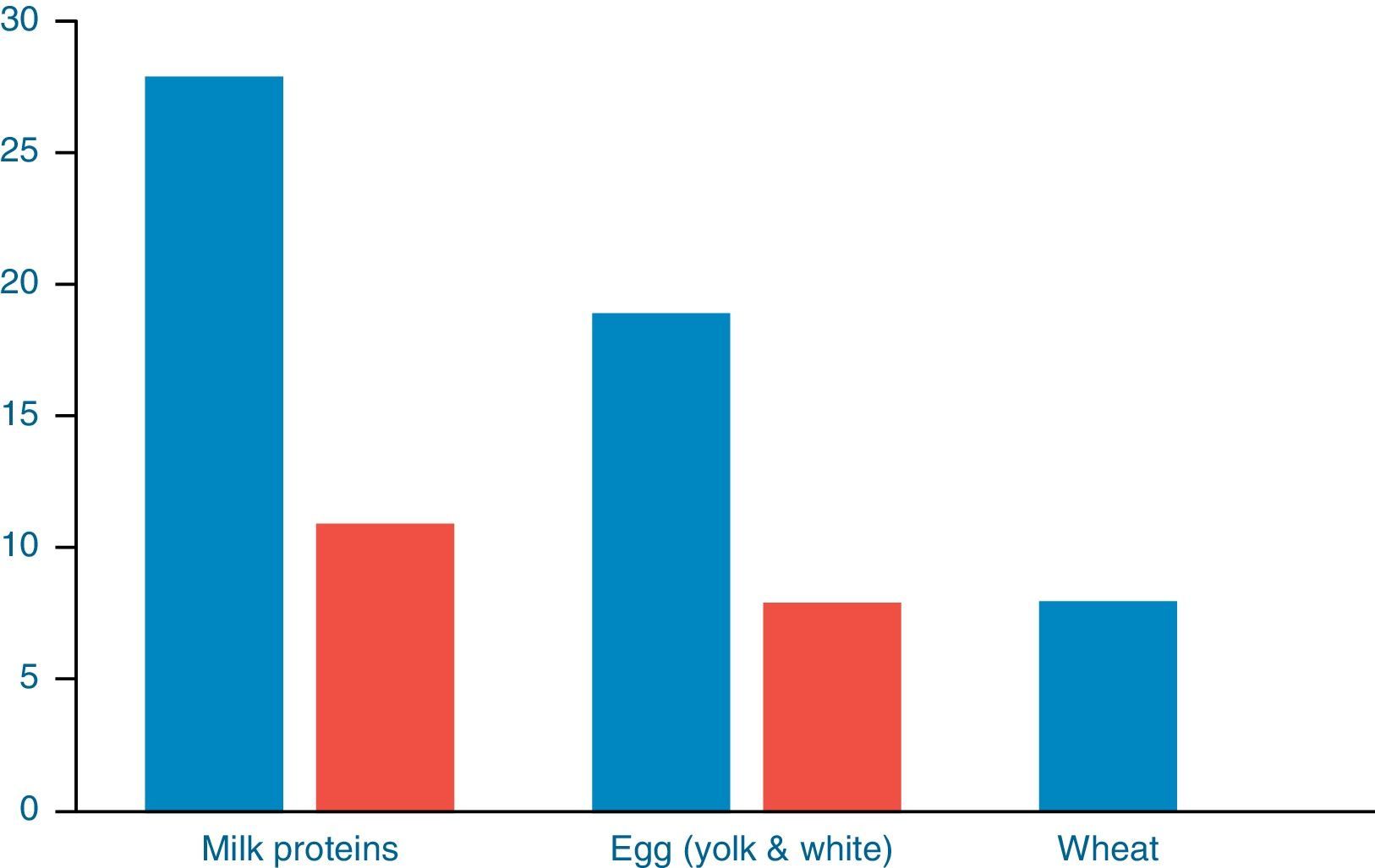
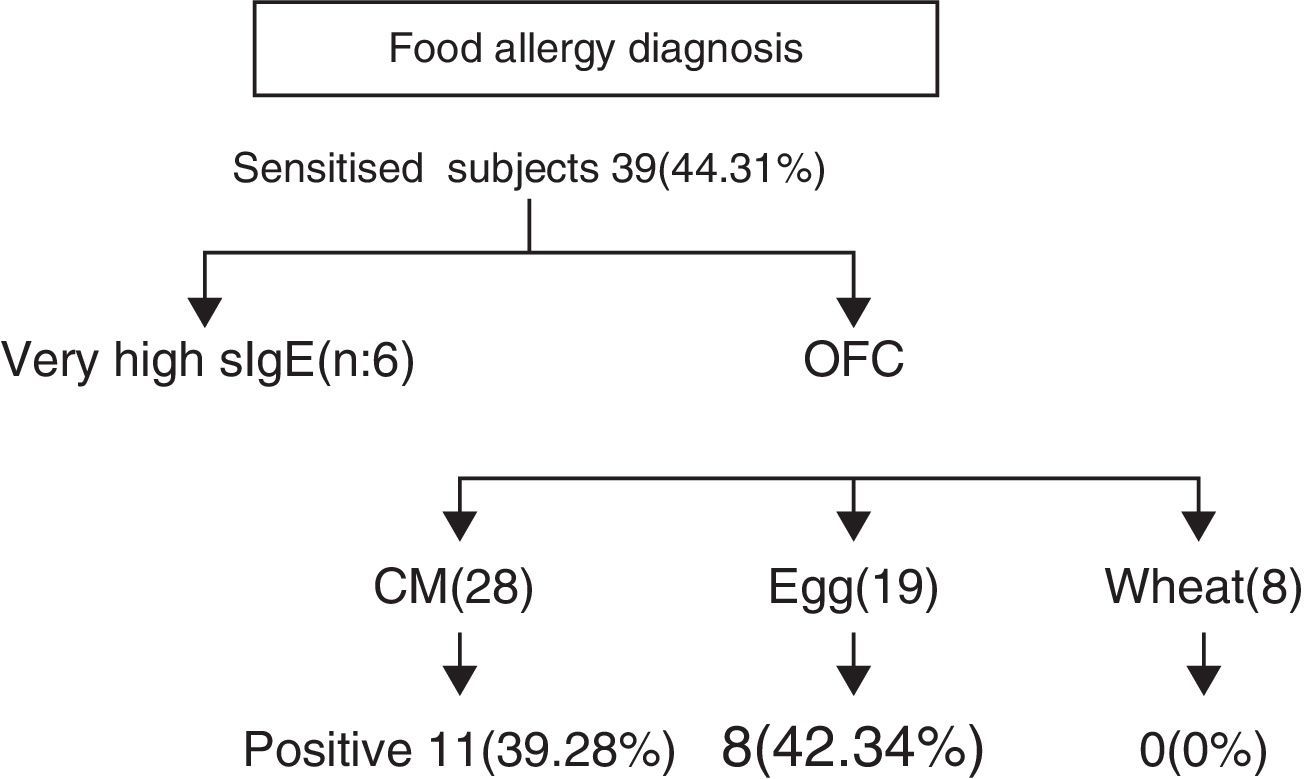
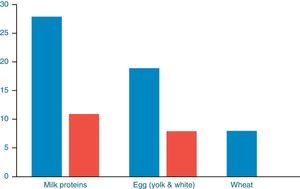
Further evaluation with OFCs was performed in subjects with cow's milk (n=28), egg (n=19) and wheat (n=8) sensitisation and were positive to milk, egg and wheat in 11/28 (39.28%), 8/19 (42.34%) and 0/8 (0%) of cases, respectively (Fig. 2). OFC was not offered in three subjects with sensitisation to cow's milk and three egg-sensitised patients with very high specific IgE levels. Seventeen subjects had positive OFCs to at least one food, while six were considered as allergic due to high levels of specific IgE antibodies to food proteins. Accordingly, a total of 23 out of 88 patients (26.13%) had a clinically active food allergy.
Forty-one of the 55 children with mild-moderate eczema (74.74%) and 29 out of 33 children with severe AD (87.87%) had increased total serum IgE (Chi-square is 0.252 (p=0.615). Increased total serum IgE was found in (29/39) 74.35% food sensitised patients and in (41/49) 83.67% of the non-food sensitised patients with eczema (Chi-square: 0.132, p=0.715).
Eleven out of 55 children with mild-moderate AD (20%) and nine out of 33 children with severe AD (27.27%) had peripheral blood eosinophilia (Chi-square is 0.385, p=0.534). Elevated peripheral blood eosinophils were found also in (11/39) 28.20% of the food sensitised patients and (10/49) 20.40% of the non-sensitised patients (Chi-square: 0.443, p=0.505). The patients’ rate of severe eczema was (9/39) 23.07% of the food sensitised patients versus (18/49) 36.07% of the patients without food sensitisations (Chi-square: 1.025, p=0.311).
Comparison of the patients sensitised to food allergens (n=39) and the non-food sensitised (n=49) showed no significant difference of their mean SCORAD (Wilcoxon–Mann–Whitney Test) (p=0.502). There was no statistically significant difference of the mean SCORAD between the group of patients with a high total IgE and the group with normal IgE (Wilcoxon–Mann–Whitney Test) (p=0.937). There was no significant mean SCORAD difference between the patient groups with and without peripheral eosinophilia (Wilcoxon–Mann–Whitney Test) (p=0.907).
DiscussionIn this study, children with atopic dermatitis were investigated for clinically active food allergy. Forty-five percent of the children with diagnosed AD were sensitised to food allergens with no statistically significant difference with regards to food sensitisation between subjects with mild-moderate and severe AD. A total percentage of one every four children, of children with AD were truly allergic to milk and or/egg proteins. A percentage of 39.28% (11/28) milk-sensitised patients and 42.34% (8/19) egg-sensitised patients with AD had clinically active food allergy on the basis of the positive OFCs. The predictive value of specific IgE in patients with AD is unclear. Fleischer et al.13 found that allergy testing has a high false positive rate. In their study, negative food challenges occurred in 89% of 364 challenges in 125 children evaluated for AD.
The majority of food sensitisation was referred to whole milk (80%) and a smaller percentage regarded egg sensitisation (55%), wheat (20%), cod and soya (2.5%). Previous studies have shown various rates of food allergy among different paediatric populations with AD.
In the study of the atopic march, 15.9% of a population of >1000 infants with AD developed one or more IgE-mediated food allergies.14 Eigenmann et al. in 1998 for the first time connected AD with food allergy.4 Among 63 patients with AD food allergy was recognised in 23 patients based upon challenge results, “convincing” history, plus a predictive food-specific IgE antibody level as determined by CAP-System FEIA, and/or prick skin test. Twenty-three of the 63 (37%) patients were allergic to at least one food. Comparison of these 23 food-allergic patients with the 40 patients with negative evaluation regarding food allergy showed no significant difference in median SCORAD. The median ages of those with and without food allergy were also similar. The study by Burks et al. showed a higher prevalence of food allergy among children with AD (37%).15 Wahn et al., in a cohort study performed in children with AD estimated that IgE responses to hen's egg proteins (42%) were most prevalent, while sensitisation to cow's milk and peanut was detected in 24–27% of the children.16
The patient rates with severe AD were not elevated among food-sensitised children versus those without sensitisation. The use of the SCORAD scale in clinical trials is considered a reliable tool which is not affected by subjective parameters.9 There was no significant mean SCORAD difference between food-sensitised and non-sensitised patients in keeping with previous studies. Guillet and Guillet reported an increasing prevalence of food allergy among patients with increasing severity of AD. However, they did not detect a difference in the prevalence of food allergy in relation to the severity of the disease. Patients with and without food allergy had similar SCORAD scores.17
The majority of the patients who were diagnosed with eczema had a high total IgE, as shown by previous studies, regardless of disease severity. Although the pathogenesis of AD is unknown, several factors suggest a role for IgE-mediated hypersensitivity. Several studies have shown that serum IgE concentrations were elevated in about 50–80% of children with AD.18–20 In the present study, the rate of a high total IgE was not related to food sensitisation and the severity of the disease. To our knowledge, there are no previous studies comparing the rate of high total IgE between food-sensitised versus non-sensitised patients or between patients with mild-moderate and severe eczema.
Peripheral blood eosinophilia was present in similar rates in the groups of patients with mild-moderate and severe AD. In a previous study it has been suggested that disease severity is an important factor in determining high blood eosinophil levels in AD.21 In this study there has not been a significant difference between food-sensitised patients and those without in terms of peripheral eosinophilia. With regard to disease severity the SCORAD value was not significantly increased among patients with peripheral eosinophilia. There are no previous studies comparing the rate of eosinophilia between food-sensitised versus non-sensitised patients or between patients with mild-moderate and severe eczema.
A limitation of the study is that it assesses the prevalence of clinically-active food allergy only among patients with IgE-mediated food allergies. However, eczema is regarded a clinical manifestation of delayed hypersensitivity. Therefore, it is likely that there must have been children among those who had a negative investigation for food allergy who would benefit from a hypoallergenic diet, such as milk and egg free diet and if challenged after a 4–6-week period of an elimination diet they would probably have had a positive OFC. In that case the percentage of children with eczema and clinically active food allergy would be higher. The association between non-IgE-mediated food allergy and eczema in children has not been well investigated and remains a target for future research.
In conclusion, food allergy was diagnosed at a rate of one every four children of the study group with AD, regardless of disease severity and while sensitisation was found in 44.31% of them. A high total serum IgE and peripheral eosinophilia was recognised in approximately 80% and 25% of the patients’ sample, respectively, regardless of the disease severity and food sensitisation. Food allergy can be hidden in a significant percentage of children with AD and may be a trigger factor that keeps on pumping water to the waterfall of AD. Clinicians need to seek from history possible suspicious allergens in foods. Further investigations and OFCs will establish the diagnosis of underlying food allergies in children with AD. It is a difficult task for the clinicians to break the vicious circle of AD and food allergy seems to be a hidden trigger in some children with AD. It is important to explore from history, such a connection and screen all cases of children with AD, regardless of disease severity.
Conflicts of interestNone.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.