Little is known about the etiology of acute liver failure (ALF) in Latin America. The objective of this paper is to investigate the main etiologies of ALF in Brazil, including Drug Induced Liver Injury (DILI) using stringent causality criteria.
Patients or material and methodsAll the cases of individuals who underwent liver transplantation (LT) in 12 centers in Brazil for ALF were reviewed. When DILI was stated as the cause of ALF, causality criteria were applied on site by the main investigator in order to rule out other etiologies.
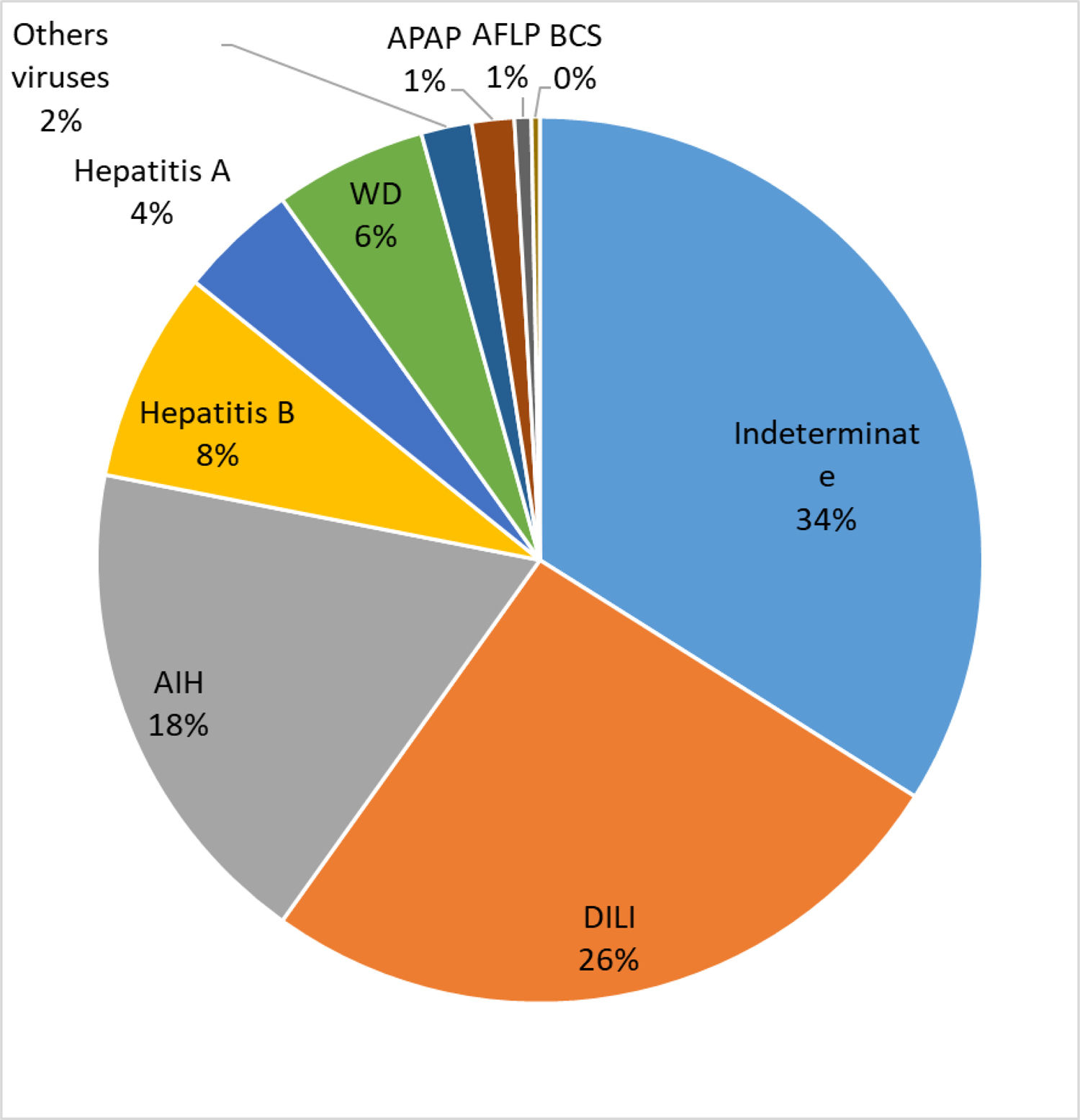
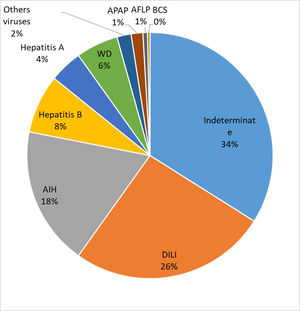
Results325 individuals had ALF mainly for unknown reasons (34%), DILI (27%) and AIH (18%). Reassessment of the 89 cases of DILI, using stringent causality criteria, revealed that in only 42 subjects could DILI be confirmed as the cause of ALF. Acetaminophen (APAP) toxicity (n = 3) or DILI due to herbal and dietary supplements (HDS) (n = 2) were not commonly observed.
ConclusionsUndetermined etiology and DILI are the main causes of ALF in Brazil. However, APAP toxicity and DILI due to HDS are mostly uncommon.
Acute liver failure (ALF) is a life-threatening potentially reversible disorder characterized by severe liver damage leading to hepatic encephalopathy (HE) and, within days to a few weeks, to the decease of patients with no previous history of chronic liver disease (CLD) [1]. It can be caused by viral hepatitis, particularly hepatitis A (HAV), B (HBV) and E (HEV) viruses; an intentional or unintentional overdose with acetaminophen (APAP); drug-induced liver injury (DILI); other diseases such as Budd-Chiari syndrome (BCS), malignancy, HELLP syndrome and acute fatty liver of pregnancy (AFLP) [1–3]. Some CLD such as autoimmune hepatitis (AIH) and Wilson disease (WD) may present ALF without any previous warning sign of liver disease and are also included in the clinical spectrum of ALF 3]. Undetermined etiology is responsible for 17%-38% of the cases of ALF [1].
There is a significant geographic heterogeneity with respect to the etiology of ALF worldwide [1]. APAP overdose is the most common cause in United States of America (USA) and United Kingdom, whereas HBV and HEV infections are the main causes of ALF in Asia and Africa [1,4]. APAP toxicity is responsible for 39%-57% of the cases of ALF in the USA and the UK, while DILI is responsible for 11%-14% of the cases of ALF usually ascribed to the use of antibiotics, herbal and dietary supplements (HDS), cardiovascular and central nervous system agents in the USA and HDS and traditional chinese medicine (TCM) and antituberculous (anti-TB) drugs in China [4–6]. Undetermined etiology is reported more frequently in Africa and Asia compared to North America and Europe.
Little is known about the epidemiology of ALF in Latin America [7]. According to an article, the most common etiologies reported were undetermined, AIH and HBV infection. Few cases were attributed to an APAP overdose or DILI and none to HDS.
There is increasing concern regarding the emergence of HDS as an important cause of ALF around the world, due to its widespread use by the general population. Recent studies have reported that 4% to 20% of all cases of DILI in Europe and USA and 26% of those in China can be ascribed nowadays to HDS [5,6,8]. The role of HDS in DILI leading to ALF in other parts of the world is currently unknown.
The purpose of the present study is to investigate the main causes of ALF leading to liver transplantation (LT) in Brazil as well as to investigate the role of APAP toxicity and DILI in the development of ALF using stringent criteria.
2Patients and methodsAll liver transplant centers in Brazil, which had performed more than 100 surgical procedures in the last 5 years, according to the Brazilian Transplant Registry, were invited to take part in the study that was sponsored by the Brazilian Society of Hepatology. After participation agreement, each center answered a survey drawn up by the principal investigator (PI), concerning the number of LTs performed and etiology of all the cases of ALF reported by each center. Etiology of ALF was determined by the local investigator. All cases which had ALF as defined by Trey and Davidson and met prioritization criteria according to Clichy or Kings College Hospital to be eligible for LT, according to the Brazilian policy regarding organ transplantation at that time [9–11], were included in the study. All cases suspected of DILI and APAP overdose were further evaluated by the central PI, who visited each LT center to review patient files and laboratory and explant pathology results in order to confirm DILI or HDS as the cause of ALF. The causality assessment was performed according to established international criteria, according to the Roussel Uclaf Causality Assessment Method (RUCAM) for evaluating the likelihood that a medication is the subjacent cause of DILI [12].
Briefly, the presence of at least three of the first four parameters, including time from drug intake and withdrawal to the apparent onset of the reaction, course of reaction after cessation of the drug and exclusion by detailed investigation of alternate etiologies had to be present in order to ascertain the occurrence of drug-related ALF. Due to the nature of the present investigation, positive rechallenge response was not taken into account. This study was approved by the Ethics Committee of the University Hospital of Bahia, Brazil. Data in the text and tables are expressed as means and standard deviation.
3ResultsTwelve LT centers in different parts of the country agreed to take part in the study. They had performed 6030 LT procedures in adult and pediatric patients between January 1, 2006 and October 31, 2015. Three hundred and twenty five patients (5.4%), including 297 adults and 28 children, underwent transplants due to ALF.
The etiology of ALF in these patients is presented in Fig. 1. The majority of the cases were undetermined (IND-ALF) (34%), either DILI (DILI-ALF) or APAP toxicity (27%) and AIH (AIH-ALF) (18%) ALF. DILI was the most common cause of ALF in certain states of Brazil, particularly the Southern and Southeastern.
Further investigation of these 89 subjects with presumed ALF due to DILI (n = 84) or APAP toxicity (n = 5) by the central PI revealed that only 42 (47%), 40 adults and 2 children, had clear-cut criteria for DILI after a detailed review of patient files, laboratory and histology data. Clinical and laboratory data of these patients (37 females, mean age 35 + 15 years) are shown in Table 1. Hepatocellular liver injury (76%) was more prevalent, as expected. Nineteen (45%) patients survived more than one year after LT. Most of the deaths (n = 23) occurred within the first eight days after transplantation.
Clinical and laboratory features of those patients with drug-related DILI leading to ALF (n = 42).
| Age (years) | 35 + 15 |
| Female sex | 37 (86%) |
| Body mass index (kg/cm³) | 25 + 5 |
| INR | 4,6 + 2,9 |
| Total bilirubin (mg/dL) | 22 + 16 |
| Alanine aminotrasferase (U/L) | 1616 + 1960 |
| Aspastate aminotransferase (U/L) | 2425 + 2713 |
| Hepatocellular liver injury | 32 (76%) |
| Mixed hepatocellular and cholestatic liver injury | 10 (24%) |
| Hypersensitivity featuresa | 4 (10%) |
| 1-year Survival | 19 (45%) |
ULN: upper limit of normal; INR: international normalized ratio.
The most common causes of drug-related ALF were: anti-TB drugs (21%), including isoniazid, rifampicin and pyrazinamide combination and non-steroidal anti-inflammatory drugs (21%), including diclofenac (n = 5), nimesulide (n = 3) and parocoxib (n = 1); antibiotics (19%), including sulfamethoxazol and trimethoprim combination (n = 2) and one each for ciprofloxacin, amoxicillin, nitrofurantoin, minocycline, imipenem and sulfasalazine; methyltopa (n = 3). Other agents (one each) implicated in DILI-ALF were phenytoin, venlafaxine, duloxetine, carbamazepine, imatinib, methotrexate, propylthiouracil and halothane. HDS was rare due to tea infusions of Bidens pilosa (n = 1) and Ruellia bahiensis (n = 1). APAP toxicity was confirmed in only 3 individuals, however, in only one it was used in a suicide attempt. More than one drug was responsible for ALF in 30% of the cases.
4DiscussionThe present study found out that 5.4% of the LTs performed in the 12 transplantation centers were due to ALF. This corroborates previous research which found that 6% of the indications for LT in Brazil were due to ALF [13]. The main causes for ALF reported by the PI were IND-ALF, DILI-ALF and AIH-ALF, which were much more frequent when compared to ALF due to viral hepatitis and APAP. Surprisingly, HDS were an uncommon trigger for DILI-ALF. IND-ALF was also reported as the most common cause for ALF in Africa, India and Japan [1], which may reflect a lack of resources for adequate laboratory investigation or the presence of unrecognized local triggers for acute liver injury. In this regard, undetermined etiology was shown to be responsible for only 11% of the causes of ALF in USA [4]. Reassessment of 303 North American patients with IND-ALF, with further determination of APAP protein adducts and additional serological and molecular analysis for viruses in stored sera, reassigned a definite etiology for ALF in 47% of those cases, including APAP toxicity (15%), AIH-ALF (11%) and DILI-ALF (8%). It is therefore possible that some of our cases of IND-ALF could be also reassigned to other etiologies if we had employed a similar strategy.
However, more than half of the patients with APAP or DILI-ALF reported by the PI had to be reassigned to undetermined etiology because they did not meet the predefined established causality criteria for DILI after extensive data review. In spite of this, it is important to highlight that some of the subjects could really have had DILI-ALF. This is due to the fact that it is almost impossible to comply with some of the aforementioned criteria in the setting of urgent LT for ALF, because there is often no time to evaluate the course of liver enzyme reactions after drug withdrawal and no feasibility for drug rechallenge.
It is important to highlight that AIH was the third cause of ALF identified in Brazil, but this corroborates previous data showing that the disease affects mainly children and its presentation in Brazil is more severe when compared to North-America [14]. In Argentina, AIH was also one of the leading causes of ALF. It is worth mentioning that, in both countries, pediatric AIH is associated with similar HLA-DRB1 alleles, namely HLA-DRB1*1301 [15].
DILI was the second most frequent etiology for ALF in the present cohort and was commonly associated with anti-TB drugs, NSAIDs and antibiotics but, surprisingly, HDS was associated with ALF in just 3 patients, as well as APAP toxicity. Two compounds, Bidens pilosa and Ruellia bahiensis were associated with DILI-ALF. Hepatotoxicity due to Ruellia bahiensis leading to ALF in the same patients have been previously reported, but not DILI-ALF due to Bidens pilosa [16]. Both are herbal compounds commonly used for tea infusions. However, both etiologies could be responsible for at least some cases of IND-ALF as patients tend not to report the use of HDS because they are generally considered safe by the majority of the population. Our results were also similar to those reported byArgentina, where undetermined etiology, AIH, HBV and DILI were the most common causes of ALF [7]. Similar to our findings, APAP toxicity was rare, reported in only two Argentinian patients. On the other hand, viral hepatitis was not a significant cause of ALF in Brazil, probably due to the decline in the incidence of HAV and HBV infections in the country owing to universal vaccination as well as the rarity of cases of hepatitis E in Brazil according to seroprevalence studies [17–19]. Mortality was higher than expected, probably due to a shortage as well as late prioritization of organs for ALF.
In summary, most of the cases of ALF in Brazil were linked to undetermined etiology, DILI and AIH. Reassessment of the cases of DILI-ALF revealed that more than 50% of these patients did not meet the stringent criteria of causality. AIH is a relevant cause of ALF in the country which is probably related to a more severe disease presentation in Latin America.
Patient consent statementNot required by the Ethics Committee due to the methodology and retrospective nature of the investigation.
Funding statementThe present study has been supported by grands of the Johnson & Johnson Foundation (Number 1321/11). And Maria Emilia Pedreira Freire de Carvalho Foundation, Brazil (CONV 7/2016 Number: 23066015723/16-10).
This funding sources had no involvement in the study design; in the collection analysis, and interpretation of data; in the writing of the report or in the decision to submit the manuscript for publication.
Ethics approval statementThis study was approved by the Ethics Committee of the University Hospital of Bahia, Brazil. CAAE number: 46361615.1.1001.0049.
Conflict of interest disclosureThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Permission to reproduce material from other sourcesNone.