Liver disease during pregnancy is more common than expected and may require specialized intervention. It is important to determine if changes in liver physiology may develop into liver disease, to assure early diagnosis. For adequate surveillance of mother-fetus health outcome, liver disease during pregnancy might require intervention from a hepatologist. Liver diseases have a prevalence of at least 3% of all pregnancies in developed countries, and they are classified into two main categories: related to pregnancy; and those non- related that are present de novo or are preexisting chronic liver diseases. In this review we describe and discuss the main characteristics of those liver diseases associated with pregnancy and only some frequent pre-existing and co-incidental in pregnancy are considered.
In addition to the literature review, we compiled the data of liver disease occurring during pregnancies attended at the National Institute of Perinatology in Mexico City in a three-year period.
In our tertiary referral women hospital, liver disease was present in 11.24 % of all pregnancies. Associated liver disease was found in 10.8% of all pregnancies, mainly those related to pre-eclampsia (9.9% of pregnancies). Only 0.56% was due to liver disease that was co-incidental or preexisting; the acute or chronic hepatitis C virus was the most frequent in this group (0.12%).
When managing pregnancy in referral hospitals in Latin America, it is important to discard liver alterations early for adequate follow up of the disease and to prevent adverse consequences for the mother and child.
Liver disease is a serious complication of pregnancy and poses a challenge for the gynecologist and hepatologist. It occurs in approximately 3% of all pregnancies, and may lead to various maternal and perinatal morbidities, some of them with fatal consequences for both mother and child [1–6].
Liver disorders may present themselves inconspicuously during gestation. Due to the physiological changes in the liver that occur during pregnancy, it is difficult to diagnose and manage a liver disease. A systematic approach is needed for its treatment and must include a detailed clinical history, examination, laboratory analyses and radiographic evaluation. The clinical history should include previous pregnancy diseases and associated liver complications, intravenous drug use, transfusions, if pregnancies were taken to term, and oral contraceptive use. Also, the patient should be evaluated for clinical data that may suggest liver dysfunction such as nausea, vomiting, jaundice, generalized pruritus, abdominal pain, polyuria and polydipsia in the absence of other morbidities as chronic metabolic disease such as diabetes [6–10].
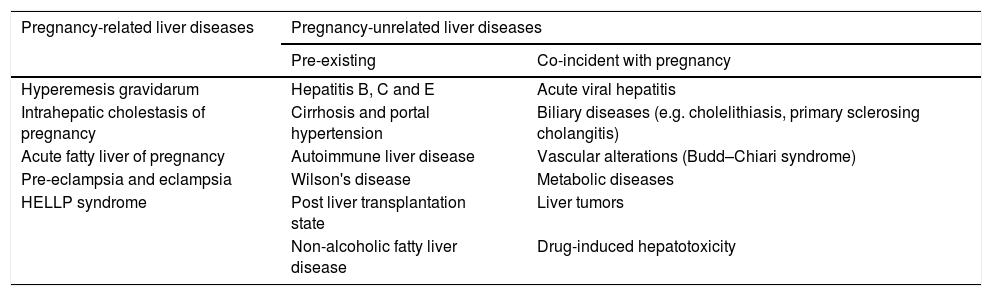
Liver disease during pregnancy is classified into two main categories: those related to pregnancy; and those non-related or coincidental to pregnancy that are present de novo, or are pre-existing chronic liver disease exacerbated by pregnancy (Table 1) [2,6,9]. Pregnancy related liver diseases include intrahepatic cholestasis of pregnancy (ICP), acute fatty liver of pregnancy (AFLP) and, hemolysis, elevated liver enzymes and low platelets count (HELLP) syndrome. In addition, pre-eclampsia (PE) and hyperemesis gravidarum (HG) are frequently associated to liver abnormalities [4,5,7]. Non-related pregnancy hepatic diseases include acute and chronic viral hepatitis, cirrhosis with or without portal hypertension, biliary diseases such as gallstones and primary sclerosing cholangitis, vascular alterations such as Budd–Chiari syndrome (BCS), Wilson's disease, autoimmune liver diseases, metabolic disorders, drug induced hepatotoxicity and post liver transplantation state (Table 1) [2,5,6,11].
Classification of liver diseases in pregnancy [2,11].
| Pregnancy-related liver diseases | Pregnancy-unrelated liver diseases | |
|---|---|---|
| Pre-existing | Co-incident with pregnancy | |
| Hyperemesis gravidarum | Hepatitis B, C and E | Acute viral hepatitis |
| Intrahepatic cholestasis of pregnancy | Cirrhosis and portal hypertension | Biliary diseases (e.g. cholelithiasis, primary sclerosing cholangitis) |
| Acute fatty liver of pregnancy | Autoimmune liver disease | Vascular alterations (Budd–Chiari syndrome) |
| Pre-eclampsia and eclampsia | Wilson's disease | Metabolic diseases |
| HELLP syndrome | Post liver transplantation state | Liver tumors |
| Non-alcoholic fatty liver disease | Drug-induced hepatotoxicity | |
HELLP: hemolysis, elevated liver enzymes and low platelets count.
Pregnancy associated liver disorders exhibit specific trimester occurrence, while non-pregnancy-related liver diseases can occur at any time. The timing of the occurrence of clinical manifestations and abnormal liver function tests (LFTs) are critical for determining diagnosis and treatment strategies. Therapeutic decisions must be made considering the implications for both the mother and the child, and rapid diagnosis is required in severe liver diseases because the decision of immediate delivery will determine maternal and fetal outcome. The main factors that determine maternal prognosis depend on the type of liver disease, the degree of impaired synthetic, metabolic, and excretory liver function, and timing of delivery [6,8,10].
In this review we will focus on the description of main liver pathologies associated to pregnancy, and an overview of some co-incidental or pre-existing liver diseases will be summarized. Additionally, we present a preliminary report of the frequencies of main liver diseases as a cause for hospitalization in pregnant women, seen at the Instituto Nacional de Perinatología (INPER) at Mexico City, during a three year period (August 2016–July 2018). We compared these data to reports of liver disorder frequencies during pregnancy.
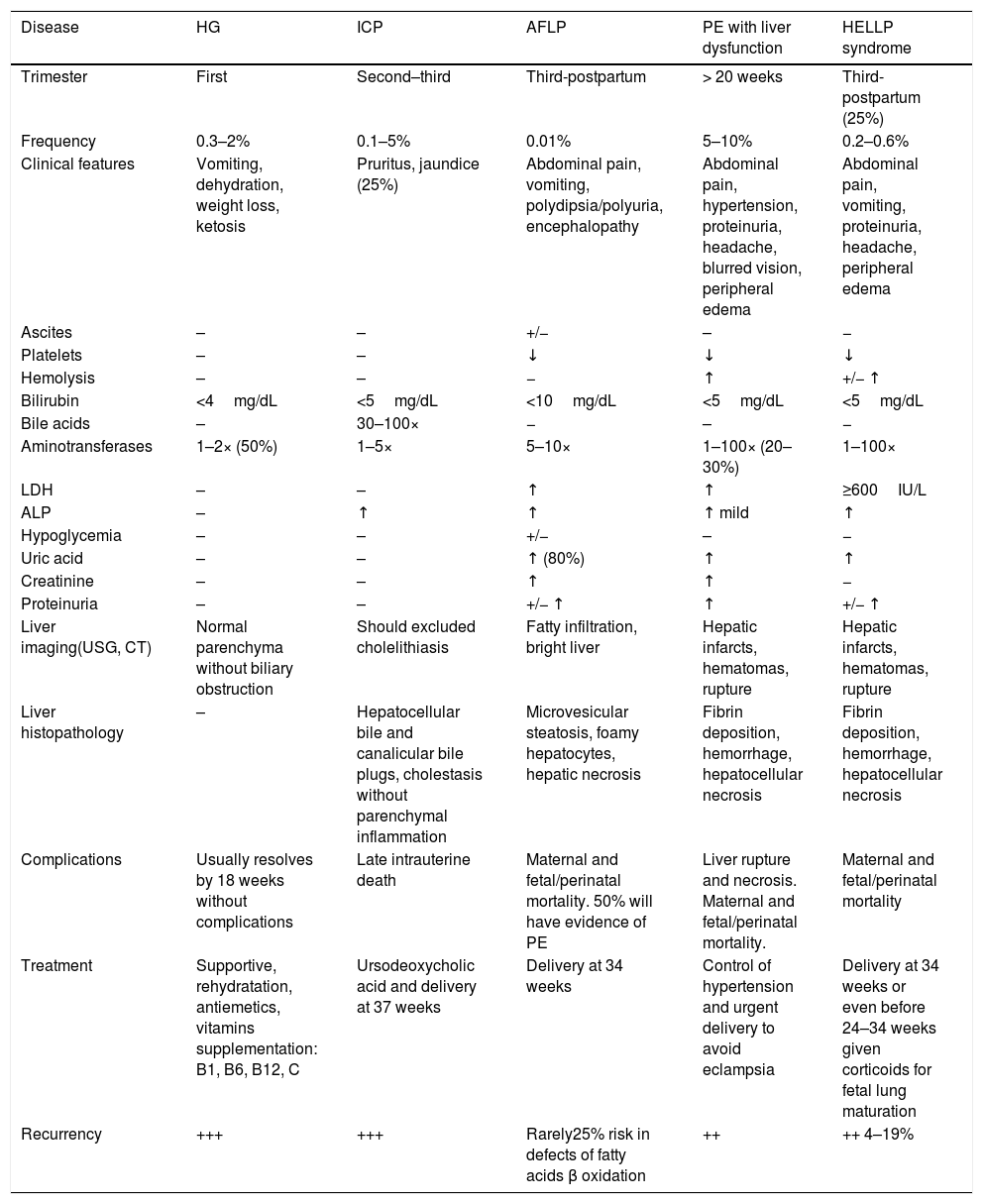
2Pregnancy related liver disordersPregnancy-specific disorders are the leading cause of abnormal LFTs during pregnancy, particularly in the third trimester. Pre-eclampsia-related disorder is the most common among these [12]. A mortality rate of 0–25% has been reported among mothers with pregnancy-related liver diseases [6,13,14]. Physiological changes in pregnancy can mimic liver disease; therefore, these must be considered when diagnosing suspected pregnancy related liver disease. However, increased levels in transaminases, bilirubin, fasting total bile acids, or the prothrombin time (PT) above the normal range during pregnancy, are abnormal and require prompt evaluation [5,6,15–17]. The main characteristics of pregnancy related liver diseases are shown in Table 2.
Major characteristics of pregnancy related liver diseases and differential diagnoses [8,9,11,15–17].
| Disease | HG | ICP | AFLP | PE with liver dysfunction | HELLP syndrome |
|---|---|---|---|---|---|
| Trimester | First | Second–third | Third-postpartum | > 20 weeks | Third-postpartum (25%) |
| Frequency | 0.3–2% | 0.1–5% | 0.01% | 5–10% | 0.2–0.6% |
| Clinical features | Vomiting, dehydration, weight loss, ketosis | Pruritus, jaundice (25%) | Abdominal pain, vomiting, polydipsia/polyuria, encephalopathy | Abdominal pain, hypertension, proteinuria, headache, blurred vision, peripheral edema | Abdominal pain, vomiting, proteinuria, headache, peripheral edema |
| Ascites | – | – | +/− | – | − |
| Platelets | – | – | ↓ | ↓ | ↓ |
| Hemolysis | – | – | − | ↑ | +/− ↑ |
| Bilirubin | <4mg/dL | <5mg/dL | <10mg/dL | <5mg/dL | <5mg/dL |
| Bile acids | – | 30–100× | − | – | − |
| Aminotransferases | 1–2× (50%) | 1–5× | 5–10× | 1–100× (20–30%) | 1–100× |
| LDH | – | – | ↑ | ↑ | ≥600IU/L |
| ALP | – | ↑ | ↑ | ↑ mild | ↑ |
| Hypoglycemia | – | – | +/− | – | − |
| Uric acid | – | – | ↑ (80%) | ↑ | ↑ |
| Creatinine | – | – | ↑ | ↑ | − |
| Proteinuria | – | – | +/− ↑ | ↑ | +/− ↑ |
| Liver imaging(USG, CT) | Normal parenchyma without biliary obstruction | Should excluded cholelithiasis | Fatty infiltration, bright liver | Hepatic infarcts, hematomas, rupture | Hepatic infarcts, hematomas, rupture |
| Liver histopathology | – | Hepatocellular bile and canalicular bile plugs, cholestasis without parenchymal inflammation | Microvesicular steatosis, foamy hepatocytes, hepatic necrosis | Fibrin deposition, hemorrhage, hepatocellular necrosis | Fibrin deposition, hemorrhage, hepatocellular necrosis |
| Complications | Usually resolves by 18 weeks without complications | Late intrauterine death | Maternal and fetal/perinatal mortality. 50% will have evidence of PE | Liver rupture and necrosis. Maternal and fetal/perinatal mortality. | Maternal and fetal/perinatal mortality |
| Treatment | Supportive, rehydratation, antiemetics, vitamins supplementation: B1, B6, B12, C | Ursodeoxycholic acid and delivery at 37 weeks | Delivery at 34 weeks | Control of hypertension and urgent delivery to avoid eclampsia | Delivery at 34 weeks or even before 24–34 weeks given corticoids for fetal lung maturation |
| Recurrency | +++ | +++ | Rarely25% risk in defects of fatty acids β oxidation | ++ | ++ 4–19% |
ALP: alkaline phosphatase; CT: computed tomography; HELLP: hemolysis, elevated liver enzyme levels and low platelet levels; LDH: lactate dehydrogenase;; USG: ultrasonography.
HG is defined as a severe form of nausea and untreatable vomiting which results in dehydration, ketosis, and weight loss of more than 5% of body weight [5,6]. HG occurs in approximately 0.3–2.0% of pregnancies during the first trimester and may require hospitalization occasionally. Symptoms usually start before the 9th week of gestation (wg) and disappear by the 20th wg [5,6,8,10]. HG should be established as exclusion diagnostic criteria in most cases. However, the evaluation of HG by exclusion and evaluation for other causes is warranted in atypical cases, especially if the vomiting begins or persists during the 2nd trimester. HG is not considered a true liver disease, but it is associated to abnormal LFTs in approximately half of patients [5,6,9,11,18]. Clinical signs lead to dehydration and increased renal values, electrolyte abnormalities, metabolic alkalosis, and erythrocytosis. The most common abnormal LFT result is elevation of amino-transferases in serum, up to 200U/L. Other biochemical abnormalities, such as increased serum amylase and lipase values may also be observed [5,15,16]. In addition, an abdominal ultrasound must demonstrate normal liver parenchyma without biliary obstruction. However, an obstetrical ultrasound is mandatory because HG may be associated with multiple and molar pregnancies. Increased body mass index, pre-existing diabetes, asthma, psychiatric illness, hyperthyroid disorders in a previous pregnancy or a previous pregnancy with HG, have also been shown to be risk factors for this disease [6,7,9,18].
The major etiologies proposed for the development of HG are: (I) High levels of human chorionic gonadotropin (HCG) exert a stimulating effect on the secretory process in the upper gastrointestinal tract; the production of thyroid-binding globulin also increases under estrogen stimulation, leading to a decrease in free thyroxine (T4). The transient decrease in the free T4 level stimulates the thyroid where the patient may develop gestational transient thyrotoxicosis, leading to vomiting. (II) HCG is similar to thyroid-stimulating hormone (TSH) and may cause hyperemesis by stimulating the TSH receptor. (III) There is a negative relationship between levels of prolactin and nausea/vomiting, and a positive relationship with estrogens. Thus, higher levels of estrogens during pregnancy can raise the risk of HG. The cause of liver dysfunction is unclear but is usually resolved when vomiting decreases [7,9].
No single therapy has been found to be significantly beneficial for HG patients. The medical approach is based on correcting electrolyte levels and preventing dehydration [23]. Sometimes patients required hospitalization requiring a complex management including vitamins, parenteral nutrition and general support medical control. If treated early, HG is usually not associated with any major adverse maternal or fetal outcome. Few studies have shown an increased incidence of low birth-weight and prematurity in the babies of these patients. No specific treatment is required for liver dysfunction and liver failure is only rarely reported [7,9,18,19].
2.2Intrahepatic cholestasis of pregnancyICP, also known as obstetric cholestasis, is characterized by cholestasis and pruritus, with onset in the late second and third trimester of pregnancy. It is associated with abnormal liver function in the absence of other liver diseases and is resolved completely after delivery. Incidence of ICP varies with geographical location and ethnicity, in 3–5% of pregnant women in Chile, 1% in Europe, 0.7% in the UK 0.3–1.0% in USA, and is rarely reported in African countries [3,4,20–22]. Seasonal variations indicate a higher incidence in the winter months in some countries [20,21]. Risk factors include advanced maternal age, a history of cholestasis secondary to oral contraceptives, personal or family history of ICP, multiple pregnancy and fertility treatment. Some studies suggest a higher prevalence in patients with hepatitis C, cholelithiasis and non-alcohol fatty liver disease (NAFLD) [8,22,23].
A typical symptom of ICP is intense pruritus, commonly localized on palms and soles, which progresses during pregnancy. Typical laboratory findings include elevated serum bile acid levels >10μmol/L (most complications occur with levels >40μmol/L) and increased activity of liver amino-transferases. Increased serum bilirubin levels have been observed in a small number of cases, while jaundice occurs in <25% of affected women and always appears after the onset of pruritus. If jaundice is the initial symptom, further evaluation is necessary [8,24]. The serum autotaxin, a lysophospholipase D, essential for angiogenesis and neuronal development during embryogenesis, was found to be a highly sensitive and specific diagnostic marker that distinguishes ICP from other pruritic disorders of pregnancy and pregnancy-related liver diseases [16,25]. Ultrasonography should be performed to exclude cholelithiasis [8,22,26].
The etiology of ICP is multifactorial, and involves genetic, hormonal, and environmental factors [21,22,24]. Estrogens and progesterone metabolites have been involved in the pathogenesis of ICP. Cholestasis, in women using oral contraceptives with high estrogen content, is similar to ICP. A high level of estrogen, in genetically predisposed individuals, may induce ICP by impaired sulfation and the transport of bile acids. Genetic predisposition in ICP has been suggested by family clustering, presence of ethnic and geographic variations, and by variants in genes coding for hepatobiliary transport proteins. Genetic predisposition may alter the bile ducts and hepatocytes cell membrane composition, as well as produce a dysfunction of biliary canalicular transporters. Variants in the genes MDR3/ABCB4, ATP8B1/FIC1, and BSEP/ABCB11 have been found in patients with ICP [20,21,24–26].
Class III multidrug resistance P-glycoproteins (MDR3/ABCB4) are canalicular phospholipid translocators that partake in biliary phosphatidylcholine excretion. ABCB4 variants with subsequent loss of MDR3 protein are associated to low levels of phospholipids in bile and a high biliary cholesterol saturation index [27]. The bile salt export pump (BSEP/ABCB11) is a member of the ATP-binding cassette superfamily and the major transporter for bile salt secretion from hepatocytes into bile in humans. High gamma glutamyl transpeptidase (γ-glutamyl-transferase, GGT) levels are present in the majority of ICP subjects with MDR3 mutations, while BSEP mutations are proposed in low GGT cases. Combined genetic variants of MDR3 and BSEP may be associated with severe phenotypic expression of ICP [24–28].
Genetic variations in ATP8B1, which encodes for phosphatidylserine flippase FIC1, have been identified in a small number of ICP patients. Other bile acid transporters such as OATP1A2, OATP1B1, and OATP1B3 expressed in placental tissues, were also found to be down-regulated in ICP. Placental gene expression profiles in ICP women have also revealed that the core regulatory genes where involved in the immune response, the vascular endothelial growth factor (VEGF) signaling pathway, and G-protein coupled receptor signaling, suggesting an essential role of the immune response and angiogenesis in the pathophysiology of ICP [29,30].
GABRA2 gene upregulation in ICP cases has led to propose, the GABA system also has an active role in its pathophysiology. Additionally, single nucleotide variants (SNVs) for the xenobiotic receptor for pregnane X, encoded by NR1I2, were identified in South American women. Bile acid homeostasis and transport in hepatocytes were found to be tightly regulated by the nuclear hormone receptor, farnesoid X receptor, encoded by NR1H4. Four rare heterozygous SNVs in farnesoid X receptor have been described in ICP. The variants in NR1H4 may possibly be implicated via downregulation of BSEP expression [24–28,31]; and during recent years increasingly ICP is recognized to be associated with an abnormal metabolic profile, including glucose intolerance and dyslipidemia, although it is considered to be secondary to maternal aberrant BA homeostasis (reviewed in [32]). However, frequencies and types of genetic variants in Latin American and Mexican population have not been described and need further investigation.
Importantly, for ICP, the first-line therapy is ursodeoxycholic acid (UDCA), which results in improved maternal symptoms and biochemistry in 75% of cases. UDCA increases the expression of bile salt export pumps and increases placental bile transporters. UDCA improves clinical symptoms, predominantly pruritus, and may even reduce the risk of premature birth. It is more effective than cholestyramine or dexamethasone in controlling pruritus. Fat malabsorption can result in fat-soluble vitamin deficiencies. Some women do not respond to UDCA. In these cases combining treatment with rifampicin can improve the symptoms and biochemical results [22,33–35]. ICP usually resolves within six weeks after delivery. Maternal outcomes are excellent, however, there is a risk of fetal distress, preterm labor, stillbirth, or even intrauterine death by fetal asphyxia. Delivery at 37wg should be considered because intrauterine death is more common in the last month of pregnancy, and fewer deaths before 37wg. Decisions should be tailored to each pregnancy [8,16,22,33].
ICP may also be associated with an abnormal metabolic profile in afflicted women, and an especially higher prevalence of dyslipidemia, impaired glucose tolerance, and maternal comorbidities (e.g., gestational diabetes and PE). ICP has a high recurrence rate. ICP affected women also have an increased risk of hepatobiliary disease later in life, most commonly gallstones, hepatobiliary malignancies and immune mediated and cardiovascular diseases. If women have ongoing symptoms or biochemical hepatic impairment for more than three months, postpartum an alternative/additional diagnosis should be sought [8,16,32,33].
2.3Acute fatty liver of pregnancyAFLP is a rare but highly threatening hepatic disease occurring during the last trimester of pregnancy, that generally begins between 30 and 38wg [5] although it can appear at early post-partum [13,36]. Prospective and retrospective studies suggest an incidence ranging from 1/7000 to 1/20,000 pregnancies [1,13,17]. This pathology is characterized by microvesicular steatosis in the hepatocytes of zone 3 (centrolobular), rapid loss of liver function, jaundice, and coagulopathy requiring maternal supportive care [1]. Delivery is necessary to assure maternal survival [17,37].
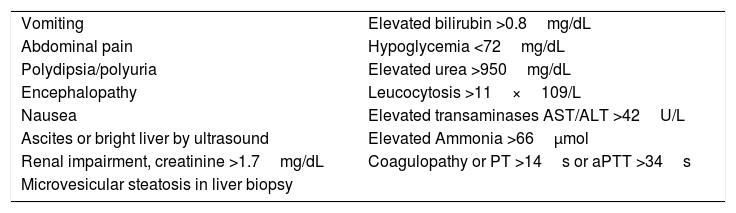
Early diagnosis is also crucial in order to save maternal and fetal lives. Liver biopsy is useful to confirm AFLP diagnosis, but has been suggested as nonessential. Clinical evaluation with at least six Swansea criteria (Table 3) is considered sufficient to accurately diagnose the disease [1,38–40]. These criteria include unspecific symptoms starting during the third trimester such as nausea, vomiting, jaundice, malaise, and anorexia, characteristic with higher levels of transaminases and bilirubins in a short period of time. Coagulopathies, including disseminated intravascular coagulation (DIC) and hemolysis, with increased lactic dehydrogenase (LDH) are frequently observed. These women often have hypoglycemia [5]. Onset of symptoms is also a good predictor to suggest AFLP; other hepatopathies in pregnancy often share some of these symptoms but at different timing during gestation (Table 2) [41]. Differential diagnosis has to be established by ruling out PE and HELLP syndrome, and having at least six of the Swansea criteria [17].
Swansea criteria for AFLP diagnosis [1,6,15,17].
| Vomiting | Elevated bilirubin >0.8mg/dL |
| Abdominal pain | Hypoglycemia <72mg/dL |
| Polydipsia/polyuria | Elevated urea >950mg/dL |
| Encephalopathy | Leucocytosis >11×109/L |
| Nausea | Elevated transaminases AST/ALT >42U/L |
| Ascites or bright liver by ultrasound | Elevated Ammonia >66μmol |
| Renal impairment, creatinine >1.7mg/dL | Coagulopathy or PT >14s or aPTT >34s |
| Microvesicular steatosis in liver biopsy |
ALT: alanine transaminase; AST: aspartate transaminase; PT: prothrombine time; aPTT: activated partial thromboplastin time.
Different risk factors for AFLP have been identified, including nullyparity, male offspring and twin pregnancies [5]. AFLP etiology is not clear, however an association with defects in mitochondrial enzyme function involved in fatty acid β-oxidation has been identified. An elevated frequency of AFLP was detected in carriers of autosomal recessive inherited mitochondrial metabolic disorders by long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD) and mitochondrial trifunctional protein (TFP) deficiencies. TFP is a mitochondrial inner membrane bound protein, which possesses three enzyme activities (long-chain enoyl-CoA dehydratase, LCHAD, and long-chain ketoacyl-CoA thiolase) and catalyzes dehydrogenation of 3-hydroxyacyl-CoA compounds with a chain length of 12–18 carbons. The HADHA and HADHB genes, localized in 2p23.3, encode the alpha and beta subunits of the mitochondrial trifunctional protein, respectively. The heterocomplex contains four alpha and four beta subunits, and catalyzes the three last steps in mitochondrial β-oxidation of long chain fatty acids. The alpha subunit harbors the LCHAD and enoyl-CoA hydratase activities [42–46].
AFLP has been found more frequently in pregnancies of LCHAD deficient fetuses (up to 79% of afflicted pregnancies) [46,47]. The predominant mutation reported in the HADHA gene is found at exon 15, c.1658G>C, pGlu510Gln (rs137852769), according to GRCh38 (previously reported as 1528G>C). This change results in a loss or dramatic decrease in LCHAD activity with normal enoyl-CoA hydratase activity [46–48].
Fetal LCHAD deficiency produces accumulation of 3-hydroxy fatty acids, such as 3-hydroxy myristic acid, 3-hydroxy palmitic acid and 3-hydroxy dicarboxylic acid in the placenta, since the fetal part of placenta is identical to the genetic makeup of the fetus. Increased accumulation of placental free fatty acids and 3-hydroxy fatty acyl-CoA causes oxidative stress, mitochondrial dysfunction and placental lipotoxicity. Further, lipolysis induced in the third trimester of pregnancy would also trigger the accumulation of fatty acid intermediates. Finally, these are shunted from the placenta to maternal circulation, where they can promote oxidative and nitrosative stress, inducing catastrophic acute maternal liver injury resulting in microvesicular steatosis, hepatic mitochondrial dysfunction and hepatocyte lipo-apoptosis. Additionally, LCHAD-deficient patients also accumulate 3-hydroxy fatty acids and develop mental retardation, developmental disabilities, ocular abnormalities and sudden infant death [46].
Maternal and fetal demise in AFLP are predicted to be 10% and 45%, respectively. As soon as the AFLP diagnosis is confirmed, maternal stabilization should be followed by delivery regardless of the gestational age. Initial recovery is observed in most patients after terminating pregnancy. However, treatment of coagulopathy and other complications may continue for days or weeks after the pregnancy has ended [14,16,33,46,47]. Monitoring subsequent pregnancies is recommended since a risk of recurrence exists particularly in cases with defects of fatty acid-β-oxidation [16,41,48]. These disorders have an autosomal recessive inheritance pattern and a recurrence risk of 25% exists. The offspring of mothers affected by AFLP should be monitored carefully for manifestations of deficiency of LCHAD, including hypoketotic hypoglycemia and fatty liver. The international recommendation suggests that all women with AFLP and their children should have molecular testing for LCHAD/TFP [16,33,46,48]. Less frequently, fetuses with LCHAD deficiency have been found in mothers with other pregnancy related diseases such as HELLP syndrome or HG [46].
2.4Pre-eclampsia with liver dysfunctionPE is a multiorgan disease process defined by the presence of hypertension (blood pressure (BP) above 140/90mmHg in two consecutive measures after the 20th wg), and proteinuria, or one of the following features when hypertension appears: thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, or cerebral or visual symptoms. PE affects 2–8% of all pregnancies [49]. Eclampsia is considered when pregnant women with pre-eclampsia develop seizures with no other explanation. Pre-eclampsia/eclampsia is a multifactorial multisystemic disorder characterized by an abnormal vascular response to placental growth associated with vasoconstriction, endothelial dysfunction, metabolic changes and increased inflammatory responses [9,50]. Etiology remains still undetermined and involves a complex network factors, mainly including angiogenic. Angiogenic markers have been identified, and might help to confirm the diagnosis of PE in women without hypertension or proteinuria. They include decreased placental growth factor, increased serum soluble endoglin, and increased soluble VEGF receptor [2,49,50]. However, identified biomarkers and risk factors are only modestly predictive [50].
In PE, there is generalized vasospasm, resulting in increased systemic vascular resistance and pressor responses to endogenous vasoconstrictors. Vascular endothelial damage results in platelet and fibrin deposition in sinusoids, causing hepatocellular necrosis and hemorrhage in zone 1. The finding of zone 3 necrosis and hemorrhage is due to shock in severe pre-eclampsia. In mild cases of pre-eclampsia, there is a mild elevation of serum AST/ALT/alkaline phosphatase as well as minor signs of DIC with thrombocytopenia. Jaundice is rare, but if present it is terminal and hemolytic in its etiology, with total serum bilirubin often not exceeding 6mg/dL [8,16,33,49].
Clinical data in affected women include persistent and severe headache with vomiting, peripheral edema, diplopia and blurred vision. Abnormal liver enzymes are present in 20–30% of cases, with mild to moderate elevation of transaminase levels (1.5–5 times greater than normal). Conjugated bilirubin, albumin and PT tend to remain normal Hepatic involvement that consists of hepatic arterial vasospasm and fibrin precipitation within the portal and periportal areas of the liver lobule, may result in lobular ischemia and hepatocyte necrosis (Table 2) [8,33,49,50].
Obstetric complications for PE include intrauterine growth restriction (10–25%), placental abruption, preterm delivery (15–65%) and fetal demise. The maternal liver condition associated with pre-eclampsia/eclampsia does not need specific treatment and medical approach is based on regulation of BP, relief of associated symptoms and prompt treatment of seizures. After 37wg, women with pre-eclampsia should deliver. If there is fetal or maternal worsening in severe cases, delivery should be considered at 24–34wg [6,8,9,16,33,49].
2.5HELLP syndromeThe acronym HELLP stands for hemolysis (with a microangiopathic blood smear), elevated liver enzymes and low platelet count. HELLP syndrome occurs in less than 1% of all pregnancies, but in 20% of pregnancies that are complicated by PE with severe features. HELLP syndrome may occur at term (18%), preterm (53%, including 11% before 27wg), or postpartum (30%). Risk factors include family history of HELLP, and HELLP or pre-eclampsia in previous pregnancies, occurring in 10–20% of patients. Diagnosis is challenging because symptoms can mimic those of other illnesses. Clinicians must consider HELLP syndrome in patients who do not have classic PE symptoms because 12–18% of women with this condition are normotensive and 13% do not have proteinuria. Although HELLP syndrome may be considered a subtype of pre-eclampsia, atypical HELLP syndrome can be diagnosed without meeting BP criteria for PE diagnosis. Evaluation includes a complete blood count and liver transaminase testing (Table 2). A DIC workup (fibrinogen, prothrombin time, partial thromboplastin time) should be ordered for women with abnormal bleeding or a platelet count less than 50×103 per μL (50×109 per L) [6,8,16,33,50].
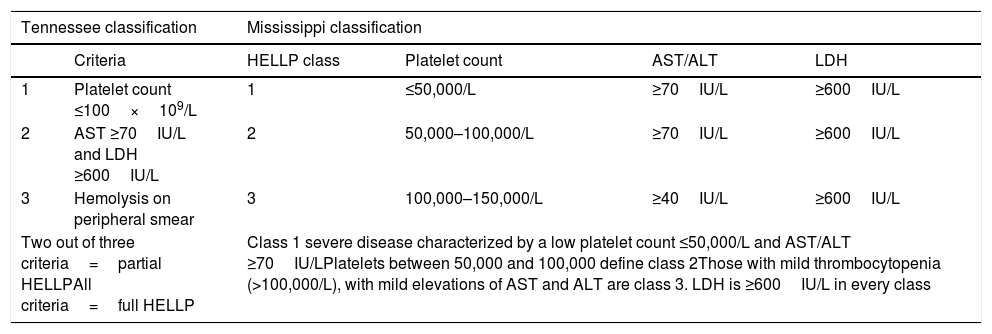
Patients with HELLP may present a plethora of clinical symptoms both in variety and severity, usually between 28 and 36wg. The laboratory findings are significant for intravascular hemolysis, schistocytes on peripheral smear, elevated LFTs (usually ALT), and a low platelet count. All three elements of the HELLP acronym need to be met in a pregnant patient before the diagnosis can be made. Serum aminotransferases are more than ten times elevated, and unconjugated hyperbilirubinemia may be present due to hemolysis Aside from aberrant LFTs, other severe hepatic manifestations have been described with HELLP including hepatic infarction, hemorrhage and rupture. Other serious complications include acute kidney injury, retinal detachment, pulmonary edema, abruption placentae and DIC [6,9,49,50]. Diagnostic criteria such as Tennessee or Mississippi Classifications should be used to diagnose and to grade HELLP syndrome for its predictive value (Table 4) [8,11].
Diagnostic criteria for HELLP syndrome [8,9].
| Tennessee classification | Mississippi classification | ||||
|---|---|---|---|---|---|
| Criteria | HELLP class | Platelet count | AST/ALT | LDH | |
| 1 | Platelet count ≤100×109/L | 1 | ≤50,000/L | ≥70IU/L | ≥600IU/L |
| 2 | AST ≥70IU/L and LDH ≥600IU/L | 2 | 50,000–100,000/L | ≥70IU/L | ≥600IU/L |
| 3 | Hemolysis on peripheral smear | 3 | 100,000–150,000/L | ≥40IU/L | ≥600IU/L |
| Two out of three criteria=partial HELLPAll criteria=full HELLP | Class 1 severe disease characterized by a low platelet count ≤50,000/L and AST/ALT ≥70IU/LPlatelets between 50,000 and 100,000 define class 2Those with mild thrombocytopenia (>100,000/L), with mild elevations of AST and ALT are class 3. LDH is ≥600IU/L in every class | ||||
HELLP: hemolysis, elevated liver enzymes and low platelet count; AST: aspartate aminotransferase; ALT: alanine aminotransferase; LDH: lactate dehydrogenase.
The pathogenesis of HELLP is not well understood but likely involves abnormal development of placental vasculature and new defects in maternal vascular endothelial cells, resulting in poor perfusion to various organs [9,49,50]. More rarely, fetal LCHAD deficiency participates in its pathogenesis [46].
HELLP syndrome carries up to 5% maternal mortality rate compared to fetal mortality rate, which can be as high as 30%. Like in ICP, prematurity remains the most common risk associated with fetal outcome. Treatment of HELLP depends on gestation age. Supportive management with anti-hypertensive medicines, and administration of magnesium sulfate should be considered for the mother. However, early delivery of the fetus remains the cornerstone of a satisfactory outcome. If gestation age is less than 34 weeks, treatment with steroids for 24–48h should be used for fetal maturity before delivery [10].
3Pre-existing and co-incident liver diseases in pregnancyThe pregnancy itself causes significant changes in liver physiological conditions; these effects can be exacerbated in women with pre-existing liver disease or with co-occurring common liver diseases [8,11]. For these conditions, there are concerns regarding not only the disease itself but also the toxicity of the medication used for treatment in both mother and fetus. Because of these concerns, the United States Food and Drug Administration has classified medication into categories according to the benefits of usage and the risks of side effects. Appropriate therapeutic interventions must be performed on the basis of these data. Yet, even taking this information into consideration, present management of pregnancy with some of these diseases, such as liver cirrhosis, is still difficult [11].
3.1Acute viral hepatitisViral hepatitis due to hepatitis A, B, C, D, and E, and herpes simplex, cytomegalovirus, and Epstein Barr virus accounts for 40% of cases of jaundice in pregnant women in the western world. Most cases of acute viral hepatitis are subclinical and anicteric. Clinical presentation is similar in all with nausea, vomiting, headache, malaise, and subsequent development of jaundice. Viruses may not be hepatotoxic however, and here the immunological response is the cause of hepatocellular necrosis. All cases of hepatitis A will recover completely, and so will most cases of hepatitis B. However, complete clinical and biochemical recovery will be seen only in a small proportion of cases of hepatitis C [2,11,15].
A recent meta-analysis demonstrated an increased risk of ICP among HCV-infected pregnant women, and also an increase in the risk of later HCV infection among ICP patients. Physicians should be aware of this association, and testing for hepatitis C in all women with signs of ICP has been suggested [23].
Several studies in developing countries have shown excess mortality in pregnant women with acute HEV-infection. Mortality ranges from 20% to 25% and usually occurs in the third trimester. The excess mortality is restricted to HEV genotype 1 and 2. Possible explanations for the severer course in pregnant women with HEV infection are hormonal, genetic and immunological changes during pregnancy. It has been suggested that a reduced expression of the progesterone receptor or a mutation of the human methylenetetrahydrofolate reductase (MTHFR) gene might be associated with development of fulminant hepatitis E in pregnant women [51].
Pregnant women are more susceptible to Herpes simplex viral hepatitis than the general population. The course of the disease is usually severe and fulminant, with high maternal mortality. Herpes simplex viral hepatitis leads to anicteric hepatitis with normal levels of bilirubin in the presence of severe derangement in LFTs. Mucocutaneous manifestation of herpes simplex infection occurs in only 50% of cases. Employing a high degree of suspicion may be lifesaving, as treatment with intravenous acyclovir is effective [15].
3.2Chronic viral hepatitisHepatitis caused by hepatitis A, B, C, D, and E viruses, cytomegalovirus, herpes simplex virus, and Epstein–Barr virus can be regarded as exacerbations of chronic hepatitis. In many developed countries, pregnant women are routinely screened for hepatitis B virus (HBV) at the initial booking visit. HBV vaccines can be given safely during pregnancy if needed. Women who are not cirrhotic but HBV-positive are at risk of transmitting the virus to the fetus. Vertical transmission remains the most common way of transmission of HBV in endemic areas and accounts for most HBV infection worldwide. Chronic HBV infection is more likely in the newborn infant when the mother is positive to both hepatitis B surface antigen and hepatitis B antigen, and also has a high HBV viral load. HBV viral load is a key factor in transmission, with high viral load being associated to 80–90% risk of transmission, compared to 10–30% transmission rates in patients with undetectable viral load. Transmission can occur directly via the placenta, during breastfeeding, or during delivery. Mode of delivery does not affect the risk of transmission, with similar rates seen in normal vaginal delivery and cesarean section. Transmission can be reduced further by administration of hepatitis B immunoglobulin to the neonate within 12h of birth. HBV vaccine should also be administered with three doses to the infant during the first six months after birth [2,11].
Antiviral treatments can be considered for the mother, however, entecavir, lamivudine, adefovir, and interferon are drugs that carry some adverse effects and ribavirin, is contraindicated due to its teratogenicity. If anti-hepatitis B virus treatment is necessary before delivery, telbivudine and tenofovir should be considered since they are classified as more secure drugs [11].
Pregnancy in patients with HCV is usually uneventful. Risk of vertical transmission of HCV remains low, except when the fetus is exposed to large volumes of mother's blood and vaginal fluid during delivery or if the mother is co-infected with HIV. Patients with genotypes 1 or 3 and with HIV co-infection are more likely to transmit HCV vertically. Data on direct-acting antivirals during pregnancy in humans is lacking. Ribavirin is teratogenic and the use of pegylated interferon in combination with ribavirin is contra-indicated during pregnancy, but not during breastfeeding [2].
3.3Budd–Chiari syndromeBCS is defined as outflow obstruction of the hepatic veins and is sometimes associated with myeloproliferative disorders. Up to 20% of cases of BCS occur in women who have taken oral contraceptives, are pregnant, or have delivered in the previous two months. As pregnancy alone represents a prothrombotic state; a physiological decrease of protein S concentration may account for an increased incidence of BCS during pregnancy. In these patients there is a risk for an exacerbation of the condition during pregnancy due to the increased concentrations of female sex hormones. Clinical features include right upper quadrant pain, jaundice, and ascites. Doppler ultrasound is very important for diagnosis. Treatment involves anticoagulation at onset, identification of procoagulant causes, and shunting, or liver transplantation in extreme cases [2,11,52].
3.4Cirrhosis and portal hypertensionHistorically, women with cirrhosis have had reduced fertility due to hormonal and metabolic changes leading to anovulation. As care of patients with liver disease has improved, fertility has increased, leading to more pregnancies in women with cirrhosis. In cirrhosis, increased portal blood flow and intrahepatic resistance cause increased pressure in the portal vein leading to portal hypertension and subsequent formation of esophageal varices. Nonetheless, pregnancy is achieved by many, and even successfully completed in those with well-compensated disease and mild portal hypertension [2,11,53].
Fetal loss, and maternal morbidity and mortality remain high in pregnant women with cirrhosis. The most common complication is variceal bleeding seen in a third to half of affected women. It usually occurs from varices near the gastroesophageal junction, especially during the second trimester and the second stage of labor, due to increased portal hypertension. All women with cirrhosis and portal hypertension planning to conceive should undergo variceal screening and banding of varices before pregnancy. Additionally, those with no varices before pregnancy should undergo endoscopy during the second trimester. If varices are found, these should be managed with beta-blocker therapy. Acute variceal bleeding is managed with endoscopic band ligation. Sclerotherapy is avoided because there is a potential risk of injecting sclerotherapeutic chemicals. If endoscopy is not available, balloon temponade can be lifesaving. Vasopressin is contraindicated during pregnancy. Other complications are hepatic decompensation with worsening synthetic liver functions, jaundice, thrombocytopenia, ascites, and rarely, rupture of splenic aneurysms. The best route of delivery is unknown. However, those with large varices are best delivered by cesarean section [2,11,53].
3.5Wilson's diseaseWD is a rare autosomal recessive hereditary disease with defective biliary copper excretion, and it leads to copper deposition in the liver, brain, and kidneys. Clinical symptoms vary widely, from asymptomatic to acute or chronic liver disease. Patients usually present hyperbilirubinaemia, high concentrations of aminotransferases, Coombs-negative hemolytic anemia, and low serum alkaline phosphatase. The cause is the malfunction of p-type ATPase ATP7B, essential for copper transport across cellular membranes. In pregnant women with undiagnosed WD, evidence of acute liver failure and hemolysis could be misinterpreted as HELLP syndrome. However, in women with known WD, serum copper levels and caeruloplasmin can rise during pregnancy without treatment, leading to a flare in symptoms. Pregnant women with WD should continue treatment with penicillamine, trientine, or zinc, with dose reduction when possible, without causing harm to the fetus. Patients should be monitored closely for hepatic and neurological symptoms [2,54].
3.6Autoimmune liver diseasesPregnant women with an autoimmune liver disease require continuous management with steroids and immunosuppressive agents. Successful pregnancies may be achieved in women with autoimmune hepatitis. Some studies have shown that flares in disease activity are more likely to occur in the first three months after delivery; although they can appear for the first time during pregnancy. If flares occur, pregnant women with autoimmune hepatitis should be managed in the conventional manner with administration of steroids or an increase in steroid dose. If an immunosuppressant is required, azathioprine remains the safest choice. Although azathioprine is teratogenic in animal models, teratogenic effects in humans have not been described. However, fetal side-effects have been reported, and include lymphopenia, hypogammaglobulinemia, and thymic hypoplasia [2,55].
3.7Liver transplantationYoung patients who have been successfully grafted can become pregnant. Pregnancy should be deferred for at least one year after transplantation for lower doses of immunosuppression and more stable graft function. Pregnancy should be managed in specialized centers. About 70% of women with liver transplantation will deliver a healthy baby. However complications during pregnancy may arise, such as an increased risk of gestational diabetes and/or pre-eclampsia. Cesarean deliveries are preferred in this group. The use of drugs such as tacrolimus, mycophenalate mofetil, prednisolone, azathioprine, and cyclosporine are common; although a risk of teratogenicity exists. Prematurity and low birth-weights (<2500g) occur more frequently in these pregnancies [2,56].
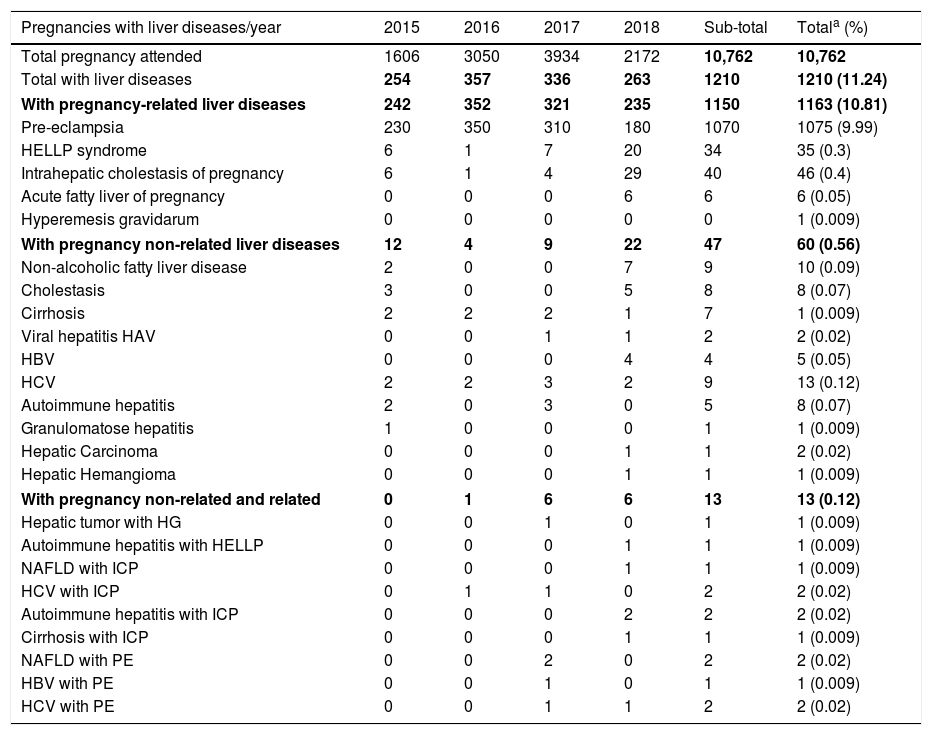
4Epidemiology of liver diseases in pregnancy at the INPERThere is little information about the epidemiology of hepatic diseases in pregnancy in the Latin American population, including our country [57–63]; thus, we reviewed the data base of the INPER, a tertiary referral hospital of Mexico City, to determine the frequencies of hospitalizations related to liver diseases during pregnancy from August 2015 to July 2018. A total of 10,762 pregnancies were attended in the three-year period of the study. The incidence of liver disease observed in our population is summarized in Table 5. Importantly, one of the most relevant findings was the high frequency (11.33%) of liver disease in our population compared to almost 3% reported around the world [1,3,4,63–65]. This may be due to the fact that our hospital is a concentration center for high-risk pregnancies. In 1163 cases (10.81% from the total of pregnancies), a pregnancy related liver disease was detected, including those in thirteen patients with a pre-existing liver pathology (1.12% of the pregnancy liver diseases). PE was the most common entity affecting pregnant women as an isolated entity in 1070 of them, and in five patients with another hepatopathy (9.98% of all pregnancies, and almost 90% of the patients with pregnancy related liver diseases). HELLP syndrome was reported in 34 women (0.32% of pregnancies) whereas ICP occurred in 40 (0.37% of pregnancies), and AFLP was only present in 6 cases (0.06% of all pregnancies). ICP frequency in our sample is lower than reported in Chile and comparable to the US Latin group [63]. No cases of HG were recorded in the period of study, except for one patient who had a hepatic tumor. Hepatopathies coincidental with pregnancy or pre-existing were diagnosed in 60 patients (0.56%); as the disease alone in 47 patients (0.44% of all pregnancies), and in 13 with a pregnancy related liver disease (0.12%). Viral hepatitis, in particular by HCV, and NAFLD were the most common entities in this group of diseases (Table 5). NAFLD is the most common chronic liver disease worldwide. It is the hepatic expression of the metabolic syndrome and shares the risks associated with its components. However, very few studies have addressed the concerns related to pregnancy in NAFLD [16].
Liver diseases in pregnancies attended in the INPER from August 1st 2015 to July 30th 2018.
| Pregnancies with liver diseases/year | 2015 | 2016 | 2017 | 2018 | Sub-total | Totala (%) |
|---|---|---|---|---|---|---|
| Total pregnancy attended | 1606 | 3050 | 3934 | 2172 | 10,762 | 10,762 |
| Total with liver diseases | 254 | 357 | 336 | 263 | 1210 | 1210 (11.24) |
| With pregnancy-related liver diseases | 242 | 352 | 321 | 235 | 1150 | 1163 (10.81) |
| Pre-eclampsia | 230 | 350 | 310 | 180 | 1070 | 1075 (9.99) |
| HELLP syndrome | 6 | 1 | 7 | 20 | 34 | 35 (0.3) |
| Intrahepatic cholestasis of pregnancy | 6 | 1 | 4 | 29 | 40 | 46 (0.4) |
| Acute fatty liver of pregnancy | 0 | 0 | 0 | 6 | 6 | 6 (0.05) |
| Hyperemesis gravidarum | 0 | 0 | 0 | 0 | 0 | 1 (0.009) |
| With pregnancy non-related liver diseases | 12 | 4 | 9 | 22 | 47 | 60 (0.56) |
| Non-alcoholic fatty liver disease | 2 | 0 | 0 | 7 | 9 | 10 (0.09) |
| Cholestasis | 3 | 0 | 0 | 5 | 8 | 8 (0.07) |
| Cirrhosis | 2 | 2 | 2 | 1 | 7 | 1 (0.009) |
| Viral hepatitis HAV | 0 | 0 | 1 | 1 | 2 | 2 (0.02) |
| HBV | 0 | 0 | 0 | 4 | 4 | 5 (0.05) |
| HCV | 2 | 2 | 3 | 2 | 9 | 13 (0.12) |
| Autoimmune hepatitis | 2 | 0 | 3 | 0 | 5 | 8 (0.07) |
| Granulomatose hepatitis | 1 | 0 | 0 | 0 | 1 | 1 (0.009) |
| Hepatic Carcinoma | 0 | 0 | 0 | 1 | 1 | 2 (0.02) |
| Hepatic Hemangioma | 0 | 0 | 0 | 1 | 1 | 1 (0.009) |
| With pregnancy non-related and related | 0 | 1 | 6 | 6 | 13 | 13 (0.12) |
| Hepatic tumor with HG | 0 | 0 | 1 | 0 | 1 | 1 (0.009) |
| Autoimmune hepatitis with HELLP | 0 | 0 | 0 | 1 | 1 | 1 (0.009) |
| NAFLD with ICP | 0 | 0 | 0 | 1 | 1 | 1 (0.009) |
| HCV with ICP | 0 | 1 | 1 | 0 | 2 | 2 (0.02) |
| Autoimmune hepatitis with ICP | 0 | 0 | 0 | 2 | 2 | 2 (0.02) |
| Cirrhosis with ICP | 0 | 0 | 0 | 1 | 1 | 1 (0.009) |
| NAFLD with PE | 0 | 0 | 2 | 0 | 2 | 2 (0.02) |
| HBV with PE | 0 | 0 | 1 | 0 | 1 | 1 (0.009) |
| HCV with PE | 0 | 0 | 1 | 1 | 2 | 2 (0.02) |
The totals include the 13 patients with both pregnancy non-related and related liver diseases. The percentages are calculated for the total of pregnancies attended in the study period. HAV: hepatitis A virus; HBV: hepatitis B virus; HCV: hepatitis C virus; HELLP: hemolysis, elevated liver enzymes, low platelets count; HG: hyperemesis gravidarum; ICP: intrahepatic cholestasis of pregnancy; NAFLD: non-alcoholic fatty liver disease; PE: pre-eclampsia. Data in bold indicated the subtotals of each category.
Any case with HEV was identified in our institution yet the frequency of HEV is particularly high in developing countries [51]. There is a 5.7% reported frequency of HEV exposure in rural pregnant women in Durango, Mexico [66] and a prevalence of HEV of 10.5% in young adults and children of different socioeconomic status from various regions in our country [67,68]. Recently, an elevated rate of G1 Hepatitis E RNA (17%) was detected in samples of children co-infected with HAV, which is endemic in Mexico. This data indicates the need for screening for HEV as a part of future health strategies, as Hepatitis E seems to be a neglected disease in Mexico [69].
5ConclusionsClinical care in pregnant women with hepatic diseases can be challenging. There are physiologic changes in pregnancy that modify liver function diagnosis and clinical follow up. For this reason, liver disease during pregnancy requires early detection for adequate monitoring. Additionally, caring for pregnant women with pre-existing liver disease, such as hepatitis B and C, is difficult to manage and treatment must acknowledge fetal and maternal concerns. Interventions from the hepatologist, gastroenterologist and/or infectologist may be required. Pregnant women may be more susceptible to develop liver disease or increased severity of certain pre-existing liver diseases (e.g., gallbladder disease/cholelithiasis). These liver diseases and those that are pregnancy-related, such as HELLP syndrome, ICP, and AFLP, may represent a significant burden to the health care system because of the increased risk of complications developing in mothers and infants. The data related to liver disease frequencies in Mexican population indicated similar patterns to those observed in other countries; however, a total frequency of 11.3% of liver disease during pregnancy was observed in contrast to 3% reported worldwide. The need to further explore liver disease in our pregnant population should be considered. These analyses may serve as a baseline for future studies that examine trends in the prevalence and management of these conditions, both in Mexican and Latin American communities and in contrast to other countries. In this sense, they can also help plan future health care policies.AbbreviationsICP
intrahepatic cholestasis of pregnancy
AFLPacute fatty liver of pregnancy
HELLPhemolysis, elevated liver enzymes and low platelets
PEpre-eclampsia
HGhyperemesis gravidarum
BCDBudd–Chiary syndrome
WDWilson's disease
LFTsliver functional tests
PTprothrombin time
T4thyroxine
TSHthyroid stimulating hormone
NAFLDnon-alcohol fatty liver disease
GGTgamma glutamyl transpeptidase
SNVssingle nucleotide variants
VEGFvascular endothelial growth factor
UDCAursodeoxycholic acid
LDHlactic dehydrogenase
LCHADlong-chain 3-hydroxyacyl-CoA dehydrogenase
TFPmitochondrial trifunctional protein
BPblood pressure
ASTaspartate aminotransferase
ALTalanine aminotransferase
HCGchorionic gonadotropin
wgweeks of gestation
DICdisseminated intravascular coagulation
FundingThis work does not received financial assistance.
Author contributionsCarmen Selene García-Romero: Substantial contributions to the conception and design of the study, the acquisition, analysis and interpretation of data, the drafting of the manuscript and critical revision at important stages, and approval of the final version to be submitted for publication.
Carolina Guzman: Substantial contributions to the conception and design of the study, the acquisition, analysis and interpretation of data, the drafting of the manuscript and critical revision at important stages, and approval of the final version to be submitted for publication.
Marco Cerbón: Design of the study, the acquisition, analysis and interpretation of data, the drafting of the manuscript and critical revision at important stages, approval of the final version to be submitted for publication.
Alicia Cervantes: Design of the study, the acquisition, analysis and interpretation of data, the drafting of the manuscript and critical revision at important stages, approval of the final version to be submitted for publication, and assumption of the responsibility of investigating and resolving all questions related to the accuracy and integrity of the manuscript.
Conflicts of interestThe authors have no potential conflicts of interest to declare.