Identification of recent human immunodeficiency virus (HIV) infection is an important tool for monitoring HIV transmission. During the last few years, several serological assays have been developed for this purpose and used in cross-sectional studies.1 The serological testing algorithm for recent HIV seroconversion (STARHS) was developed in 19982 and used with the Abbott HIVAB 3A11 assay (Abbott Laboratories, Abbott Park, Chicago, Illinois, USA) and with the Vironostika HIV-1 Microelisa System (bioMérieux SA, Marcy l’Etoile, France). The sensitivity of both assays was lowered in order to obtain a negative result in specimens with low antibody titers, such as those from individuals with a recent infection. These assays are no longer available, and laboratories have turned to new methods. The BED assay (Calypte Biomedical Corporation, Portland, Oregon, USA) measures anti-HIV IgG titers3 and includes a calibrator to ensure comparability of results. Furthermore, the BED assay is included in an external quality program offered by the Centers for Disease Control and Prevention (CDC, Atlanta, Georgia, USA).
In Catalonia, the STARHS was introduced using the Vironostika assay as part of the enhanced HIV/STI surveillance program in 2003. As the Vironostika assay has no longer been available since 2007, our laboratory changed to the BED assay in 2008. Our aim was to assess whether the results obtained by both techniques were comparable.
A total of 101 serum specimens from HIV-1–positive individuals were selected from a previous study.4 The selection criteria were sufficient sample volume and minimum available clinical and laboratory information (CD4+ T-cell count, HIV viral load, HIV infection stage, previous antiretroviral treatment). Patients were classified into three groups. The first group included 15 recently infected individuals (sera were drawn no more than 6 months after seroconversion in nine patients, and the rest had a diagnosis of acute HIV infection). The second group included three patients with long-standing infection (subjects infected for >12 months) and 45 patients with a diagnosis of AIDS (clinical criteria or CD4+ T-cell count under 200 cells/μl). Finally, 38 specimens were unable to be classified as either recent infections, long-standing infections, or AIDS.
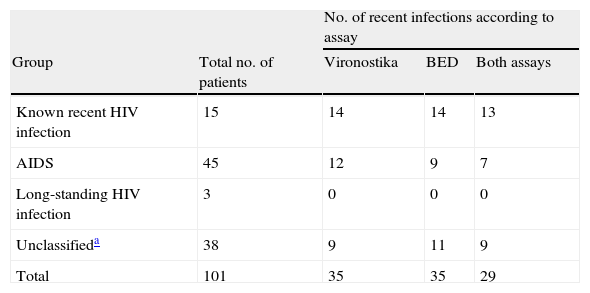
The agreement between Vironostika and BED assays was good (κ=0.738, P<.005), which is consistent with the results of two published studies.5,6 Sensitivity to detect recent infection was 93.3% (95% CI: 68.1 – 99.8) for both the Vironostika and the BED assays. Specificity for detecting long-term infections was 75.0% (95% CI: 60.4 – 86.4) using the Vironostika, and 81.3% (95% CI: 67.4 – 91.1) using the BED. Positive predictive values were 60.9% (95% CI: 38.5 – 80.3) using the BED, and 53.8% (95% CI: 33.4 – 73.4) using the Vironostika. Negative predictive values were 97.5% (95% CI: 86.8 – 99.9) using the BED, and 97.3% (95% CI: 85.8 – 99.9) using the Vironostika. Table 1 shows the samples identified as recent infections by both BED and Vironostika according to their clinical characteristics and laboratory information.
Performance of both assays according to clinical information.
| No. of recent infections according to assay | ||||
| Group | Total no. of patients | Vironostika | BED | Both assays |
| Known recent HIV infection | 15 | 14 | 14 | 13 |
| AIDS | 45 | 12 | 9 | 7 |
| Long-standing HIV infection | 3 | 0 | 0 | 0 |
| Unclassifieda | 38 | 9 | 11 | 9 |
| Total | 101 | 35 | 35 | 29 |
The BED assay correctly classified a greater proportion of recent infections and patients with AIDS than Vironostika. These results are similar to those of a previous study,6 in which the Vironostika kit also tended to misclassify more individuals with long-standing infections or AIDS as recently infected in a comparison with the avidity index method.7 The misclassification of patients with AIDS or CD4+ T-cell counts ≤200 cells/μl is explained by the low anti-HIV IgG titers. Hence the importance of excluding those samples belonging to patients fulfilling these criteria from STARHS testing when this information is available.
The BED assay offers several advantages over the Vironostika: i) it has been reported to have better reproducibility, since it is based on the HIV IgG/non-HIV IgG ratio and uses a simple 1:100 dilution;8 ii) the BED assay can also be automated providing more precise results than the Vironostika assay, which is performed manually; and iii) the window period of the Vironostika assay differs for B and non-B HIV-1 subtypes, whereas these differences are less pronounced with the BED.9 For all those reasons, the BED assay offers a good alternative to the discontinued Vironostika assay.
FundingThis work was supported by the “La Marató de TV3” Foundation grant for the development of the AERI-HIV (Algoritme Estandarditzat per la detecció de Recent Infectats pel VIH) project for epidemiological research on AIDS by applying the STARHS technique (project #022010). This work was partially funded by the Direcció General de Salut Pública de la Generalitat de Catalunya and by the Fundación para la Investigación y Prevención del Sida en España (FIPSE, Madrid, Spain, National AIDS Plan Secretariat of the Spanish Ministry of Health) and grant CD05/00258 (EM) (post-doctoral training contracts) from the “Ministerio de Sanidad y Consumo”, within the “Plan Nacional de Investigación científica, Desarrollo e Innovación Tecnológica (I+D+I)”.
The authors acknowledge partial funding of this research from the CIBER Epidemiología y Salud Pública (CIBERESP), Spain”.
We thank Joanne V Mei (Centers for Disease Control and Prevention, Atlanta, Georgia, USA) for technical assistance and advice. We would also like to thank Thomas O¿Boyle for his assistance with the English version of the manuscript.
The Recent HIV Infections (AERIVIH) study group includes the following:
Coordinating Center (CEEISCAT): Jordi Casabona, Anna Esteve, Anabel Romero, Núria Ortega, Alexandra Montoliu, Eva Puchol, Rafael Muñoz, Joan Masip, Núria Vives, Berta Ortiga, Meritxell Granell, Diana Puente, M. Jesús Casado, Àngels Jaen, Jesús Almeda, Vanessa Espurz.
STARHS Laboratory (Microbiology Service, Hospital Universitari Germans Trias i Pujol): Victoria González, Elisa Martró, Lurdes Matas and Vicenç Ausina
Peripheral Centers
Primary Health Care Laboratories: Isabel Rodrigo (Laboratori Clínic Manso, Barcelona), Àngels Bosch (Laboratori Intercomarcal de l’Alt Penedès, l’Anoia i el Garraf, Igualada), Rosa López (Laboratori Clínic Bon Pastor, Barcelona), Eva Dopico (Laboratori Clínic l’Hospitalet de Llobregat,), Josep Ros (Laboratori Clínic Barcelonés Nord i Maresme, Badalona), Rosa Navarro (Laboratori Clínic Cornellà de Llobregat), Conrad Vilanova (Laboratori Clínic El Maresme, Mataró)
Hospitals
Laboratory Staff: Tomàs Pumarola (Hospital Clínic-IDIBAPS, University of Barcelona, Barcelona), Aurora Casanova (Hospital Universitari de Bellvitge-IDIBELL, Hospitalet de Llobregat); Elisa Martró, Lurdes Matas, Victoria González and Vicenç Ausina (Hospital Universitari Germans Trias i Pujol, Badalona); Estrella Caballero (Hospital Universitari Vall Hebron, Barcelona); Núria Margall (Hospital de la Santa Creu i Sant Pau, Barcelona); Joan Farré (Hospital Universitari Arnau de Vilanova, Lleida); M. Goretti Sauca (Hospital de Mataró); Xavier Ortín (Hospital de Tortosa Verge de la Cinta, Tortosa); M. José Amengual (Corporació Sanitària Parc Taulí, Sabadell); Josep M. Prat (Hospital de Palamós); Josep M. Euras (Hospital General de Vic); José Ramón Blanco (Hospital San Pedro - CIBIR La Rioja); Josep M. Simó (Hospital Universitari de Sant Joan de Reus); Carlos Toro (Hospital Carlos III, Madrid) M. Carme Villà (Hospital General de Granollers); Eugenia Márquez (Hospital General de l’Hospitalet, Hospitalet de Llobregat).
Clinical Staff: Josep M. Miró, Fernando Agüero, Omar Sued, María López-Diéguez and José M. Gatell (Hospital Clínic-IDIBAPS, University of Barcelona, Barcelona); Elena Ferrer and Daniel Podzamczer (Hospital Universitari de Bellvitge-IDIBELL, Hospitalet de Llobregat); Cristina Tural and Bonaventura Clotet (Hospital Universitari Germans Trias i Pujol, Badalona); Esteve Ribera (Hospital Universitari Vall Hebron, Barcelona); Jordi Altès and José Manuel Guadarrama (Hospital Alt Penedès, Vilafranca); Pere Domingo (Hospital de la Santa Creu i Sant Pau, Barcelona); Teresa Puig (Hospital Universitari Arnau de Vilanova, Lleida); Carmen Bernal (Hospital Universitario San Cecilio, Granada); Pilar Barrufet and Lluís Force (Hospital de Mataró); Carolina Gutiérrez (Hospital Ramón y Cajal, Madrid); Amat Ortí (Hospital de Tortosa Verge de la Cinta, Tortosa); Gemma Navarro and Ferran Segura (Corporació Sanitària Parc Taulí, Sabadell); Àngels Masabeu (Hospital de Palamós); Vicente Soriano (Hospital Carlos III, Madrid); Josep Vilaró (Hospital General de Vic);José Antonio Iribarren (Hospital de Donostia, San Sebastián); José Antonio Oteo (Hospital San Pedro - CIBIR La Rioja); Blai Coll and Carlos Alonso Villaverde; (Hospital Universitari de Sant Joan de Reus); Santiago Montull (Hospital General de Granollers) and Isabel Garcia (Hospital General de l’Hospitalet, Hospitalet de Llobregat).
Sexually transmitted infections clinic: Carmen Rodríguez and Jorge del Romero (Centro Sanitario Sandoval, Madrid)
Site-testing nongovernmental organizations: Roser Sala (Laboratori Sabater Tobella, Barcelona); Olga Díaz (Servei d’Atenció i Prevenció Sociosanitària: SAPS - Creu Roja, Barcelona); Kati Zaragoza (Stop Sida, Barcelona); Ferran Pujol and Jorge Saz (Projecte dels Noms – Joves positius, Barcelona); Mercè Meroño (Àmbit Prevenció, Barcelona); Jasmina Becerra (Associació Ciutadana Antisida de Catalunya – ACASC, Barcelona); Rosa Ros (Centre Jove d’Anticoncepció i Sexualitat – CJAS, Barcelona); Anna Avellaneda and Montse Sité (Actua Vallès, Sabadell) and Anna Rafel (Associació Antisida de Lleida).