The disbalance between the number of candidates to liver transplant and the number of liver grafts leads to waiting list mortality. Two potential ways of increasing the number of liver grafts are split liver transplantation and the transplantation of grafts from non-heart beating donors. Both of them were discussed in a consensus meeting of the Spanish Society of Liver Transplantation in October 2012. This paper outlines the conclusions of that meeting.
El desequilibrio entre el número de candidatos a trasplante hepático y el número de injertos hepáticos disponibles conduce a la mortalidad en lista de espera para trasplante. Dos posibles fuentes de aumentar el número de injertos hepáticos son la bipartición hepática y los donantes en asistolia. Ambas fueron estudiadas en una reunión de consenso de la Sociedad Española de Trasplante Hepático en octubre de 2012. Este artículo recoge las conclusiones de esta reunión.
The marked imbalance between the growing number of liver transplant candidates on the waiting list and the limited number of available grafts for this procedure is resulting in considerable mortality. Once liver transplant has been established as consolidated therapy, graft sources which are considered unconventional have been explored with the intention of alleviating this mortality, such as split liver transplantation, non-heart beating donor organs (NHBD), live donor transplants and domino transplants. Liver transplant (LT) from a live donor has already been reviewed in the consensus documents of the Spanish Society of Liver Transplantation (SETH) published in 2011.1,2
Split Liver TransplantationSpain is exceptional, in terms of its high rate of donation and LT (the highest in the world in 2011) and also for its very infrequent use of split liver grafts.3 This document will chiefly address a cadaveric split liver graft for a paediatric recipient, who will receive segments II and III, and an adult recipient, who will receive the remaining liver.
It is important to highlight that, in the SETH consensus conference held in 2008,4,5 clear guidelines were recommended to alleviate mortality among paediatric LT candidates on the waiting list, proposing strategies for the distribution of organs in order to promote the development of split liver transplantation; yet these recommendations have not materialised at a national level.
NecessityLT waiting list mortality affects both adult and child candidates. However, this problem is particularly serious for a subgroup of the paediatric population. This is how the transplant team from the University Hospital of La Paz in Madrid6 describes the situation, observing a critical risk for children under one year of age. These children are candidates with few options for complete cadaveric organ transplant. This mortality has never been completely eradicated since transplantation started in Spain and, year after year, a variable number of child candidates die on the waiting list.
Over the past 10 years (2002–2011), the annual average of children dying on the waiting list has been 7.1, with a range of 4–10 as can be observed from the transplant records of the National Transplant Organisation (ONT).7 This implies that if the transplants performed with a very small number of split livers were added to the regular activity of paediatric LT, child mortality on the waiting list would be completely eradicated.
The majority of child candidates in recent years have been transplanted using complete cadaveric grafts, whereas the second graft source for child candidates by order of use, and nationwide, is partial grafts from live donors. Transplants performed using this technique offer excellent outcomes with higher survival than complete cadaveric grafts or split liver grafts.8 While the rate of transplants with partial live donor graft was 23% in Spain between 2004 and 2011, the usage rate of grafts from split livers, was only 14%.7 Even with the aforementioned good outcomes of live donor grafts for child recipients, we should not forget the risk of morbimorbidity for the donor. In this regard, in a recent study recording live donor mortality in the United States, a donor death frequency of 1.7/1000 donations was demonstrated, with 2 cases of death after donation of left lateral segments, even though this type of donation is theoretically less compromising for the donor. This study concludes that the mortality observed for live LT donation is irrespective of the type of graft donated.9
Therefore, it is concluded that there is a need to extend donation for child recipients through the split liver technique with the objective of eliminating paediatric mortality on the LT waiting list.
PotentialSpain has a high rate of donation per million inhabitants and occupies the first place globally. However, the solid organ donor profile has significantly changed compared to earlier periods of transplant activity.3 An older donor population, with cardiovascular disease-related causes of mortality, makes extending transplant using split liver transplantation challenging, as the donors considered for split liver transplantation have to meet strict standards in relation to age, weight, length of time spent in intensive care, absence of cardiac arrest (CRA), vasoactive support, lack of obesity or relevant steatosis, absence of serious hypernatraemia and minimal alteration of liver biochemistry.10–12
Different studies have assessed donation potential, and have estimated varying numbers of donors eligible for split liver transplantation, from 23% to 43% for donors weighing more than 70kg.13
The rate of pancreatic transplantation can be analysed14 in order to reach an approximate calculation of the potential for this resource in Spain, as the level of requirements to evaluate split liver donation potential can be likened to that of pancreatic donation. In Spain over the last 10 years, an average 89.5 pancreatic transplants have been performed annually (and 8.5 split liver transplants); therefore, there are more than 80 optimal donors who could undergo split liver transplantation per year.
The ONT studied the number of donors under 55 years of age, weighing more than 70kg, with a stay in ICU of less than 5 days, and transaminase counts of less than 70 throughout 2009 among a total of 1.431 donors.15 This year 84 donors were recorded with these characteristics and meanwhile 7 children died on the waiting list.
In conclusion, there is sufficient potential to develop the necessary transplant activity using liver grafts from split liver transplantation to eradicate child mortality.
JustificationSplit liver transplantation allows simultaneous LT of 2 patients. Its benefit can be simply quantified by estimating the sum of the years of survival achieved with both grafts.16
Furthermore, different studies show that survival rates of grafts from split liver transplantation are comparable to those achieved with LT performed with complete cadaveric grafts, despite the fact that a greater incidence of vasculobiliary complications are observed.10,17–29
The current national policy for assigning donors does not favour LT using grafts from split liver transplantation, which means that Spain performs very few of these types of transplant (from 0.3% to 2.4% of the LT performed in the past 10 years),7 unlike the situation in other countries where there are lower donation and transplantation rates.30–32
Nonetheless, prioritisation systems have been successfully established which clearly favour split liver transplantation in the Andalusian Community33 and in Catalonia.34 In both cases the mandatory assessment is stipulated of optimal child or adult donors in order for split liver transplantation to be allowed as long as there is a potential child candidate who might benefit from this procedure.
The national centres which are the largest paediatric providers perform transplants on candidates many of whom come from distant geographical areas of Spain, whereas the assignation of grafts for these transplants is arranged via the most restrictive local, area or regional distribution algorithms. This considerably reduces the possibility of performing split liver transplantation. The national burden of paediatric transplantation has to be shared via the donation network which is also national. Agreements such as those made in Andalusia and Catalonia should be extended nationwide.
The abovementioned agreements, established in Catalonia and Andalusia, detail the algorithms for coordination between paediatric and adult LT teams which could also be easily reproducible nationally (technical ex situ or in situ division of vasculo-biliary parts, etc.).
Extending this source of donation would entail minimal impact on the transplantation of adult recipients (4 split liver transplantations during 2011 would have been sufficient for there to have been no paediatric mortality on the waiting list).
RecommendationsEvery donor aged above 15 with suitable characteristics has to be considered divisible for split transplantation irrespective of the centre at which they present, if they fulfil the agreed requirements.
It is recommended that similar policies to those of Andalusia and Catalonia should be implemented which favour split liver transplantation from donors who meet the requirements. Such policies would be taken to a regional (when the recipient reference area of the paediatric hospital is regional) or inter-regional level (when the paediatric recipient reference area covers more than one region). Once these policies have been developed, regional and later national priorities will be considered when assigning these donors.
A register should be made of potential adult extended right liver lobe recipients, chosen by the various transplant teams, who have accepted the procedure by informed consent.
Every effort should be made to standardise criteria (assignation of donor, division of pedicles, recipient selection) for LT with split liver graft.
It is not advisable to assign a split liver graft to a very seriously ill adult recipient (e.g. MELD >24).
Liver Transplantation With Non-Heart-Beating DonorsIntroductionSituation Regarding Non-Heart Beating Donors in SpainIn 2012 there were 161 NHB donors in Spain, representing 10% of the total number of donors. In regions such as Madrid the figure reaches 40%. This has generated a great interest on the part of transplant programmes; currently 17 programmes in 9 autonomous regions have already started NHB donor programmes.35
However, despite there being great potential to increase the number of transplants, only 17 LT were performed from NHB donors in Spain during 2012; a total of 1,084. Twelve of the NHB donors were uncontrolled (u NHBD) and 5 were controlled (c NHBD).35
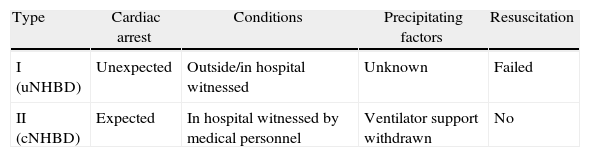
Classification of Non-Heart-Beating DonorsFor practical purposes, non-heart-beating donors can be classified into 2 basic groups, as shown in Table 1. In uNHBD there has been a witnessed cardiac arrest, irreversible despite cardiopulmonary resuscitation (CPR), and they correspond to type 2 NHBD of the Maastricht classification. Ventilator support has been withdrawn from cNHBD which will determine subsequent cardiac arrest and they correspond to type 3 NHBD of the Maastricht classification.36
Proposal for Classification of Non-Heart-Beating Donors (SETH).
| Type | Cardiac arrest | Conditions | Precipitating factors | Resuscitation |
| I (uNHBD) | Unexpected | Outside/in hospital witnessed | Unknown | Failed |
| II (cNHBD) | Expected | In hospital witnessed by medical personnel | Ventilator support withdrawn | No |
cNHBD: controlled non-heart beating donor; uNHBD: uncontrolled non-heart beating donor.
Although, due to ethical reservations, a moratorium was called for on the assessment of organ donation for transplantation after what is termed controlled cardio circulatory death (Maastricht classification type 3 donors36 or the 2011 Madrid amendment37), as reflected in the Spanish Consensus Document38 drawn up by Spanish transplant groups and the National Transplant Organisation in 1995, there are several factors which oblige a review of the current situation. Spain performs the highest number of organ transplantation using type 2 Maastricht39–41 donors, uncontrolled donation after cardio circulatory death, yet LT activity using type 3 Maastricht donors, or donation after controlled cardio circulatory death, has only just started to be developed.42,43
ApplicabilityThis type of transplant which is increasingly common in some European countries has provided results which are comparable to those obtained with the use of donors after brain death (DBD).44,45
Recent changes in the legal framework, in line with draft bill 121/000132 Proyecto de ley reguladora de los derechos de la persona ante el proceso final de la vida (draft bill regulating end-of-life rights),46 could favour the development of this type of donation in the future.
In light of this situation, the ONT has prepared a National Consensus Document2 in direct collaboration with the national transplantation groups, with the intention of laying the bases for this type of donation and promoting its development, undertaking a thorough analysis of all aspects of this type of activity, including the ethical and legal aspects, which initially constituted a major area of debate.
These changes have allowed some Spanish centres to successfully perform their first LT from controlled donation after cardio circulatory death.
The complications which are traditionally associated with this type of donation, such as primary graft failure and ischaemic cholangiopathy, which are hypoxic stress-dependent, appear to have considerably reduced, along with their impact on graft and patient survival. In the most recent series, survival exceeds 80%47–49 in the first year after transplantation and the rate of ischaemic cholangiopathy (a frequent complication which makes retransplantation necessary) has reduced,45 even to below 3%.
The decision to withdraw life support measures for a critical patient has to have been made prior to and independently from the donor request process and the criteria of the intensive care specialists responsible for the patients will be determining factors in the development of this type of donation.37 Nevertheless, the phenomenon of the possible indiscriminate substitution of DBD for this type of donation (which has been witnessed in other countries) is of concern to all transplant practitioners. It is considered an adverse circumstance, as such substitution could lead to a quantitative and qualitative restriction of LT.44,50
In any event, it is advisable that centres undertaking this sort of activity should prepare protocols agreed by the healthcare professionals from the different areas involved in the transplantation, supervised by the relevant ethical committee.37
With regard to tactical action protocols, it is worth noting that the National Consensus Document37 constitutes a reference which describes in detail all the relevant points once the donation has been decided, such as possible actions prior to the withdrawal of measures (heparinisation, cannulation) or maximum donor warm ischaemia times. This document also includes guidelines for the extraction and preservation of organs according to the different methods, either by fast extraction and cold preservation51 or using normothermic recirculation52 prior to cold preservation. Both alternatives are equally valid and therefore shall be chosen according to availability and each hospital's preference.
RecommendationsControlled donation after cardio circulatory death could be considered a useful and applicable source of liver grafts at a national level.
This source of donors must extend organ donation and never enter into competition with DBD.
It is recommended that in-hospital protocols should be promoted and agreed by the different healthcare professionals involved in transplantation activity, with the participation of the hospital's ethical committees, detailing the specific procedures to be followed for this type of donation.
Uncontrolled Non-Heart-Beating DonationUNHBD have several advantages as donors. This is because: (1) CPR has generally been performed outside hospital and not after a prolonged stay in ICU; (2) they can never progress to DBD like some cases of cNHBD and therefore their number will never decrease and (3) they die from natural causes and not due to withdrawal of life support.53 Furthermore, prestigious healthcare organisations such as the USA's Institute of Medicine have highlighted the enormous potential that uNHBD would have to increase the number of transplants. During 2006 it was estimated that at least 22000 people who died as a result of a cardiac arrest outside hospital could have been potential donors.54
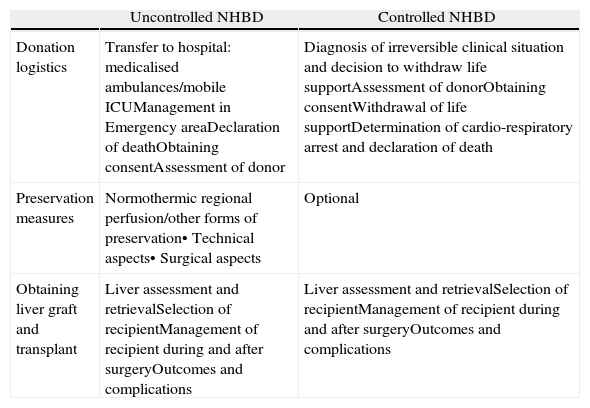
LogisticsThe logistics of NHBD are specific to this context and there are significant differences depending on whether the NHBD are controlled or uncontrolled55,56 (Table 2).
Logistics of the Procedure for Donation and Transplant in the Event of Non-Heart-Beating Donation.
| Uncontrolled NHBD | Controlled NHBD | |
| Donation logistics | Transfer to hospital: medicalised ambulances/mobile ICUManagement in Emergency areaDeclaration of deathObtaining consentAssessment of donor | Diagnosis of irreversible clinical situation and decision to withdraw life supportAssessment of donorObtaining consentWithdrawal of life supportDetermination of cardio-respiratory arrest and declaration of death |
| Preservation measures | Normothermic regional perfusion/other forms of preservation• Technical aspects• Surgical aspects | Optional |
| Obtaining liver graft and transplant | Liver assessment and retrievalSelection of recipientManagement of recipient during and after surgeryOutcomes and complications | Liver assessment and retrievalSelection of recipientManagement of recipient during and after surgeryOutcomes and complications |
The development of specific action protocols in this first NHBD phase does not fall within the direct competence of the experts taking part in this consensus meeting and has been widely discussed in other forums.37 Transplant coordinators, intensive care and out-patient doctors play an essential role in the development of protocols for use inside and outside hospital which could enable a subsequent donation. The ethical and legal aspects must also be known and respected, with the huge variety of laws worldwide, including those of our neighbouring countries, which sometimes prevent the implementation of these types of programmes.57 The legal bases of uNHBD in Spain are included in ley general 30/1979 and in the recent real decreto 1723/2012 which clearly set out the cardio respiratory criteria and conditions for the diagnosis of death on which to base protocols for cNHBD and uNHBD.
Logistics of Uncontrolled Non-Heart-Beating Donation and the Necessary Resources for Their ImplementationAfter CPR, life support measures have been carried out on uNHBD, following the recommendations of competent healthcare authorities such as the European Resuscitation Council.58 Once these measures have been completed, the patient may spontaneously resuscitate and complete their recovery in hospital, or they may not: in the latter scenario it might be possible to activate the donation procedure. The process of death in uNHBD entails, therefore, the initial cessation of spontaneous circulatory and respiratory function, after which advanced CPR is performed by healthcare personnel prior to and during transfer of the patient to hospital. Once in hospital, these measures are continued until it is considered that these functions have irreversibly ceased. A five minute period of observation follows, during which there must be an absence of spontaneous electrocardiographic activity. After this procedure, the diagnosis of the patient's death is made.
The implementation of uNHBD programmes in Spain should take place in areas of population where there is sufficient potential to ensure its viability. Ideally this should be established in metropolitan areas with adequate infrastructure in terms of advanced and intermediate life support units and rapid medical assistance units. Experience gained in Granada, with a minimum population of 600000 inhabitants demonstrates the possibility of obtaining 8 potential liver donors per year. Experience in Catalonia demonstrates that developing specific programmes for non-heart-beating donation produces a significant increase in the number of potential donors over the years. There were 5 potential donors in 2002 which had increased to 83 by 2010 in the Hospital Clinic of Barcelona.41
Activation of the Protocol for Uncontrolled Non-Heart-Beating DonationWhenever a competent expert has made a diagnosis of death, the procedure for non-heart-beating donation can be activated. This procedure starts with taking samples for serology and microbiology, biochemistry and immunology. At this time, the donor can be heparinised, at a dose of 3mg/kg.
Characteristics of Potential Liver Donors in Uncontrolled Non-Heart-Beating DonationThe initial characteristics which the uNHBD must fulfil in order to activate the preservation and assessment protocol are summarised as follows:
- -
Age: 14 (40kg) – 65 years of age
- -
Cardio respiratory Arrest (CRA): witnessed, <15min until establishing CPR measures. Cases of violent death or where there is an indication of criminal activity are excluded.
- -
CPR: total duration <150min until abdominal recirculation is established.
- -
Transfer to hospital: at all times with orotracheal intubation and continuous manual or mechanical CPR. The use of LUCAS-type mechanical compression devices is highly recommended during transfer, as this has been demonstrated to be more effective than manual CPR in ensuring adequate tissue perfusion.59–61
- -
Contraindications: an external appearance indicative of parenteral drug addiction, active haemorrhage from injuries to the chest or abdomen, a history of systemic infections or malignant disease which would contraindicate donation.
4 techniques have been developed to date for preserving the NHBD in order to ensure appropriate preservation of the liver graft for transplantation, prior to its extraction:
- -
In situ perfusion, where perfusion is started using a cold preservation solution through the inguinal vessels. This is an easy and fast technique, but it has shown inferior results in renal transplantation.
- -
Simultaneous chest (mechanical) and abdominal (manual) compressions in order to maintain MAP ≥70mmHg and PaO2 ≥100mmHg.39,62
- -
Hypothermic recirculation, which has mainly been used to maintain cNHBD, with varying results, and high rates of delayed graft function in some renal series, there is little experience in uDCD.63–68
- -
Normothermic recirculation, also currently termed normothermic regional perfusion (NRP), which appears to ensure better immediate functioning of organs after transplant and has become the technique of choice in the preservation of abdominal organs.52,55
Prior to undertaking NRP it is recommended that a Fogarty® catheter is placed to occlude the supracoeliac aorta. This achieves better blood flow to the relevant abdominal organs, in particular the liver. It also avoids restoration of cardiac or cerebral circulation.
A lack of venous return to the circuit making it impossible to perform NRP constitutes the greatest contraindication for uNHBD.41 Depending on the cause of death, there are different factors which may affect this event, such as the presence of internal haemorrhaging, or vascular problems, such as a severe atheromatosis or abdominal aortic aneurysms. In turn, there are technical factors which can determine NRP failure. In this regard, the use of multi-perforated cannulas, placed in a retrohepatic position, centrifugal pumps or removing the reservoir in the circuit are alternatives which might improve the application of this technique. Personnel trained in this type of perfusion with capacity for rapid response when the uNHBD protocol is activated are essential if a programme of this type is to be implemented.
Assessment of the Uncontrolled Non-Heart-Beating Donor During Normothermic Regional PerfusionThe characteristics which NRP must present and which will affect acceptance of the liver graft for transplant are summarised as follows:
- -
Circuit temperature: 37°C
- -
pH: 7.35–7.45
- -
PaO2: 100–150mmHg
- -
Haematocrit: above 20%
- -
Initial AST, ALT: <3 times the upper limit of normal
- -
Final AST, ALT: <4 times the upper limit of normal
- -
Recirculation pump flow: >1.7l/min with Fogarty® in the supracoeliac aorta
- -
Heparinisation: 1.5mg/kg every 90min
- -
Recommended duration: under 4h
In the Hospital Clínic of Barcelona during the period from April 2002 to December 2010, NRP was established using criteria similar to the above in 290 uNHBD and 145 of these were excluded during the course of the NRP. This indicates that assessing the donor during this period is essential in assessing suitability.41
Final Assessment of the Non-Heart-Beating Donor During the Extraction ProcessFinal intraoperative assessment of the graft is very important and especially relevant in these types of donors. For it to be accepted, the liver, the bile duct and the gallbladder must have an appropriate appearance before and after cold perfusion.
Performing a liver biopsy to assess the liver microscopically is not recommended, as this is subject to a wide margin of error in this context and is unlikely to facilitate decision making.69–71
It is recommended that a fast technique is used during the process of liver graft retrieval, and that NRP should be maintained until the preservation solution is introduced in uNHBD cases. The use of preserving solutions which do not contain colloid has been controversial. Although these solutions can improve lavage of the peribiliary arterial tree, in some studies it has been demonstrated that they significantly increase the risk of losing the liver graft from the NHBD.72–74
Intraoperative Management of the Recipient During Liver Transplant From a Non-Heart-Beating DonorThe intraoperative haemodynamic management of a LT recipient from a NHBD has to be particularly careful. It should be no different to that for recipients of an organ from a DBD, but it may require more intense application, given the possibility of haemodynamic instability after reperfusion of the graft. The handling of fluids is particularly important, as it is recommended that a central venous pressure of around 5mmHg should be maintained, and that vasopressor support should be started early, prior to reperfusion of the graft, and renal protection measures should be taken (maintenance of an arterial pressure above 70mmHg and diuresis above 0.5ml/kg/h).
Given the greater risk of severe coagulopathy after reperfusing grafts from uNHBD, the following measures, according to the action protocol of the Hospital Clínic of Barcelona, are recommended: maintain a haemoglobin above 8g/l and haemocrit above 24%, administer tranexamic acid (10mg/kg bolus on induction and 10mg/kg/h up to biliary anastomosis), maintain a platelet count above 50000 and fibrinogen level above 2g/l prior to reperfusion and at the time of closure, administer fresh frozen plasma 15mg/kg if the INR is above 1.7 or the prothrombin time is below 40%, administer factor VIIa in the event of uncontrollable haemorrhage, and packing for 48h for oozing haemorrhage.
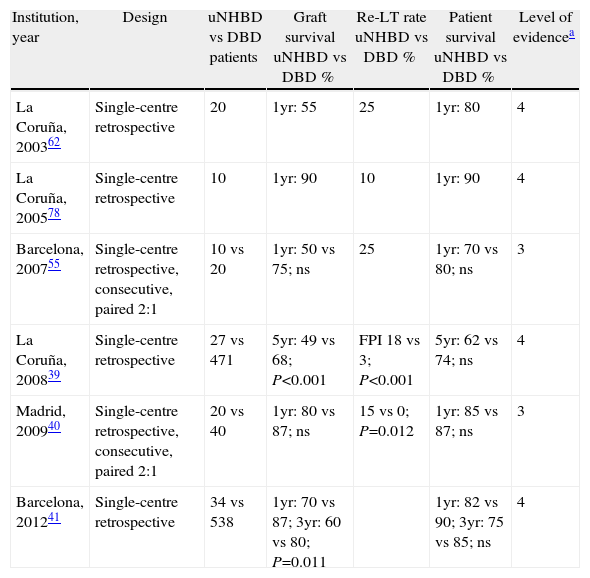
Outcomes and Complications of Liver Transplant With Grafts From Non-Heart-Beating DonorsSurvival After Liver Transplant From Uncontrolled Non-Heart-Beating DonorsSome studies published in Spain compare post transplant outcomes after using grafts from uNHBD and from DBD (Table 3). In a study by the Hospital 12 de Octubre in Madrid, which included 20 uNHBD where a NRP system was used, the survival of the graft and the patient was no different to that achieved in 40 patients transplanted with grafts from DBD.40 In another study by the Hospital Clínic of Barcelona, which included 34 patients transplanted from uNHBD with a median follow-up of 24 months (range 0–111), the survival of the graft after one year was 70%, significantly below that achieved with grafts from DBD which was 87% (P=0.011). However, the number of grafts surviving more than six months after LT was significantly greater in the second half of the series, 15 (88%) compared to those of the first half, 9 (53%) (P=0.024), which suggests a learning curve with the NHBD process and a significant improvement in the survival of the graft over time. Moreover, survival of the patient one year and 5 years after transplant was similar between the uNHBD and the DBD.
Survival of Graft and Patient in Liver Transplant From uNHBD Compared to DBD.
| Institution, year | Design | uNHBD vs DBD patients | Graft survival uNHBD vs DBD % | Re-LT rate uNHBD vs DBD % | Patient survival uNHBD vs DBD % | Level of evidencea |
| La Coruña, 200362 | Single-centre retrospective | 20 | 1yr: 55 | 25 | 1yr: 80 | 4 |
| La Coruña, 200578 | Single-centre retrospective | 10 | 1yr: 90 | 10 | 1yr: 90 | 4 |
| Barcelona, 200755 | Single-centre retrospective, consecutive, paired 2:1 | 10 vs 20 | 1yr: 50 vs 75; ns | 25 | 1yr: 70 vs 80; ns | 3 |
| La Coruña, 200839 | Single-centre retrospective | 27 vs 471 | 5yr: 49 vs 68; P<0.001 | FPI 18 vs 3; P<0.001 | 5yr: 62 vs 74; ns | 4 |
| Madrid, 200940 | Single-centre retrospective, consecutive, paired 2:1 | 20 vs 40 | 1yr: 80 vs 87; ns | 15 vs 0; P=0.012 | 1yr: 85 vs 87; ns | 3 |
| Barcelona, 201241 | Single-centre retrospective | 34 vs 538 | 1yr: 70 vs 87; 3yr: 60 vs 80; P=0.011 | 1yr: 82 vs 90; 3yr: 75 vs 85; ns | 4 |
uNHBD: uncontrolled non-heart-beating donor; DBD: donor after brain death; PGF: primary graft failure; re-LT: retrasplantation.
Level of evidence based on the classification of the Centre for Evidence-based medicine at the University of Oxford (www.cebm.net).
Currently, the appearance of ischaemic cholangiopathy after LT from NHBD has a great impact on the need for retransplantation, as the need for retransplantion for other reasons has been reduced, such as a primary failure of the graft or acute vascular complications. In a series from Ianjin (China), where 80 cases of re-LT were studied after a first LT from an uNHBD, in 45% of these cases retransplantation was indicated due to the appearance of biliary complications.75
Managing ischaemic cholangiopathy after LT is complex and is associated with a significant increase in costs as it requires interventional radiology procedures that in most cases are palliative and prolonged readmission to hospital.76
Following a strict uNHBD selection protocol as we recommend in this article can result in a significant reduction in the development of ischaemic cholangiopathy. From the experience of the Hospital Clínic of Barcelona, its appearance was reduced to 8% (3 cases), a figure which is below the rates referred to in centres where LT were performed with cNHBD.41 The 3 patients who developed the condition were retransplanted 5, 8 and 13 months post-transplant.
With regard to surgical technique, there is no evidence whatsoever to recommend the use of a T tube in bile duct reconstruction in the recipient, although it could facilitate serial postoperative cholangiography for diagnosis. At present, cholangio-MRI can replace cholangiography via T tube. In the experience of the Barcelona and La Coruña groups the use of biopsies and cholangio-MRI depends on the clinical evolution. However, in the Hospital 12 de Octubre in Madrid a mandatory biopsy is performed one year after transplant and a cholangio-MRI 6 months after transplant.
Selection of RecipientsDue to the limited experience with this type of NHBD, there are no published studies to date which are based on the multivariate analysis of factors associated with recipient characteristics which might affect post-transplant outcomes.
Therefore we can only analyse the characteristics of patients transplanted from uNHBD in Spanish hospitals with experience. From the experience reported by the Hospital 12 de Octubre in Madrid, the age of the recipient was 59±6 (range 48–67), the median Child–Pugh score 10±2 (range 6–13) and the MELD score 19±6 (range 10–34), respectively. The main indication for transplant was alcoholic cirrhosis in 60% and VHC cirrhosis in 30%.40
In the series by the Hospital Clínic of Barcelona, the median age was 55 (range 49–60) and the median MELD score 19 points (range 14–21). Recipients were classified as Child-Pugh A and B in 47% of cases and Child-Pugh C in 53%. The principal indications were alcoholic cirrhosis (24%), CHC (32%) and VHC cirrhosis, 74% of recipients were infected withVHC. It is worth noting that these characteristics changed over time as experience was gained; in the first half of the series 71% of the patients were Child-Pugh C, compared to 35% of the patients in the second half of the series (P=0.039). Furthermore the pre-transplant MELD score tended to be higher in the first half of the series compared to the second half: 20 (range 18–23) compared to 1614–19 (P=0.112). Moreover, 53% of the recipients in the first half of the series and 18% of those of the second half were admitted to hospital prior to transplant due to decompensated cirrhosis (P=0.031).41
Furthermore, the cold ischaemia time of the graft appears to have a negative impact on outcomes of LT from NHBD as it is far more deleterious to grafts which have suffered an initial period of warm ischaemia prior to preservation.76,77 In light of this, there are factors relating to the recipient which could produce an increase in cold ischaemia time and which, a priori, should be avoided.
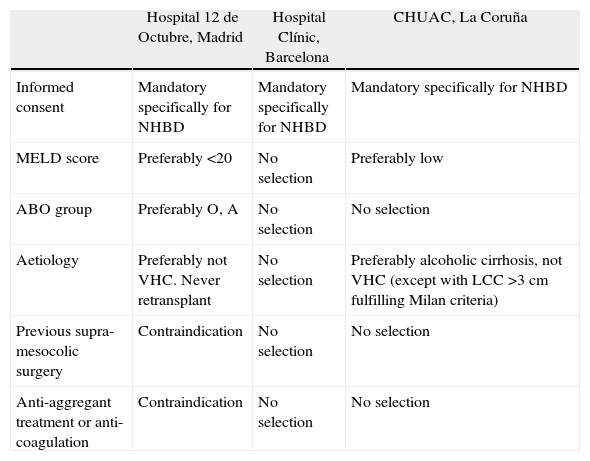
Given this scant evidence, amending the selection criteria of recipients is variable in Spain, and will depend on the policies applied to the waiting list in each hospital and region. Table 4 shows the recipient selection criteria in the 3 Spanish hospitals which are currently the most active in this type of donation.
Selection of Recipient for Transplant With Grafts From Non-Heart-Beating Donation.
| Hospital 12 de Octubre, Madrid | Hospital Clínic, Barcelona | CHUAC, La Coruña | |
| Informed consent | Mandatory specifically for NHBD | Mandatory specifically for NHBD | Mandatory specifically for NHBD |
| MELD score | Preferably <20 | No selection | Preferably low |
| ABO group | Preferably O, A | No selection | No selection |
| Aetiology | Preferably not VHC. Never retransplant | No selection | Preferably alcoholic cirrhosis, not VHC (except with LCC >3cm fulfilling Milan criteria) |
| Previous supra-mesocolic surgery | Contraindication | No selection | No selection |
| Anti-aggregant treatment or anti-coagulation | Contraindication | No selection | No selection |
LCC: liver cell carcinoma; CHUAC: La Coruña University Hospital Complex; UHBD: non-heart-beating donor; VHC: viral hepatitis C.
To date, the outcomes of LT using uNHBD in VHC positive recipients have not been specifically assessed; therefore it is not possible to establish any conclusion in this regard. However, the series by the Hospital Clínic of Barcelona shows satisfactory outcomes despite the fact that 74% of recipients are VHC positive, which suggests that perhaps this is not a factor that particularly affects outcomes. Obviously larger scale studies are necessary to reach any conclusion in this regard.
RecommendationsDue to the current low applicability of LT from uNHBD, its more complex organisation and taking into account the waiting lists of each hospital on an individual basis, the implementation of this type of donation cannot be recommended in every transplant centre.
A specific place should be assigned in centres which activate a donation programme of this type to perform preservation measures and for the donor to be kept during the assessment process, prior to transfer to the operating theatre for organ extraction. The procedure for preserving organs includes starting cardio compression and mechanical ventilation until cannulation in order to initiate blood recirculation in the abdominal area.
The duration of the different donor phases is an essential parameter which will greatly affect the viability of the organs for transplantation. No more than 150min should have passed once CPR measures have been established and until NRP is started; this period includes the time taken to cannulate the femoral vessels. NRP time should not exceed 240min.
NRP prior to retrieval, using a similar system to ECMO, is essential in uNHBD to maintain and select the suitability of the liver grafts for transplant. The use of NRP allows liver grafts to be rejected based on viability parameters. If the initial transaminases are high or continue to increase during the preservation period, this is indicative of irreparable liver damage.
Liver assessment in NHBD is more complex than with DBD due to the additional period of ischaemia. This assessment is a dynamic process and is not based on a single parameter. Macroscopic assessment should weigh up 2 principal aspects. Firstly, when there is any additional parameter of poor prognosis, such as the existence of liver steatosis, it is advisable not to use these grafts. Furthermore, as the great sensitivity of the biliary tree to warm ischaemia is well known, its careful assessment during retrieval is essential. The presence of intestinal areas of ischaemia during retrieval can be an indirect indicator which would assist in making the decision as to whether or not to accept the liver graft.
In LT from uNHBD, there can be severe haemodynamic instability and coagulopathy after reperfusion and therefore it is advisable to start vasopressor support early and to use prophylactic antifibrinolytics and to recover a minimum amount of platelets and fibrinogen prior to reperfusion, at the end of surgery and during the stay in ICU. Transfusion of fresh plasma should be based on clinical evidence of haemorrhage rather than laboratory tests.
In order to reduce the incidence of biliary complications and optimise the use of NHBD, it is recommended that strict selection criteria are applied. Limiting the selection of NHBD according to the parameters outlined earlier in this document can minimise the risk of biliary complications. The length of time that there is inadequate tissue perfusion and oxygenation of organs during cardiac arrest and CRP is key to the appearance of biliary complications and should be precisely known. Similarly, it is essential to administer heparin to the donor prior to starting liver preservation measures.
In the event of the appearance of ischaemic cholangiopathy in NHBD recipients, in centres where the waiting list is organised according to MELD score, it is recommended that NHBD graft recipients are prioritised whose MELD score did not reflect the morbimortality associated with this clinical situation.
A better selection of recipients might have contributed to the improved outcomes of LT from uNHBD over time; therefore it might be advisable to offer this type of transplant to low-risk recipients.
Prior abdominal surgery, portal vein thrombosis or having undergone retransplantation, although not clearly assessed in clinical studies, could be considered relative contraindications for LT from NHBD.
Conflict of InterestsThe authors have no conflict of interests to declare.
Manuel Abradelo (coordinator). Hospital Doce de Octubre (Madrid)
Constantino Fondevila (coordinator). Hospital Clinic (Barcelona)
Patricia Ruiz Ordorica. Hospital de Cruces (Bilbao)
Gerardo Blanco. Hospital Infanta Cristina (Badajoz)
Javier Briceño. Hospital Reina Sofía (Cordoba)
Ramón Charco. Hospital Vall d’Hebrón (Barcelona)
Daniel Garrote. Hospital Virgen de las Nieves (Granada)
Miguel Ángel Gómez Bravo. Hospital Virgen del Rocío (Seville)
Rafael López Andújar. Hospital La Fe (Valencia)
Manuel López Santamaría. Hospital La Paz (Madrid)
Alejandra Otero. Complejo Hospitalario Universitario de A Coruña (La Coruña)
Baltasar Pérez Saborido. Hospital Río Hortega (Valladolid)
Víctor Sánchez Turrión. Hospital Puerta de Hierro (Majadahonda, Madrid).
Corresponding author.
E mail: iherrero@unav.es (J.I. Herrero).
Please cite this article as: Abradelo M, Fondevila C, on behalf of the working group of the Spanish Society of Liver transplantation. IV Reunión de consenso de la Sociedad Española de Trasplante Hepático (SETH) 2012. Trasplante hepático con injertos no convencionales: bipartición hepática (split) y donante en asistolia. Cir Esp. 2014;92:157–167.
The working group members are listed in Appendix A.