Both bacteremia and infective endocarditis caused by Staphylococcus aureus are common and severe diseases. The prognosis may darken not infrequently, especially in the presence of intracardiac devices or methicillin-resistance. Indeed, the optimization of the antimicrobial therapy is a key step in the outcome of these infections. The high rates of treatment failure and the increasing interest in the influence of vancomycin susceptibility in the outcome of infections caused by both methicillin-susceptible and -resistant isolates has led to the research of novel therapeutic schemes. Specifically, the interest raised in recent years on the new antimicrobials with activity against methicillin-resistant staphylococci has been also extended to infections caused by susceptible strains, which still carry the most important burden of infection. Recent clinical and experimental research has focused in the activity of new combinations of antimicrobials, their indication and role still being debatable. Also, the impact of an appropriate empirical antimicrobial treatment has acquired relevance in recent years. Finally, it is noteworthy the impact of the implementation of a systematic bundle of measures for improving the outcome. The aim of this clinical guideline is to provide an ensemble of recommendations in order to improve the treatment and prognosis of bacteremia and infective endocarditis caused by S. aureus, in accordance to the latest evidence published.
Tanto la bacteriemia como la endocarditis infecciosa causada por Staphylococcus aureus son infecciones graves y frecuentes. El pronóstico puede verse ensombrecido por la presencia de dispositivos cardíacos o por la resistencia a meticilina. La optimización del tratamiento antimicrobiano es clave en los resultados. Las considerables tasas de fracaso terapéutico y la influencia de la susceptibilidad a vancomicina en el pronóstico, tanto de los episodios causados por cepas resistentes como sensibles a meticilina, ha conducido a la investigación de nuevos esquemas terapéuticos. Específicamente, el interés que en los últimos años han generado los nuevos antibióticos con actividad frente a cepas resistentes a meticilina se ha extendido a las cepas sensibles, que son aún responsables de la mayoría de los casos. Recientes estudios en el ámbito clínico y experimental se han centrado en la actividad de nuevas combinaciones, cuyo papel e indicación clínicas son aún objeto de debate. Por otro lado, la importancia de un tratamiento antibiótico empírico precoz y adecuado ha cobrado interés en los últimos años. Finalmente, cabe destacar el impacto que la instauración de un conjunto sistemático de medidas en el manejo de la bacteriemia estafilocócica tiene en el pronóstico global de la infección. Esta guía clínica reúne un conjunto de recomendaciones a la luz de la última evidencia científica, con el objeto de mejorar el tratamiento y pronóstico de la bacteriemia y endocarditis infecciosa causada por S. aureus.
Staphylococcus aureus is the second most frequent microorganism causing bloodstream infection, thus leading to significant morbidity and mortality.1,2 Incidence rate ranges between 15 and 40 episodes per 100,000 inhabitants and year, according to several population-based studies performed in the last decade, with death rates of 15–25%.1–3
The notable increase of cases caused by methicillin-resistant S. aureus (MRSA) accounts for one of the most important epidemiological changes occurred in recent years. This has been observed especially among hospitalized, elderly patients with various intrinsic and extrinsic risk factors such as diabetes, immunosuppressant therapy and the performance of invasive procedures.4,5 Also, a higher incidence has been observed in non-nosocomial health-care environments and, to a lesser extent, in the community.1,3 Many recent studies have focused in bloodstream infections caused by MRSA, due to its inherent therapeutical difficulties and higher mortality.
Notwithstanding, the burden of infection caused by methicillin-susceptible S. aureus (MSSA) is still enormous. In a recent multinational study collecting more than 18,000 cases of S. aureus bacteremia (SAB), the annual incidence of episodes caused by MSSA was 24.2 per 100,000 inhabitants, while the corresponding annual rate for MRSA was 1.9. The incidence rates of SAB in the various countries and regions were homogenously distributed for community-acquired MSSA (around 15 episodes per 100,000 inhabitants and year), whereas those of community-acquired and nosocomial MRSA infections changed widely.1 In another population-based study, the case fatality ratio of SAB was 20.3% (MSSA 20.2%, MRSA 22.3%), while the mortality rate per 100,000 inhabitants and year was 3.4 (MSSA 3.1, MRSA 0.3).2 In the specific scenario of hospitalized patients with MRSA bacteremia, data from our country indicate that the case fatality ratio may be higher than 30%, death frequently occurring early in the course of bacteremia.6
Thus, bloodstream infection caused by both MSSA and MRSA is a serious concern for public health due to its frequency and severity. Given the discrepancies found in some studies evaluating the clinical relevance of the staphylococcal loss of susceptibility to glycopeptides and other antibiotics,7–10 it is evident that the best antimicrobial treatment of SAB is yet to be elucidated, the efficacy of some current treatments being suboptimal. On the other hand, both the availability of new antibiotics with activity against MRSA, and the efficacy of various antimicrobial combinations currently under evaluation raise our hopes on a near improvement of SAB prognosis. Randomized clinical trials are urgently needed in order to better precise the role of the available antimicrobials and their combinations against SAB, and against staphylococcal infectious endocarditis (IE). In addition, novel strategies will be needed, both therapeutical (antibacterial antibodies) and preventive (vaccines against S. aureus) in order to reduce the incidence of SAB and improve the prognosis of these severe infections.
Six years ago a Consensus Statement entitled ‘Consensus document for the treatment of bacteremia and endocarditis caused by methicillin-resistant Staphylococcus aureus’11 was published in this journal. The above mentioned reasons illustrate the presence, persistence and complexity of the problem, thus the Spanish Society of Clinical Microbiology and Infectious Diseases (SEIMC) has requested to a panel of experts to update the previous Statement, this time including both MRSA and MSSA bloodstream infections.
Aims, methods and definitionsThe main objective of this Consensus Statement is to provide an ensemble of recommendations in order to improve the diagnosis and treatment of bacteremia and IE caused by S. aureus, in accordance to the latest evidence published.
The source of bacteremia is crucial in order to plan the best diagnostic and therapeutical strategy. In the hospital and health-care system a high proportion of SAB are catheter-related. Less commonly the origin of bacteremia is not evident, for the patient has not signs or symptoms pointing to a specific source of the infection, nor does not carry any intravascular catheter. This last scenario is more frequent in community-acquired bacteremia caused by MSSA, and is commonly referred as primary bacteremia. It is usual that the clinical evolution and/or the complementary tests performed in the following days help to reach a definite diagnosis. In other cases, SAB occurs as a consequence of a localized infection, obvious from the beginning. This is nominated secondary bacteremia and is frequent in typical staphylococcal infections such as septic arthritis, osteomyelitis, cellulitis and pneumonia, among others, due to the ability of S. aureus to reach and invade the bloodstream.
In any of these three scenarios, an appropriate antimicrobial treatment usually leads to patient's clinical improvement, along with sterile new blood cultures. However, sometimes bacteremia does not clear, this commonly indicating the existence of IE or other metastatic distant foci. This situation is referred as complicated bacteremia. In fact, infective endocarditis, which is the paradigm of bacteremic staphylococcal infection, may be secondary to a catheter-related bacteremia (CRB), may present initially as primary bacteremia or may be evident from the beginning with a typical clinical picture.
This Consensus Statement will review important microbiological and genetic concepts of SAB pathogenesis and epidemics. It will also analyze the management of specific clinical scenarios, namely the clinical suspicion of SAB, confirmed non-complicated and complicated SAB, and staphylococcal IE. The management of secondary bacteremia in specific staphylococcal infections are beyond the scope of this Statement. Finally, the role of care bundles that may contribute to ameliorate the prognosis of SAB will be also analyzed.
The present Statement has been written following the SEIMC guidelines for consensus statements (www.seimc.org), as well as the recommendations of the Agree Collaboration (www.agreecollaboration.org) on evaluation of the methodological quality of clinical practice guidelines. During various meetings, a set of questions, which were meant to be the base of the document, was chosen by the authors. Their recommendations are based on a systematic and critical review of the literature and, when necessary, in the opinion of experts, who are SEIMC members. Their recommendations have been adjusted according to the scientific evidence available12 (Annex 1). The contents of the document and the conclusions have been agreed by all the authors and the coordinators of the Statement. Before publication, the manuscript was published in the SEIMC intranet and open to suggestions and comments by any of the SEIMC members.
Microbiological aspects of SABWhat are the available techniques to identify MSSA or MRSA in positive blood cultures?When positive blood cultures are detected, the implementation and early reporting of Gram staining has a significant impact on the empirical antibiotic therapy of bacteremic patients.13 However, more accurate identification of S. aureus and its methicillin susceptibility can be achieved within a few hours combining different laboratory techniques.14,15 These combinations would significantly reduce the turnaround time for these processes compared to the reference techniques (i.e. culture and antibiotic susceptibility testing by microdilution, E-test or disk-diffusion) that can delay the final results for 24–48h.
Among the available techniques to accurately differentiate S. aureus from coagulase-negative staphylococci and other Gram-positive cocci directly on blood cultures,14,15 the matrix-assisted laser desorption ionization time-of-flight spectrometry (MALDI-TOF MS) technology has shown excellent results.14,16 A reliable identification of S. aureus can be reached from positive blood cultures in less than an hour in 95% of the cases with a 100% of specificity.14 Other techniques (i.e. based on antigen detection) are also available for S. aureus identification on blood cultures with Gram-positive cocci in clusters.15,17 Sensitivity and specificity of these techniques are good (92–95% and 99%), although lower than the values reached by MALDI-TOF MS, and they cannot differentiate between MRSA and MSSA.15,17
Though, the detection of methicillin-susceptibility by MALDI-TOF MS has not been clinically validated to date.18,19 Accordingly, the time to report the antibiotic susceptibility testing would remain unmodified. For that purpose, tests aimed to detect the mecA gene for rapid diagnosis of MRSA that could be applied to the positive blood culture media have been introduced into clinical practice. There are several commercial approaches: GeneOhm MRSA (BD, Franklin Lakes, NJ), LightCycler MRSA (Roche, Basel, Switzerland), and GenoType MRSA Direct (Hain, Lifescience, Nehren, Germany), among others.20 Of these, the GeneXpert MRSA/SA blood culture test (Cepheid, Sunnyvale, CA, USA), a real time polymerase chain reaction (PCR) based technique, has proven to identify methicillin resistance with a sensitivity of 99% and a specificity of 100% on blood cultures samples previously identified as S. aureus by other methods.14,21 Although the GeneXpert assay has shown a high specificity compared with other molecular techniques,14,21–23 false-positive results were described in the coexistence of a methicillin-susceptible S. aureus carrying an staphylococcal cassette chromosome (SCC) element that does not contain the mecA gene, and a coagulase-negative staphylococci carrying the mecA gene.21,24 False-positive results were described from active surveillance cultures (nasal and cutaneous swabs),21 the presence of an S. aureus SCC element lacking the mecA gene along with a mecA positive coagulase-negative staphylococci is less likely in positive blood cultures.
The application of PCR-based procedures as a routine to positive blood cultures with Gram-positive cocci in clusters would be expensive because of the frequency of coagulase-negative staphylococci.14 Therefore, the implementation of early detection of S. aureus in the positive blood cultures by MALDI-TOF MS, or other rapid techniques, combined with the detection of methicillin susceptibility by PCR-based methods has proven to be a convenient combination for early diagnosis of S. aureus bacteremia and methicillin susceptibility, once the blood cultures are positive.14,15,20,21
Evaluation of these diagnostic procedures has been addressed in recent publications, concluding that the patient's clinical outcome can be improved by decreasing the time to identification of coagulase-negative staphylococci, MSSA and MRSA and by allowing for earlier and more effective antimicrobial therapy.25,26 Moreover, decreased costs associated with more rapid adjustment of the definitive antibiotic therapy and a decrease in the length of stay have also been reported.26–28
Finally, it should be noted that rapid methods are not intended to replace the conventional microbiological methods, which are still needed to recover the pathogenic strain.Recommendation:
The implementation of early detection of S. aureus in positive blood cultures by MALDI-TOF MS, or other rapid techniques, combined with the detection of methicillin susceptibility by PCR-based methods has proven to be a convenient combination for the early diagnosis of S. aureus bacteremia and methicillin susceptibility (A-II).
What actions would improve reporting of results to the clinician?The implementation of rapid testing methods for early identification of MSSA or MRSA bacteremia alone is not enough to improve the management of staphylococcal infections.28 These processes should become part of a bundle of associated interventions such as the timing of batched laboratory analysis, improved reporting of results and the integration of rapid diagnosis into an antibiotic stewardship program, to fully impact timely patient care decisions.28
Actions improving quality of information from the laboratory to the clinicians, like introducing written or oral-alert reports with clinical advice complementing traditional microbiological reports, may have a positive impact on the quality of clinical care.14,29 A clinical trial reported the benefits of active infection notification regarding adequacy of antimicrobial therapy (patients under active microbiological notification were on correct therapy for 92% of the infectious episode, whereas patients under traditional report were only for the 66% of the duration of the episodes).29 In addition, active reporting of bloodstream infection saved approximately 25% of the economic cost per episode.29
On the other hand, the report to an infectious diseases specialist and the management of the bacteremic episode by qualified personnel has shown improved outcomes30,31 as well as a mortality decrease.32,33 Recently, Lopez-Cortés et al. described a multicentre intervention with the identification of six indicators of quality-of-care for the treatment of S. aureus bacteremia.34 This study, described in Section 6, shows a reduction in overall mortality following a bundle aimed to improve adherence of specialists to the evidence-based indicators of quality of care.34 Similar results have been published in different clinical settings in other geographical areas.35,36Recommendation:
The active notification of the microbiological results is recommended, as part of a bundle of interventions aimed to improve the management of patients with SAB (A-I).
What are the recommended techniques for determining the resistance or diminished susceptibility of S. aureus to antimicrobial agents of clinical importance?Antimicrobial susceptibility testing of S. aureus can be determined either by disk diffusion or by broth dilution following the ISO 20776-1 guidelines,37 the European Committee of Antimicrobial Susceptibility Testing (EUCAST) guidelines,38 or the Clinical and Laboratory Standard Institute (CLSI) guidelines.39,40 In addition susceptibility testing can also be determined by different automated systems and by gradient tests. Information concerning the compliance of manufacturers of antimicrobial susceptibility testing materials and devices with EUCAST guidelines has been published (www.eucast.org; June 20 2014, date last accessed).
For the detection of specific antimicrobial resistance mechanisms of clinical and/or epidemiological importance in S. aureus (i.e., methicillin resistance and glycopeptide non-susceptibility) we recommend the EUCAST specific methods that are summarized below.38
Methicillin resistanceMethicillin/oxacillin resistance can be detected both phenotypically, by disk diffusion tests, MIC determination or latex agglutination to detect the PBP2a protein, and genotypically using PCR for the detection of the mecA gene. Latex agglutination tests are not useful for the detection of the recently discovered alternative PBP2 encoded by the mecC gene, which can also be detected by a specific PCR technique.41 Disk diffusion using oxacillin must not be used. Cefoxitin is a very sensitive and specific marker of mecA/mecC-mediated methicillin resistance and is the agent of choice for disk diffusion. Strains with a cefoxitin (30μg disk) zone diameter <22mm should be reported as methicillin-resistant, as well as strains with cefoxitin MICs >4mg/L. The heterogeneous expression of methicillin resistance particularly affects MICs of oxacillin. Strains with increased MICs of oxacillin (MIC >2mg/L), but remaining susceptible to cefoxitin (zone diameter ≥22mm, MIC ≤4mg/L) are uncommon. If oxacillin is tested and gives a different interpretation than that of cefoxitin, the interpretation should be as follows: (a) oxacillin-susceptible and cefoxitin-resistant isolates must be reported as oxacillin-resistant; (b) oxacillin-resistant and cefoxitin-susceptible isolates must be reported as oxacillin-resistant. It is recommended to subject such strains to phenotypic or genotypic investigations for mecA or mecC.
Glycopeptide resistanceThe vancomycin MIC should always be determined when using vancomycin for treating a patient with SAB or IE. Glycopeptide MICs are method-dependent and should be determined by the broth microdilution methodology as recommended by the ISO 20776-1 guidelines, which is the gold standard. However, MICs may also be determined by gradient strip methods, agar dilution or automated systems. It should be noted that the results with gradient strip methods may be 0.5-1 two-fold dilution steps higher than the results obtained by broth microdilution.42 The EUCAST and CLSI clinical MIC breakpoint for resistance to vancomycin in S. aureus is >2mg/L, according to broth microdilution. However, S. aureus isolates with vancomycin MIC values in the upper part of the susceptible range (MIC >1.5mg/L) are associated with poorer outcomes and may be linked to increased mortality.10,43 Isolates of S. aureus can also be vanA-mediated high-level glycopeptide resistant [glycopeptide-resistant S. aureus (GRSA); vancomycin MIC >8mg/L], and non-vanA mediated low-level resistant isolates, the latest including glycopeptide intermediate S. aureus [glycopeptide-intermediate S. aureus (GISA); vancomycin MIC 4–8mg/L], and heteroresistant glycopeptide intermediate S. aureus [heteroresistant glycopeptide-intermediate S. aureus (hGISA); vancomycin MICs ≤2mg/L]; hGISA isolates are susceptible to vancomycin but with minority populations (1 in 106 cells) with vancomycin MIC >2mg/L, as judged by population analysis profile investigation. The prevalence of hGISA is frequently associated with spread of specific clonal lineages.44 This phenotype is often unstable in the laboratory, but hGISA have the ability to develop into GISA in vivo.44 Since hGISA are not detected by MIC determination, a number of screening methods have been developed. Confirmation is done by analysing the population profile of the isolate on agar plates containing a range of vancomycin concentrations (PAP-AUC), this being usually performed in reference laboratories.45 Disk diffusion cannot be used to test for either hGISA or GISA, but can be used to test for GRSA.Recommendations:
The EUCAST specific methods for the detection of antimicrobial mechanisms of resistance of clinical and/or epidemiological importance are recommended (B-III). For the detection of methicillin-resistance by disk diffusion, cefoxitin is the agent of choice (B-III). Broth microdilution is the gold standard method for determining vancomycin MIC, but it can also be determined by strip methods, agar dilution or automated systems (B-III).
How often the studies of surveillance of resistance of S. aureus should be performed?S. aureus is a constantly evolving pathogen that rapidly develops antimicrobial resistance. In addition to methicillin resistance, S. aureus has adapted to glycopeptide exposure and has been able to produce infections. Such adaptations allow for the development of resistance and may select for virulence properties.46 In addition, high level vanA-mediated vancomycin resistance has recently been described in S. aureus in Europe.47 Although linezolid and daptomycin are available alternatives, resistance to these agents is emerging and evolving changes in the patterns of resistance to other antimicrobials are frequent over time.48–50 All these circumstances ask for a continuous surveillance of the resistance of S. aureus to all antimicrobial agents, including linezolid, daptomycin and ceftaroline, and not only in certain units or hospitals, but also at a nationwide level in order to know the overall rates of resistance in different geographical areas. Surveillance of resistance of S. aureus to antimicrobials must be performed, if possible, on a monthly basis in high-risk units, and at least once per year in a whole institution.
It is also important to monitor the evolution of susceptibility to vancomycin, daptomycin and linezolid in successive isolates from the same patient, since loss of susceptibility to these agents has been observed after prolonged therapy with these antimicrobials in patients with severe S. aureus infections.51–53 Loss of daptomycin susceptibility has been described in patients after prolonged treatment with vancomycin, too.53 Furthermore, recent outbreaks of linezolid-resistant S. aureus at different institutions in Spain, create a new challenge and the need for continuous monitoring.54,55Recommendation:
The constant changes in the patterns of antimicrobial resistance in S. aureus must be regularly monitored. Surveillance must be performed on a monthly basis in high-risk units, and at least once per year in a whole institution (B-III).
It is also recommended to monitor the evolution of susceptibility to vancomycin, daptomycin and linezolid in successive isolates from the same patient (B-III).
Empirical treatment of a clinical suspicion of SABWhat is the impact of an appropriate empirical treatment in the prognosis of SAB?While some studies have failed to prove the benefits of an appropriate initial treatment,56–59 the majority highlight that a delay in the administration of antibiotics is an independent predictor of mortality in the setting of bacteremia caused by MSSA60–62 or MRSA.6,63–67 In this regard, a significant reduction in mortality was observed when treatment was administered before 44.75h among 167 patients with nosocomial SAB.68 Also, in a recent cohort study including 579 patients with MRSA aimed at identifying predictors or early and delayed mortality, the appropriate empirical treatment was the only distinctive risk factor of early mortality (OR 3.59 [95% CI 1.63–7.89]).6
The discrepancies found between studies have been attributed to specific definitions of appropriate treatment, along with the presence of confounding factors and other biases.63,69,70 Finally, some authors conclude that the appropriateness of the empirical treatment may only have impact in particular subpopulations, namely those with severe or complicated SAB,68 non-removable source of SAB,71 or patients with less severe SAB.59Recommendation:
With the available evidence, it seems reasonable prescribing early appropriate treatment to any patient suspected to have SAB, although some subpopulations may have a more significant benefit as compared to others (A-II).
Who is at higher risk of presenting with bacteremia caused by MRSA?Some recent studies have identified the risk factors of receiving inappropriate empirical therapy, resistance to methicillin being the most frequently reported,60 with odds ratio (OR) ranging from 3.7 to 21.7.56,68,71 Other predictors of inappropriate empirical treatment are previous hospital admission and a length of stay >2 weeks, underlying hematological malignancy, acquisition of bacteremia in a non-critically ill ward, and patients with chronic pulmonary obstructive disease; previous MRSA nasal carriage decreased the likelihood of inappropriate therapy.56,68,71
Early reports on MRSA bacteremia pointed to intravenous catheters in ICUs as the most frequent source of infection,72,73 but the epidemiology has significantly changed in the last years. Nowadays, non-nosocomial bacteremia by MRSA accounts for 40–60% of all cases of SAB.74,75 In a recent study including 8987 patients with invasive infection caused by MRSA, the majority of health-care related cases (both nosocomial and in the community) had at least one of the following characteristics: recent hospitalization (77% and 58%, respectively), recent surgery (37% and 38%), previous colonization or infection by MRSA (30% and 17%), and living in a nursing home (39% and 22%).75 In a case–control study on patients with SAB on admission, MRSA was more likely in patients with previous colonization this microorganism (OR 41 [95% CI 4.0–350] or those coming from nursing homes (OR 37 [95% CI 4.5–316]).76 Another study identified previous infection or colonization by MRSA (OR 17 [95% CI 5.0–58.3]), the presence of a central venous catheter (CVC) (OR 3 [95% CI 1.7–6.4]), cutaneous ulcers (OR 3 [95% CI 1.4–7.1]), or cellulitis (OR 4 [95% CI 1.5–11.9]) as independent predictors of SAB caused by MRSA.77 Patients undergoing hemodialysis are also at significant risk of bacteremia and IE caused by MRSA.78–80 Of note, many of these studies observed that MRSA bacteremia in patients with no previous contact with the health-care system was anecdotal.6,77Recommendations:
Bacteremia by MRSA should be suspected in the following circumstances: (1) nosocomial episodes, especially if occurring in wards with high prevalence (depending on each centre's local epidemiology) (A-II); and (2) non-nosocomial episodes in patients previously colonized by MRSA (A-II), coming from nursing homes (A-II) or hemodyalisis centers (B-II), with CVC (B-II) or chronic cutaneous ulcers (B-II).
Including antibiotics with activity against MRSA in community-acquired episodes with none of the former risk factors seems not necessary (B-II).
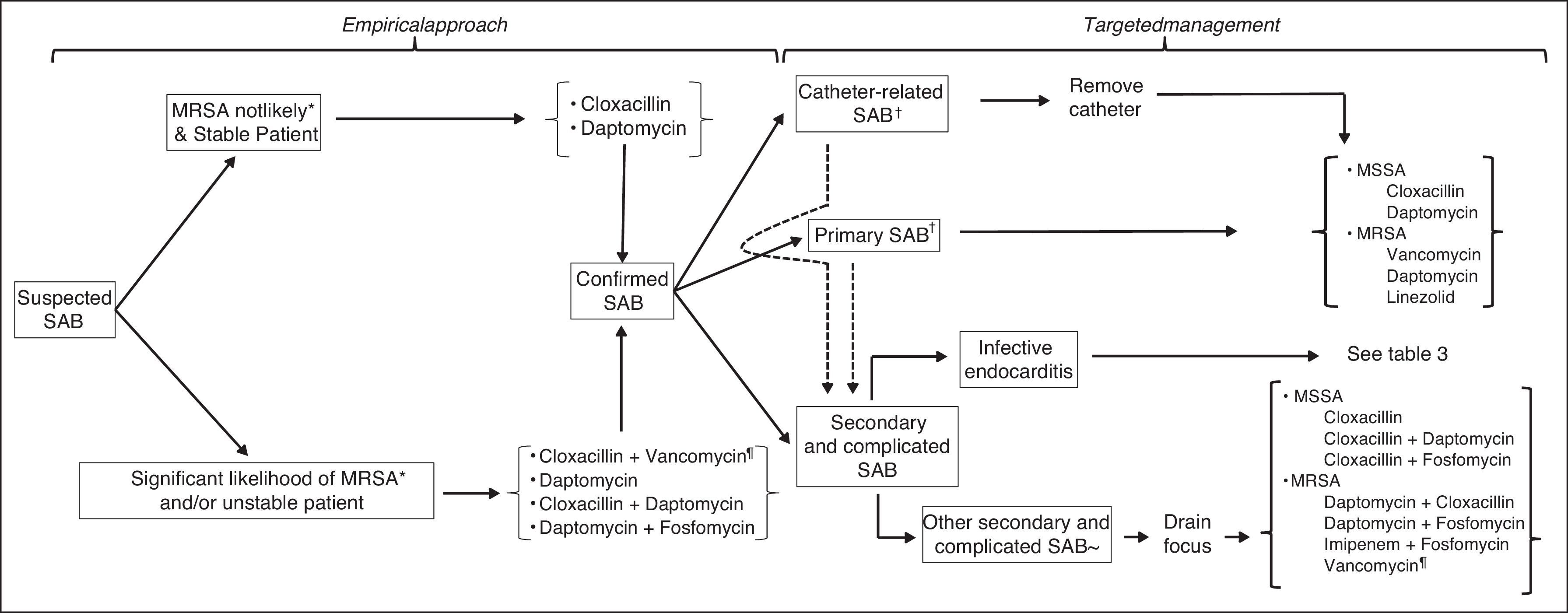
What is the most appropriate empirical antibiotic treatment when suspecting SAB?An algorithm for antimicrobial therapy when SAB is suspected is proposed in Fig. 1. In the setting of SAB, it is commonly accepted that the in vitro bactericidal activity of any given antibiotic is associated with the clinical prognosis, although few studies have proven so, especially in regard to β-lactams and glycopeptides.81–84 β-Lactams have the fastest and most intense bactericidal activity against MSSA. Against most MRSA strains, vancomycin and daptomycin are the only bactericidal antibiotics, the activity of the latter being more intense and faster.
Empirical treatment when suspecting bacteremia by MSSASo far, β-lactams have been the treatment of choice for MSSA bacteremia. More than 90% of isolates produce β-lactamases, thus the β-lactam used must be stable in the presence of these enzymes. Isoxazolic penicillins (cloxacillin) and 1st-generation cephalosporins (cefazolin) are the most frequent choices. In contrast, a higher mortality was observed among patients treated with 2nd- or 3rd-generation cephalosporins (OR 2.24 [95% CI 1.23–5.85]) or β-lactamase inhibitors plus β-lactams.85
Several in vitro studies have observed a lower activity of vancomycin in front of MSSA isolates as compared with β-lactams,86,87 and various authors have reported a worse clinical prognosis in patients with SAB or IE caused by MSSA when treated with vancomycin.86–93 Also, the initial use of glycopeptides in MSSA bacteremia has been associated with a higher likelihood of complications.8
In contrast with vancomycin, a study comparing daptomycin with standard of care therapy for SAB found a similar efficacy for patients treated with cloxacillin or the lipopeptide.94
Empirical treatment when suspecting bacteremia by MRSAVancomycin has been the most common treatment for MRSA bacteremia for the last decades.95 There are very few studies comparing the efficacy of various glycopeptides as empirical treatment in MRSA bacteremia, but there seems to be no significant differences. A randomized clinical trial comparing teicoplanin (loading dose 12mg/kg, followed by 6mg/kg/d) and vancomycin (12mg/kg/d) in invasive infections by Gram-positive microorganisms needed to be stopped because 6 out of 7 patients with endovascular staphylococcal infections in the group of teicoplanin died.96 Thereafter, the dose of 6mg/kg/d was considered to be insufficient for endovascular infections.97 A posterior meta-analysis showed that teicoplanin was non-inferior than vancomycin, while the rate of associated nephrotoxicity was lower.98
Daptomycin is a lipopeptide antibiotic with in vitro activity against Gram-positive bacteria, which is faster and more intense than that of vancomycin.99–102 The only randomized study comparing daptomycin with the standard of care for SAB – vancomycin or a β-lactam – included 246 cases (39% with a probable or definite diagnosis of IE) and concluded that daptomycin was non-inferior than vancomycin.94 In that study there was no data regarding empirical treatment. In a recent cohort study including 579 episodes of bacteremia caused by MRSA, no significant differences were observed regarding the mortality of patients treated with vancomycin or daptomycin (OR 1.42 [95% CI 0.83–2.44]).6 In another case–control study focused in cases of bacteremia caused by MRSA with a vancomycin MIC≥1.5mg/L, a higher survival rate was observed in the group of patients treated with daptomycin.103 The use of suboptimal doses of daptomycin, the presence of non-drained purulent collections and previous exposure to vancomycin – favoring the selection of VISA strains – are parameters associated with a lesser efficacy of daptomycin.104
Antibiotic combinations for empirical treatmentAntimicrobial combinations may be considered in order to widen the antibacterial spectrum, especially when there is no information on the antibiotic susceptibility. It may also increase the bactericidal activity of the treatment, too. The role of combined empirical treatment in the setting of SAB is yet to be defined, though.
The combination of β-lactams and vancomycin has proven no synergy nor antagonism in most in vitro studies.105,106 There is no clinical information on the role of this regime as empirical treatment.
The association of gentamycin with either β-lactams or glycopeptides has shown synergism in several in vitro studies107–110 and also a shorter duration of bacteremia in the setting of left-IE as compared with monotherapies.111 These findings have supported the recommendation of administering combined treatments including a low dose of aminoglycosides for 3–5 days in order to accelerate the clearance of bacteremia.112 However, a clinical benefit in patients with SAB or IE has not been proven, while a higher rate of nephrotoxicity has been observed.111,113,114 In the case of glycopeptides, the combination of two nephrotoxic drugs carries a risk that clearly overcomes the potential benefits proven so far.113,115,116
The combination of daptomycin with antibiotics which block the cell wall synthesis has shown synergy in vitro and promising results in patients with bacteremia and IE caused by MRSA.117–119
In a randomized study, the combination of levofloxacin with the standard-of-care treatment in patients with SAB did not show to improve the clinical outcome.120 The addition of rifampin does not seem to be beneficial due to its potential antagonism with β-lactams and vancomycin.109,121,122Recommendations:
In a suspected episode of SAB, a treatment with bactericidal activity against S. aureus must be started so effective bactericidal concentrations are available as soon as possible, especially for cases presenting with severe sepsis or shock (C-III).
The empirical treatment must include, if possible, a penicillinase-stable β-lactam (A-II).
When the presence of MRSA seems likely, a second antibiotic with bactericidal activity against MRSA should be added (C-III). The following possibilities would be advisable: (1) vancomycin in combination with a β-lactam (B-III); (2) in cases of severe sepsis or shock (C-III), recent use (previous 30 days) of vancomycin (C-III), a higher local prevalence of S. aureus isolates with vancomycin MIC≥1.5mg/L (measured by E-test) (C-III) and/or previous renal impairment (B-III) the use of daptomycin in combination with a β-lactam is preferred (C-III); (3) alternatively, patients may be treated with daptomycin alone at recommended doses of≥10mg/kg/d (A-II).
Management of non-complicated SABCatheter related bacteremiaIn what cases the catheter must be removed?Indications for removing intravenous catheters causing SAB are the same, regardless of the microorganism's methicillin susceptibility.123,124 Three prospective observational studies observed that the removal of the CVC (including those without complications) was associated with an earlier clinical response and with a lower rate of relapse.31,125,126
Tunneled CVC (i.e. Hickman® catheters) or surgically placed CVC (i.e. Port-a-cath®) may be kept only when they are highly needed and no easy alternative vascular access is available; when there are no signs of infection in the skin, the tunnel or the surgical incision; on the grounds of hemodynamic stability; and when there are no metastatic distant complications of the SAB. In these circumstances a conservative treatment may be attempted by local (antibiotic-lock) and systemic therapy.127,128 However, failure rates higher than 50% with this conservative approach have been reported in various observational studies.127,129–132 Keeping the CVC responsible for SAB during more than 72h has been identified as an independent risk factor of persistent bacteremia and death.133 Among patients undergoing hemodialysis via CVC with catheter-related MRSA bacteremia, not removing the intravenous line is an independent predictor of complications.134Recommendations:
The presence of inflammatory signs at the site of insertion of any intravenous line responsible for SAB forces the prompt removal of the catheter (A-II). Catheters should be also removed if infection is suspected (presence of catheter and no other obvious focus), and the catheter is easily replaceable (A-II).
A conservative approach to CRB caused by S. aureus should be only attempted in exceptional circumstances (i.e.: absolute impossibility of removing the catheter for technical reasons) and taking into account the clinical and baseline characteristics of the patient (B-II). In these cases, the antibiotic lock therapy must be administered in combination with an effective systemic antimicrobial treatment (B-II). Anyway, the persistence of bacteremia beyond the first 72hours of a conservative management will lead to the immediate removal of the catheter (B-II).
Who should be screened for ruling out complications of SAB?Initial assessment of a patient with SAB should include a detailed history investigating the presence of predisposing factors for IE, prosthetic material, penicillin allergy; possible primary focus, and data of severe sepsis or shock.
Patients with CRB by S. aureus are at high risk of presenting with distant hematogenous complications, especially when the catheter cannot be removed and/or if the antibiotic treatment is not appropriate.134 The risk of developing IE may be higher than 25% in some types of SAB, this leading to prolonged therapy.135–137
Recent observational studies, including all cases from a cohort of patents with SAB, have found that the risk of developing IE is strongly associated with the presence of complications of this infection, the persistence of bacteremia >72h after the onset of adequate therapy, the recurrence of bacteremia or the presence of intracardiac devices.138–140 The systemic performance of transesophageal echocardiography (TEE) to all patients presenting with CRB caused by S. aureus is some controversial, due to the lack of prospective studies specifically addressing this question.141
One of the most important predictive parameters of complicated SAB is the persistence of bacteremia after removing the intravenous catheter.142,143 The treatment of persistent bacteremia is discussed in the next chapter. In these circumstances, it is necessary performing TEE in order to rule out IE.142 Since septic thrombophlebitis may give place to a similar clinical picture, the absence of echocardiographic signs of IE makes it necessary to perform an ultrasound study of the central veins or other vascular accesses where the responsible catheter had been placed, and eventually extend the length of therapy.
In recent years, the use of new molecular imaging methods such as 18F-fluoroglucose (18F-FDG) PET-CT has shown higher sensibility than TEE for the detection of an inflammatory valvular or perivalvular process among patients carrying intracardiac devices, with a very high negative predictive value, and it seems reasonable to recommend its performance in patients with suspected intracardiac device-associated infections when TEE has not been diagnostic.144–146 The usefulness of this technique for the systematic evaluation of patients with SAB has not yet been well defined.Recommendations:
A careful evaluation of the patient's symptoms and an exhaustive clinical examination are essential in cases of catheter related SAB in order to rule out possible sources of the infection. The presence of eventual metastatic septic foci must be identified (B-II).
Blood cultures must be taken after 72h of the onset of appropriate antimicrobial therapy in order to rule out complicated bacteremia (A-II).
Systematically performing TEE to all patients with CRB by S. aureus in order to decide the length of therapy remains controversial. The absence of valvular risk (no valvular disease, neither previous nor diagnosed at the moment of SAB) along with a clinical and microbiological response (negative blood cultures) to therapy within the first 72h after the catheter removal and onset of adequate antibiotics are associated with a favorable outcome (absence of complications or relapse) in more than 95% of patients receiving treatment for at least 14 days after negative blood cultures (B-II).
The length of therapy needs to be adapted to the findings of the TEE or central veins ultrasonography, when indicated (A-II).
The role of new imaging molecular techniques for the diagnosis of intracardiac device-associated infections has not been fully elucidated (C-II).
What is the definitive antibiotic treatment of catheter-related bacteremia?The treatment of choice for CRB caused by MSSA will be a β-lactamase resistant isoxazolic penicillin, such as intravenous cloxacillin at a dose of 2g/4h.147 In the case of intolerance or allergy to β-lactams, vancomycin may be used. However, this antibiotic is less effective than β-lactams for MSSA bacteremia, the incidence of relapse and persisting bacteremia being higher.88,91,92,148,149
Some recent studies (but not all) have observed a worse prognosis in cases of MSSA bacteremia when vancomycin MIC is ≥1.5mg/L (measured by E-test), regardless of the antibiotic chosen as treatment.8,9,150 Although there are no definitive information in this field., alternative antimicrobial regimes could be considered, such as daptomycin, alone or in combination with β-lactams or fosfomycin, especially in the case that blood cultures remain positive and/or clinical improvement is not evident after catheter removal (C-III).
In the case of MRSA, susceptibility to vancomycin must be considered in the prognosis of bacteremia,65,81 along with the clinical response. In patients with no fever and clinical stability after 24–48h of antibiotic treatment and removal of the catheter, therapy with glycopeptides could be continued regardless of the isolate's MIC to these antibiotics (as long as it is within the susceptibility range). Although there are no controlled studies, in patients with not so favorable clinical response and isolates with low susceptibility to vancomycin (MIC≥1.5mg/L measured by E-test) it seems reasonable to treat with alternative antibiotics.103,151
In this regard and with the available data, daptomycin and linezolid are the two possible candidates to be considered. Daptomycin is a rapidly bactericidal antibiotic against MRSA. In a large, randomized trial including patients with SAB, daptomycin showed a similar efficacy as cloxacillin and vancomycin for bacteremia caused by MSSA and MRSA, respectively.94 In the subgroup of patients with non-complicated SAB, good results were obtained with the currently recommended dose of 6mg/kg/d. Also, the rate of renal toxicity was lower in patients treated with daptomycin as compared with those treated with vancomycin.113
Linezolid is a bacteriostatic antibiotic with activity against MRSA, a 100% bioavailability and good diffusion to tissues (including poorly vascularized areas). Its efficacy has been proven in the setting of pneumonia, bacteremia and severe skin and soft tissue infections. In a meta-analysis of 5 randomized controlled trials in patients with bacteremia caused by MRSA, linezolid was non-inferior to vancomycin.152 A more recent meta-analysis found that linezolid had a higher efficacy against Gram-positive microorganisms, as compared with glycopeptides or β-lactams153; however, poor information on the specific pathogens or sites of infection was provided, thus strong conclusions on the efficacy of linezolid for SAB could not be drawn. In a posterior randomized clinical trial, linezolid proved to have a similar efficacy as comparators for CRB by S. aureus, including MRSA.154 No data are available for treatment with linezolid in patients with SAB due to strains with low sensitivity to vancomycin.Recommendations:
- •
The treatment of choice of an episode of CRB caused by MSSA is cloxacillin (B-I).
- •
Alternatively, patients may be treated with daptomycin (A-I) or a glycopeptide (B-II).
- •
The best antimicrobial treatment in episodes caused by a strain of MSSA with low susceptibility to vancomycin (MIC≥1.5mg/L measured by E-test) has not been elucidated. This panel suggests to use a combination of cloxacillin and daptomycin when blood cultures remain positive and/or clinical improvement is not evident after catheter removal (C-III).
- •
In the case of CRB caused by MRSA, vancomycin is the treatment of choice (B-II). It may be continued in stable patients with negative blood cultures after 72h of treatment, regardless of the susceptibility of vancomycin (C-III).
- •
Alternatively, patients may be treated with daptomycin (A-I).
- •
Linezolid should be only used in patients who cannot take the previous agents (B-II).
The identification of patients with SAB that are evolving favorably – thus may be classified as having non-complicated bacteremia – may be helpful for deciding not to extend the therapy beyond 14 days. This will consider the patient's baseline features, the clinical evolution and the microbiological parameters.
The absence of diabetes, intravascular devices (such as pacemakers or vascular prosthesis), or any immunosuppressant condition (such as neutropenia, or being under therapy with corticoids or other immunosuppressant drugs) is associated with a good prognosis. The removal of the catheter and the absence of complications such as IE, septic thrombophlebitis or any metastatic foci are also associated with a good outcome. Early defervescence and negative blood cultures within the first 72h are also associated with a non-complicated evolution and, all together, may support a treatment not longer than 14 days.31,80,88,134,135,142,155,156Recommendations:
An episode of CRB caused by S. aureus may be considered as non-complicated in the basis of several characteristics of the host (such as absence of diabetes, immunosuppressant conditions and intravascular devices), of the clinical presentation, and of the clinical and microbiological evolution (clearance of bacteremia in less than 3 days of treatment).
For how long must the patients be treated?The length of therapy for episodes of non-complicated CRB caused by S. aureus has not been well defined by controlled studies. A meta-analysis showed that, after 10–14 days of systemic antimicrobial therapy, the rate of relapse was only 6.1%.157 In more recent series including well-selected cases of non-complicated CRB, the rate of recurrence has been higher in patients treated for less than 14 days, as compared with patients treated for a longer period.155 There is no available information regarding length of therapy in CRB caused by MRSA. However, according to various controlled trials,94,158 it seems reasonable to treat these cases for a similar period of time, as long as the bacteremia is non-complicated and the catheter has been removed. Sequential oral therapy with drugs such as linezolid could be considered in clinically stable patients, with no metastatic complications and with negative blood cultures after the onset of treatment and the removal of the intravenous line.154Recommendations:
Systemic antibiotics in cases of non-complicated CRB caused by S. aureus must be administered for a period not shorter than 14 days (A-II). In patients with favorable clinical and microbiological evolution, sequential oral antibiotics may be considered (A-II).
Primary SABWhat tests should be performed in patients with apparent primary SAB?In patients with no apparent source of SAB an exhaustive anamnesis should be made in order to rule out potential origins of the infection. The presence of permanent devices or foreign bodies, such as catheters, pacemakers, valve prosthesis or orthopedic prostheses must be specifically addressed. Also, patients must be carefully searched for unnoticed skin lesions, or symptoms suggesting distant infectious metastasis (which may occur in up to 30% of cases), such as back pain (indicating vertebral osteomyelitis or epidural abscess), low back pain (in the case of renal or psoas abscesses) and prolonged fever or sweating (suggesting IE). Adequate complementary tests will be performed according to these findings.
The frequency of IE among patients presenting with SAB ranges between 10% and 30%, the higher rate being associated with community-acquired primary SAB or in patients with intracardiac medical devices.142,159,160 Therefore, these patients should undergo an echocardiography. Initially it may be a transthoracic echocardiography (TTE), but if no vegetations are found, the performance of a TEE is recommended.136,161Recommendations:
A careful evaluation of the patient's symptoms and an exhaustive clinical examination are essential in cases of primary SAB in order to rule out possible sources of the infection (C-I). A reliable echocardiographic test should be performed in carriers of intracardiac devices and in cases of community-acquired SAB (A-II).
What is the length and type of the definitive antimicrobial treatment?The basis for the choice of the antimicrobial regime for patients with primary SAB is not different from that of CRB caused by S. aureus (see above). The majority of studies have analyzed heterogeneous samples of patients, including cases with no identifiable source of SAB.8,9,65,81,88,91,92,94,103,113,147–153 Therefore, recommendations on treatment under this circumstance may be the same.
The absence of an identifiable origin of SAB is a risk factor for complication, especially in community-acquired cases. The length of therapy depends on whether the bacteremia is complicated or not. For non-complicated cases, a minimum of 14 days of therapy is recommended in order to avoid relapse of the infection. However, not all authors agree with this opinion, some of them suggesting that community acquired SAB, or cases with no identifiable source should be always considered as complicated.155,162,163
Patients carrying intravascular devices (either valvular or vascular prostheses) should receive a longer treatment (4–6 weeks). Patients with previous valvular heart disease where a TEE (performed after 5–7 days of the onset of bacteremia) has found no vegetations may be treated for 14 days.Recommendations:
Recommendations for the specific definitive antimicrobial treatment for primary SAB do not differ from those of CRB by S. aureus (B-II). The duration of antibiotics should be no shorter than 14 days (B-II). In patients carrying intravascular prostheses, the length of therapy will depend on the findings of the complementary tests performed to discard a secondary involvement of these devices (C-I).
Management of complicated SABComplicated SAB is defined as the persistance of positive blood cultures after three or more days of adequate tretment (including catheter removal), and/or the development of septic thrombophlebitis, IE, or other metastatic distant foci.
Which clinical and microbiological evaluation must be made in patients with complicated SAB?Repeated blood culturesThe persistence of positive blood cultures after 72h of appropriate antimicrobial treatment indicates complicated SAB, leading to the repetition of new blood cultures for assessing the clearance of bacteremia. There are no solid recommendations on the frequency for repeating these cultures. However, the persistence of bacteremia beyond 7 days of adequate therapy is an important landmark. When this happens, treatment failure must be considered, as well as the need for changing the antibiotics and double check for the presence of non-drained infectious foci.
Identification and removal of primary or secondary fociThe optimal management of complicated SAB includes the administration of appropriate antimicrobial therapy and the identification and drainage of infectious foci, either primary or secondary. The intravenous line catheter is one of the most frequent origins of SAB.
Apart from the intravenous catheter, and given the high frequency of subclinical venous thrombosis in the setting of CRB, some authors support the systematic performance of vein ultrasounds.164 However, this may detect many abnormalities which may be difficult to interpret. A more pragmatic approach is to perform such ultrasound test only in cases of persistent bacteremia. In such cases, the finding of thrombosis is diagnostic of septic thrombophlebitis, leading to a minimum of 4 weeks of antimicrobial treatment.
A frequency of 30–40% of hematogenous seeding of a foreign body in the course of SAB has been reported.165,166 Therefore, patients carrying a device or prosthesis must follow a careful evaluation. The eventual infection of the prosthesis will need to be managed accordingly, the odds of therapeutic failure being higher. In the case of prosthetic valve endocarditis, early surgery is associated with a better prognosis.167
The performance of an 18F-FDG-PET/CT in the context of complicated SAB with neither evident source nor IE may help to find the origin of the infection and/or distant septic metastasis.168
EchocardiogramThe risk of having IE in the setting of SAB is very high, especially among carriers of prosthetic valves (5–20% of all episodes).142,169 Some studies advocate for the performance of TEE to all patients with SAB (either complicated or not), arguing that it is cost-benefit in terms of length of therapy,170 while some groups prefer a more conservative approach, and indicate TEE in the case of persistent bacteremia or in carriers of intracardiac devices.138Recommendations:
Blood cultures must be repeated every 72hours in order to monitor the microbiological response to antibiotic therapy (A-II). Make it sure that an intravenous catheter left in place is not the origin of the persistent bacteremia (A-II). When a foreign-body (i.e. prosthetic joints or prosthetic valves) becomes infected, the indication of surgery for debridement and/or removing the device must be considered (A-II). It is necessary to perform an echocardiography to all patients with complicated SAB. In patients carrying an intracardiac device or in those with persistent bacteremia, a TEE is preferable (A-II).
What is the treatment for complicated bacteremia caused by MSSA?In this setting, the treatment of choice is intravenous cloxacillin or cefazolin, either as intermittent bolus or in continuous infusion.85,149 Failures using cefazolin have been described in infections with a high inoculum when the strain produces class A beta-lactamase.85,149 The combination of antimicrobials pursuing a higher bactericidal activity has been used in the past, especially in cases of IE. However, there is scarce clinical evidence supporting combined therapy, and currently it is only recommended in particular clinical contexts.
Recently, various studies have observed a higher frequency of complicated SAB and mortality in cases of MSSA bacteremia when vancomycin MIC≥1.5mg/L (measured by E-test), regardless of the antimicrobial treatment used.8,9,171 Some non-randomized studies have shown that the combinations of daptomycin with either β-lactams or fosfomycin may be synergistic for SAB.118,172 These studies suggest that these combinations could be useful for MSSA bacteremia, both in severe sepsis or in cases caused by isolates with vancomycin MIC≥1.5mg/L (measured by E-test).
Combination with aminoglycosidesSome studies published decades ago, including intravenous drug users, observed a 1-day reduction in the duration of bacteremia with combined therapy. However, the addition of an aminoglycoside in the first days of therapy had no impact on mortality, and was significantly associated with renal toxicity.111,113,173
Combination with rifampinThe good intracellular and tissue diffusion of rifampin could potentially lead to a faster control of complicated bacteremia. However, there is no clinical evidence supporting that the combination with rifampin is more active than β-lactam monotherapy.122 Notwithstanding, an ongoing randomized study will try to confirm whether the addition of rifampin to the standard treatment of SAB is able to decrease the mortality.174Recommendations:
The treatment of choice for complicated bacteremia caused by MSSA is cloxacillin, either 2g every 4h, or administered in continuous infusion (A-I). Combined therapy is recommended in the following scenarios: (1) persistence of fever; lack of improvement of signs and symptoms (B-III); (2) microbiological failure detected by the positivity of subsequent blood cultures, especially in episodes by an isolate with vancomycin MIC≥1.5mg/L (measured by E-test). The possible options for combined therapy are: (1) cloxacillin 2g/4h iv+daptomycin 10mg/kg/d iv; (2) cloxacillin 2g/4h iv+fosfomycin 2g/6h iv (A-III). The length of therapy in complicated bacteremia is variable, ranging between 4 and 6 weeks according to the clinical evolution and the source of infection. The length of combined therapy is not established, but it seems reasonable to maintain at least it until blood cultures became negative.
What is the treatment for complicated bacteremia caused by MRSA?Until recently, vancomycin has been the treatment of choice for bacteremia caused by MRSA, either complicated or not.175 However, vancomycin has been associated with renal toxicity, treatment failure and high mortality.176 Various strategies have tried to ameliorate vancomycin's results, such as modifying the dosage, or treating with alternative antibiotics, namely daptomycin and antimicrobial combinations. Herein we describe the options for the treatment of MRSA complicated bacteremia, their rationale and their efficacy.
VancomycinVancomycin MIC is a key parameter for the efficacy of this antibiotic in MRSA complicated bacteremia, the ratio AUC/MIC over 400 being associated with optimal antimicrobial activity. When the MIC value is ≥1.5mg/L, this PK/PD goal may not be easily achieved with the standard dose of vancomycin (15mg/kg/12h), this probably leading to treatment failure, especially in complicated bacteremia.65,177 In order to achieve this AUC/MIC ratio, doses of vancomycin may be increased so the trough levels are over 15mg/L. However, this has been associated with higher renal toxicity, especially in long treatments.178 Therefore, this strategy is no longer recommended.
Importantly, it has been observed that the vancomycin MIC creeping is associated with a higher likelihood of treatment failure, regardless of the method for determining the MIC value and also the antibiotic treatment administered.43 While this has been questioned by a recent meta-analysis,7 this would suggest that MRSA isolates with low susceptibility to vancomycin may associate other unknown virulence parameters which may lead to treatment failure.179
DaptomycinAs previously discussed, daptomycin possesses a faster bactericidal effect and less toxicity than vancomycin. The efficacy is similar,94 or even superior when vancomycin MIC is higher than 1.5mg/L (measured by E-test).103,151,180 Doses of daptomycin have been increased since its approval (from 6 to 8–10mg/kg/d) in order to avoid the emergence of resistance,181 and to reduce the mortality, although the latter point has not been clearly proven.6,182
Daptomycin plus β-lactamsSeveral in vitro studies have observed synergy with the combination of daptomycin plus β-lactams against MRSA,183,184 including VISA isolates. In addition, the combination may avoid the emergence of resistance185 and also increase the activity of β-lactams by a mechanism which is independent from the gene mecA (seesaw effect) in isolates with low susceptibility to daptomycin.186
Clinical experience with this combination is scarce. In a study including 7 consecutive patients with persistent MRSA bacteremia who had been previously treated with daptomycin or vancomycin, the combination of daptomycin (8–10mg/kg/d) plus oxacillin or nafcillin (2g/4h) led to a rapid clearance of bacteremia in all seven cases. In vitro studies performed on 3 isolates from this study showed an enhanced membrane daptomycin binding and a higher bactericidal activity.117 In patients registered in the Cubicin® Outcomes Registry and Experience (CORE, 2005–09), with mild or medium renal impairment and treated with daptomycin for SAB (mainly caused by MRSA), a trend toward a better outcome was observed among patients that were also treated with β-lactams.172 Thus, this combination may be more effective than monotherapy for complicated bacteremia caused by MRSA. However, more studies supporting this hypothesis are necessary, and also defining which β-lactams are synergistic and what doses should be used.
Daptomycin plus fosfomycinFosfomycin is a bactericidal antibiotic with activity in the early steps of the cell wall peptidoglycan synthesis. Its unique mechanism of action makes cross-resistance with other antibiotics very rare, most isolates of MRSA being susceptible. However, fosfomycin must be administered in combination with a second drug in order to avoid the rapid development of resistance.
The clinical experience with the combination daptomycin-fosfomycin is limited. Notwithstanding, in vitro studies have proven synergy with this combination.118 In addition, avoidance or delay of daptomycin-resistance emergence has been observed.185 Various experimental models have confirmed the synergy of the combination, one of the first being a model of experimental enterococcal endocarditis.187 More recently, a model of experimental foreign-body infection caused by MRSA has also proven the activity of the combination.188
Clinical experience with daptomycin plus fosfomycin is still anecdotal. The most important experience included three patients with left-side endocarditis (one by MSSA on a prosthetic valve and two by MRSA) treated with daptomycin (10mg/kg/d) plus fosfomycin (2g/6h); a surgical management was considered, but outcome was favorable for the three patients with only medical treatment.118 Another case of IE caused by daptomycin-resistant MRSA was treated with daptomycin (12mg/kg/d) plus fosfomycin (6g/6h), also with good results.189 It must be stressed that the intravenous administration of fosfomycin includes a high sodium concentration, therefore caution is recommended in patients with liver cirrhosis or heart failure.
As with the combination with β-lactams, daptomycin plus fosfomycin seems to be promising in the management of MRSA complicated bacteremia, but again further prospective studies proving the superiority of this treatment are necessary.
Fosfomycin plus imipenemThe mechanism of synergy of this combination is not fully understood. The early inhibition of the cell wall synthesis due to fosfomycin's activity may produce impairment in PBP2a. The wall synthesis would depend again on the activity of PBP2, and therefore the bacteria would become susceptible to imipenem. Various in vitro, experimental studies and clinical experience support the efficacy of the combination of fosfomycin and β-lactams,190–192 and synergy has been observed for the combination with imipenem.193 However, clinical experience is still very scarce. In a Spanish multicentre study, fosfomycin-imipenem was administered to 16 patients as salvage therapy. Clearance of bacteremia was observed within 72h in all patients.194 This supports the use of this combination as salvage therapy for patients with complicated bacteremia caused by MRSA.Recommendations:
The best treatment for complicated MRSA bacteremia has not been elucidated. The treatment with vancomycin is associated with a high rate of treatment failure, especially in the following situations: (1) if vancomycin MIC≥1.5mg/L (measured by E-test) (A-II); (2) if the patient has renal impairment or is at risk of renal toxicity (A-II).
Doses of 6mg/kg/d of daptomycin have been associated with treatment failure and emergence of resistance. Daptomycin at doses of 10mg/kg/d is the treatment of choice for MRSA complicated bacteremia (A-III).
Patients with persistent bacteremia or severe sepsis or shock in the setting of treatment with high doses of daptomycin may benefit from combined therapy. The options are: (1) daptomycin (10mg/kg/d)+fosfomycin (2g/6h) (A-III); (2) daptomycin (10mg/kg/d)+cloxacillin 2g/4h (A-III); imipenem (1g/6h) plus fosfomycin (2g/6h) (A-III).
The administration of high doses of fosfomycin may lead to sodium overload and hypokalemia (1g of fosfomycin-disodium carries 13.5mEq [330mg] of Na). The duration of treatment for complicated bacteremia is variable, ranging from 4 to 6 weeks, depending on the clinical evolution and the source of the infection.
How is treatment failure in complicated SAB defined clinically and microbiologically?As it happens with other serious infections, a narrow monitoring of clinical (blood pressure, heart rate, respiratory rate and temperature) and analytical parameters (leukocyte and polymorphonuclear cell count, serum C-reactive protein and serum creatinine) must be performed. There is not detailed information on the evolution of these parameters during the early phase of SAB (first 3–5 days) in order to know whether the antimicrobial treatment is appropriate or should be changed. However, we might extrapolate our knowledge from patients with severe sepsis caused by other microorganisms. In a prospective study on 891 patients admitted in the ICU for community-acquired sepsis, the reduction of C-reactive protein (CRP) after 5 days of treatment was significantly associated with the intrahospitalary mortality, after adjusting by the grade of sepsis severity. The authors were able to classify the patients in three groups, according to the CRP ratio between day 1 and day 5: mortality rate was 14%, 20% and 30% for patients with a <40%, 40–80% and 80% ratio, respectively (<0.001).195 These data suggest that, in the setting of severe infections such as complicated SAB, the dynamics of CRP during the first 5 days of treatment may be useful to evaluate the response to antibiotics and/or the need for draining a purulent foci (i.e. abscess) or removing an infected device (i.e. pacemaker or prosthetic valve).
The persistence of positive blood cultures beyond the third day after the onset of appropriate antimicrobial therapy is associated with the risk of presenting distant septic metastasis89 or other complications, including death.142,196 Therefore, blood cultures should be systematically taken every 48–72h in order to acknowledge the clearance of bacteremia. The persistence of SAB has been related with: (1) host's baseline features89; (2) microbiological characteristics of the staphylococcal isolate197; (3) the management of the source of the infection134; and (4) the initial antimicrobial therapy.89 We can only modify the two last. Thus, the confirmation of persistent bacteremia forces to drain any existent focus of infection or to remove an infected device, if this had not been done before. It is less evident when to consider that the initial antimicrobial treatment has failed and should be modified.Recommendations:
In patients with complicated SAB, a daily monitoring is necessary for evaluating the response to the antimicrobial therapy (A-III). Consecutive determinations of CRP (every 24–48h) during the first week of treatment may be a useful marker for an early evaluation of the treatment efficacy (B-III). It is also recommended to take new blood cultures every 48–72hours until they are negative (C-III). In cases of persistent bacteremia, the antimicrobial treatment should be reevaluated (A-III).
Is it necessary to administer the whole treatment by the intravenous route?In the setting of complicated SAB, most guidelines recommend to start antibiotics by the intravenous route. On clinical grounds, an oral treatment could be considered if blood cultures have became sterile, the patient has had no fever for more than 24h, the origin of the infection has been drained and systemic inflammatory parameters (i.e. CRP) have significantly improved. There is clinical experience with oral treatments after a variable period of intravenous antibiotics (1–2 weeks) in IE and other staphylococcal infections with β-lactams,198 clindamycin199 and linezolid.200
The choice of the oral antibiotic depends in its intrinsic activity (MIC) and its pharmacokinetic characteristics (oral bioavailability, half life, protein binding and tissue diffusion), which should guarantee, at the recommended dose, enough free concentrations in both serum and the focus of infection, in order to accomplish the PK/PD parameter predicting its efficacy.
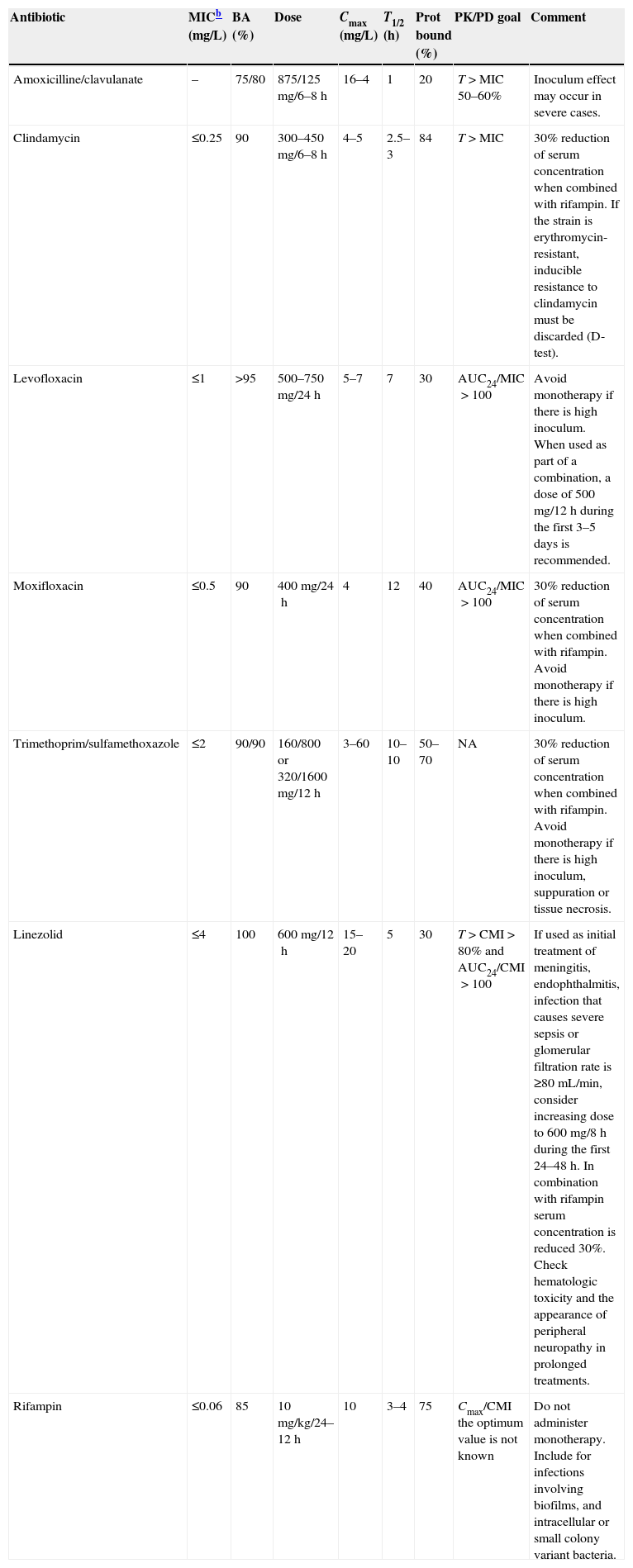
Finally, attention should be paid on the potential development of biofilm (especially if there are foreign bodies involved), and on the eventual presence of intracellular bacteria or small colony variants, which have been reported in episodes of bacteremia, abscesses, osteomyelitis and prosthetic material infections.201,202 Treatment with a rifampin-based combination should be considered under these circumstances. The possible options for oral treatment are summarized in Table 1.
Oral antimicrobial alternatives for patients with SAB candidates to sequential treatment.a
| Antibiotic | MICb (mg/L) | BA (%) | Dose | Cmax (mg/L) | T1/2 (h) | Prot bound (%) | PK/PD goal | Comment |
|---|---|---|---|---|---|---|---|---|
| Amoxicilline/clavulanate | – | 75/80 | 875/125mg/6–8h | 16–4 | 1 | 20 | T>MIC 50–60% | Inoculum effect may occur in severe cases. |
| Clindamycin | ≤0.25 | 90 | 300–450mg/6–8h | 4–5 | 2.5–3 | 84 | T>MIC | 30% reduction of serum concentration when combined with rifampin. If the strain is erythromycin-resistant, inducible resistance to clindamycin must be discarded (D-test). |
| Levofloxacin | ≤1 | >95 | 500–750mg/24h | 5–7 | 7 | 30 | AUC24/MIC>100 | Avoid monotherapy if there is high inoculum. When used as part of a combination, a dose of 500mg/12h during the first 3–5 days is recommended. |
| Moxifloxacin | ≤0.5 | 90 | 400mg/24h | 4 | 12 | 40 | AUC24/MIC>100 | 30% reduction of serum concentration when combined with rifampin. Avoid monotherapy if there is high inoculum. |
| Trimethoprim/sulfamethoxazole | ≤2 | 90/90 | 160/800 or 320/1600mg/12h | 3–60 | 10–10 | 50–70 | NA | 30% reduction of serum concentration when combined with rifampin. Avoid monotherapy if there is high inoculum, suppuration or tissue necrosis. |
| Linezolid | ≤4 | 100 | 600mg/12h | 15–20 | 5 | 30 | T>CMI>80% and AUC24/CMI>100 | If used as initial treatment of meningitis, endophthalmitis, infection that causes severe sepsis or glomerular filtration rate is ≥80mL/min, consider increasing dose to 600mg/8h during the first 24–48h. In combination with rifampin serum concentration is reduced 30%. Check hematologic toxicity and the appearance of peripheral neuropathy in prolonged treatments. |
| Rifampin | ≤0.06 | 85 | 10mg/kg/24–12h | 10 | 3–4 | 75 | Cmax/CMI the optimum value is not known | Do not administer monotherapy. Include for infections involving biofilms, and intracellular or small colony variant bacteria. |
Resistance breakpoint, according to EUCAST (www.eucast.org).
SAB, Staphylococcus aureus bacteremia; MIC, minimal inhibitory concentration; BA, bioavailability; T1/2, half life; Prot bound, protein bound; PK/PD goal, pharmacokinetic/pharmacodynamic parameter predicting the efficacy of the antibiotic; T>MIC, time that the serum concentration is over the MIC; ABC24/MIC, ratio of the area under the curve of serum concentration during 24h and MIC; NA, no data available.
The use of oral antibiotics from the beginning in complicated SAB has been tested in patients in whom an intravenous access was not possible or very difficult (i.e. intravenous drug users). Two randomized open studies compared the efficacy of oral rifampin plus ciprofloxacin vs. conventional treatment with vancomycin or a β-lactam (oxacillin or flucloxacillin) among patients with right-side IE or other infections.203,204 In both studies the oral treatment was similar to the intravenous therapy, in terms of efficacy and tolerance. Currently, the most active fluoroquinolone against S. aureus is moxifloxacin, followed by levofloxacin. However, rifampin decreases moxifloxacin's levels in 30%,205,206 while this does not happen with levofloxacin.207 This has also been reported for clindamycin,208 cotrimoxazol209 and linezolid210 in a similar degree. Therefore, levofloxacin is the antibiotic of choice to be combined with rifampin.
There is also some experience with linezolid as initial or salvage therapy for SAB. A systematic review published in 2006 included 18 cases of IE caused by S. aureus receiving linezolid either for prior treatment failure or toxicity, or due to absence of intravenous access. In 13 cases (72%) the outcome was favorable. This review included two case series with similar results.211 More recently, Muñoz and cols.200 reported 9 cases of IE (8 left-sided and 1 right-sided) treated with linezolid due to failure (n=2) or intolerance (n=4) of previous treatment, or as sequential oral treatment (n=3). Again, more than 70% of cases presented a good evolution.
Cotrimoxazole is a highly bioavailable antibiotic with a fast bactericidal activity. However, in a double-blind randomized clinical trial including 101 patients with infection caused by S. aureus, an oral dose of 320/1600mg/12h was significantly less effective than vancomycin 1g/12h 212. A possible reason for this would be that, in infections with high inoculum and in the presence of tissular necrosis, the concentration of thymidine may be increased, this being used by bacteria to antagonize the effect of cotrimoxazole.201 Therefore, this antibiotic should be reserved as sequential oral therapy, once blood cultures are negative and the infectious foci has been drained or the necrotic tissues have been removed.Recommendations:
In complicated SAB, antimicrobial treatment should be administered entirely by the intravenous route. An oral sequential treatment may be considered for patients accomplishing the following requirements: (1) the patient has presented no fever for at least 24h; (2) blood cultures are negative; (3) the origin of infection has been drained; and (4) the parameters of systemic inflammation (i.e. CRP) have significantly decreased (C-III). In exceptional situations where an intravenous access is not possible, there is some experience supporting the use of oral fluoroquinolones plus rifampin (B-II).
Management of infective endocarditis caused by S. aureusEmpirical antimicrobial treatment in IE caused by S. aureusHow frequent is S. aureus in IE and how important is to include this etiology in the empirical treatment of IE?S. aureus is the most frequent etiology of IE worldwide, both in native valve endocarditis and in early prosthetic valve endocarditis, as well as in the infection of intracardiac devices.213 In the study by Murdoch and cols.213 including 2781 cases diagnosed from 2000 to 2005 in several countries from the five continents, S. aureus was the cause in 31%, it being methicillin-resistant in 27%. More recent studies report increasing rates of staphylococcal IE,214 despite the decrease of cases in intravenous drug users (IVDUs). The increasing rate is probably in relation with more prevalent health-care associated cases,215 implantation of intracardiac devices, patients in hemodialysis, diabetes mellitus or MRSA skin colonization.80
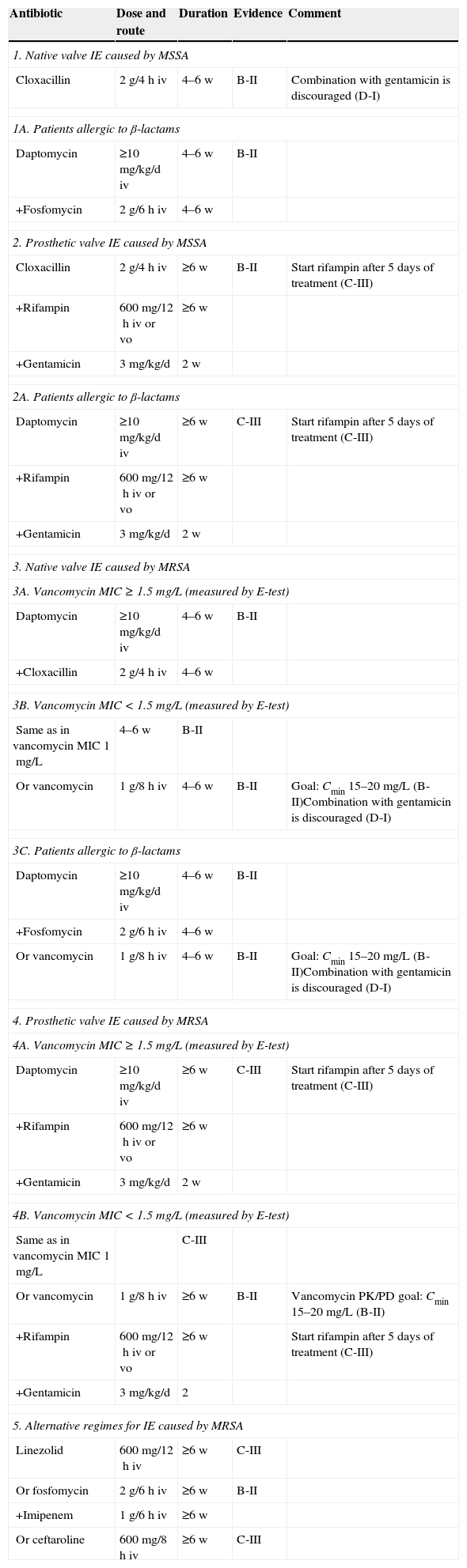
In spite of the increasing incidence of IE caused by S. aureus, therapeutical innovations and the indication for surgery have barely improved the prognosis of this infection over the last 25 years, current mortality rates being over 20% and around 40% for MSSA and MRSA IE, respectively.80,216 Surgery is performed in 25 to 35% of cases, with no differences between MSSA and MRSA episodes.80,216 This is observed in many old and recent studies, where S. aureus has proven to be an independent risk factor for mortality in left side IE.80,213 Therefore, providing appropriate antimicrobial treatment during the first 24–48h is key in the approach to patients with suspected IE caused by S. aureus, until definitive identification of the etiology and its antibiotic susceptibility profile are available. Empirical treatment recommendations for IE are summarized in Table 2.Recommendations:
Empirical antimicrobial treatment for infective endocarditis (IE) caused by S. aureus.
| Antibiotic | Dose and route | Evidence | Comment |
|---|---|---|---|
| 1. Community-acquired IE (native valve or late IE on prosthetic valve) | |||
| Cloxacillin | 2g/4h iv | B-II | Combine with daptomycin (≥10mg/kg/d) in severe sepsis or septic shock (C-III).Combination with gentamicin is discouraged in native valve endocarditis (D-I)IVDUs with non-complicated right-side IE and negative blood cultures in 72h may be treated for 2 weeks (A-I) |
| 1A. Patients allergic to β-lactams | |||
| Daptomycin | ≥10mg/kg/d iv | B-II | In cases of non-anaphylactic allergy, treatment with cefazolin may be considered. |
| +Fosfomycin | 2g/6h iv | ||
| 2. Nosocomial or health-care related IE (native and prosthetic valve) | |||
| Daptomycin | ≥10mg/kg/d iv | B-II | |
| +Cloxacillin | 2g/4h iv | ||
| 2A. Patients allergic to β-lactams | |||
| Daptomycin | ≥10mg/kg/d iv | B-II | |
| +Fosfomycin | 2g/6h iv | ||
| 2B. Alternative | |||
| Vancomycin | 1g/8h iv | C-III | Goal: Cmin 15–20mg/L (B-II) |
| +Cloxacillin | 2g/4h iv | ||
iv, intravenous; IVDUs, intravenous drug users.
The empirical antimicrobial treatment of an episode of complicated bacteremia or IE should include S. aureus whenever there are reasonable doubts on its potential role as etiology, given its high and increasing incidence and severity.
Therefore, active antibiotics against S. aureus should be included in the empirical treatment in the following cases: suspicion of community-acquired IE (either in IVDUs or not); suspicion of acute IE or presenting with severe sepis (B-II); and early IE, associated to pacemakers or defibrillators (B-II), or in nosocomial cases or in health-care associated cases (B-II).
In which patients with SAB the possibility of IE should be taken into account when choosing empirical treatment?To date, the clinical evidence suggests that the appropriateness of the empirical antibiotic treatment has an important impact in the prognosis of complicated SAB.6 In this context, the choice of the most appropriate empirical antibiotics may be guided by three fundamental principles: (i) patients with a clinical presentation strongly suggesting IE and having particular epidemiological and/or clinical characteristics making S. aureus a probable etiology, the results of blood cultures not being available at the time of starting the antibiotics; (ii) patients with a clinical presentation strongly suggesting IE and blood cultures being positive for Staphylococcus spp, the species and the susceptibility to methicillin still unknown; (iii) SAB in a patient with risk factors for IE, the susceptibility to methicillin still unknown.
In the setting of IE, the clinical features suggesting S. aureus as the etiology include acute clinical presentation, with severe sepsis or septic shock, the presence of major embolic events (i.e., ictus), a new regurgitating murmur and, in the case of right side IE, the presence of pulmonary emboli.80,216 In the setting of SAB, the features associated with a higher likelihood of having IE are: community-acquired primary SAB; SAB of unknown source; hospital-acquired SAB; persistent fever and bacteremia; the presence of acute skin lesions; previous valve diseases or the presence of prosthetic valves; IVDUs; and previous episodes of IE.142,217 In a study including 736 patients with hospital-acquired SAB (mainly CRB episodes), independent risk factors for IE were the presence of a prosthetic valve or other intracardiac devices, and prolonged bacteremia.138 In the absence of persistent bacteremia, intracardiac devices, hemodialysis or osteomyelitis, the likelihood of IE is very low (negative predictive value near 100%), therefore TEE would not be necessary.138Recommendations:
In the setting of SAB, it is recommended considering the diagnosis of IE until it has been ruled out by complementary tests (namely TEE) in the following scenarios: community-acquired episodes (B-II); IVDUs (B-II); presence of skin lesions suggesting hematogenous seeding (B-II); and nosocomial bacteremia in the presence of prosthetic valves or intracardiac devices (B-II).
What clinical and epidemiological characteristics may lead to include MRSA in the empirical treatment?Risk factors for MRSA have been previously discussed. To date, there are few papers addressing the likelihood of MRSA as etiology of staphylococcal IE. In a recent study including 72 episodes of IE caused by S. aureus, 22% were caused by MRSA, the risk factors being: nosocomial origin, surgical procedures within the previous 6 months, surgical wound infection and the presence of an intravenous catheter.218-222 In the prospective International Collaboration on Endocarditis (ICE) study, including 424 episodes of IE caused by S. aureus, 33% of cases were due to MRSA and were significantly associated with diabetes, chronic immunosuppressant therapy, cancer, acquisition in a health-care environment, presence of intravascular devices, and recent invasive procedures.80
MSSA is more frequent in community acquired SAB with no apparent source (>90%)80 and in right side native valve IE among IVDUs. However, except for rare cases, the clinical presentation is rarely able to predict whether the etiology of IE will be MSSA or MRSA. Persistent bacteremia is more frequently caused by MRSA.80,218Recommendations:
The empirical antimicrobial treatment for IE should include activity against MRSA in any of the following instances: nosocomial cases (B-II), previous nasal or skin colonization by MRSA (B-II); patients from nursing-homes (B-II) or in hemodialysis (B-II), surgical procedure within the 6 months preceding the bacteremia (B-II), or the presence of certain baseline conditions (diabetes, cancer, immunosuppressant therapy) (B-II).
What is the most appropriate empirical antimicrobial treatment for community-acquired IE caused by S. aureus?From a practical point of view, the previous considerations may be simplified according to the place of acquisition of the infection. For community-acquired cases, empirical treatment should focus MSSA, the episode frequently involving a native valve. However, if the infection has been acquired in the hospital (or in a health-care related environment), empirical treatment should also include MRSA, and prosthetic valves (fundamentally early prosthetic valve IE) or intracardiac devices may be involved.
As previously discussed, the treatment of choice for IE caused by MSSA are anti-staphylococcal penicillins, such as cloxacillin or nafcillin.85,112,226 Cefazolin's activity may be impaired in high-inoculum infection such as staphylococcal IE, so caution in this setting is required.85 Notwithstanding, efficacy was similar in a case–control study including 49 patients treated with cefazolin and 84 with nafcillin, tolerance being higher for cefazolin.149 In addition to these β-lactam antibiotics, some experts support the addition of daptomycin in severely ill patients, in accordance with the excellent results reported in small case series and in experimental studies.117,183Recommendations:
When community-acquired IE caused by S. aureus is suspected, the treatment of choice is cloxacillin (B-II).
In critically ill patients, or in patients with severe sepsis or septic shock, many experts recommend adding daptomycin to the treatment with cloxacillin (C-III).
Patients allergic to β-lactams may be treated with cefazolin (if no previous anaphylaxis has been reported) (B-II), or with the combination of daptomycin plus fosfomycin (C-III).
In the setting of community-acquired IE caused by S. aureus, should gentamicin be added to the empirical treatment?Current guidelines recommend adding gentamicin to cloxacillin for the treatment of native valve IE caused by MSSA.112,226 As previously discussed, there is scarce clinical evidence proving a better activity of this combination, while some studies have proved that this increases the risk of renal toxicity at the end of the episode, even when the aminoglycoside is administered at low doses.113Recommendation:
The addition of gentamicin in the empirical treatment of community-acquired IE caused by S. aureus during the first 3–5 days is not recommended (D-I).
What is the empirical treatment of hospital-acquired or health-care related IE caused by S. aureus?In this setting, the treatment should include activity against MRSA. If information on blood cultures yielding S. aureus is available, the goal is to provide effective antibiotic treatment for both MSSA and MRSA, taking into account the considerations previously discussed. If no blood cultures have been taken or are not available (i.e. fever and new valve prosthetic dehiscence in the post-operative period), antibiotics with activity against Gram-negative microorganisms should also be included in the treatment. As previously mentioned, experimental studies on the combination of daptomycin and β-lactams (cloxacillin, nafcillin, ampicillin, carbapenems, ceftriaxone or piperacillin/tazobactam) also observed that synergy occurred with a wide range of these drugs, and also with fosfomycin.118,183Recommendations:
In the setting of health-care related IE caused by S. aureus, monotherapy with vancomycin nor daptomycin are not recommended (D-II).
In this context, daptomycin in combination with cloxacillin is recommended (B-II). For patients allergic to β-lactams, cloxacillin may be substituted by fosfomycin (C-III).
Faced a suspected case of IE but no available blood cultures, the use of daptomycin in combination with a β-lactam with activity against nosocomial Gram-negative microorganisms is recommended (C-III).
Definitive antimicrobial treatment for IE caused by S. aureusDefinitive treatment recommendations for IE are summarized in Table 3.
Definitive antimicrobial treatment for infective endocarditis (IE) caused by S. aureus.
| Antibiotic | Dose and route | Duration | Evidence | Comment |
|---|---|---|---|---|
| 1. Native valve IE caused by MSSA | ||||
| Cloxacillin | 2g/4h iv | 4–6 w | B-II | Combination with gentamicin is discouraged (D-I) |
| 1A. Patients allergic to β-lactams | ||||
| Daptomycin | ≥10mg/kg/d iv | 4–6 w | B-II | |
| +Fosfomycin | 2g/6h iv | 4–6 w | ||
| 2. Prosthetic valve IE caused by MSSA | ||||
| Cloxacillin | 2g/4h iv | ≥6 w | B-II | Start rifampin after 5 days of treatment (C-III) |
| +Rifampin | 600mg/12h iv or vo | ≥6 w | ||
| +Gentamicin | 3mg/kg/d | 2 w | ||
| 2A. Patients allergic to β-lactams | ||||
| Daptomycin | ≥10mg/kg/d iv | ≥6 w | C-III | Start rifampin after 5 days of treatment (C-III) |
| +Rifampin | 600mg/12h iv or vo | ≥6 w | ||
| +Gentamicin | 3mg/kg/d | 2 w | ||
| 3. Native valve IE caused by MRSA | ||||
| 3A. Vancomycin MIC≥1.5mg/L (measured by E-test) | ||||
| Daptomycin | ≥10mg/kg/d iv | 4–6 w | B-II | |
| +Cloxacillin | 2g/4h iv | 4–6 w | ||
| 3B. Vancomycin MIC<1.5mg/L (measured by E-test) | ||||
| Same as in vancomycin MIC 1mg/L | 4–6 w | B-II | ||
| Or vancomycin | 1g/8h iv | 4–6 w | B-II | Goal: Cmin 15–20mg/L (B-II)Combination with gentamicin is discouraged (D-I) |
| 3C. Patients allergic to β-lactams | ||||
| Daptomycin | ≥10mg/kg/d iv | 4–6 w | B-II | |
| +Fosfomycin | 2g/6h iv | 4–6 w | ||
| Or vancomycin | 1g/8h iv | 4–6 w | B-II | Goal: Cmin 15–20mg/L (B-II)Combination with gentamicin is discouraged (D-I) |
| 4. Prosthetic valve IE caused by MRSA | ||||
| 4A. Vancomycin MIC≥1.5mg/L (measured by E-test) | ||||
| Daptomycin | ≥10mg/kg/d iv | ≥6 w | C-III | Start rifampin after 5 days of treatment (C-III) |
| +Rifampin | 600mg/12h iv or vo | ≥6 w | ||
| +Gentamicin | 3mg/kg/d | 2 w | ||
| 4B. Vancomycin MIC<1.5mg/L (measured by E-test) | ||||
| Same as in vancomycin MIC 1mg/L | C-III | |||
| Or vancomycin | 1g/8h iv | ≥6 w | B-II | Vancomycin PK/PD goal: Cmin 15–20mg/L (B-II) |
| +Rifampin | 600mg/12h iv or vo | ≥6 w | Start rifampin after 5 days of treatment (C-III) | |
| +Gentamicin | 3mg/kg/d | 2 | ||
| 5. Alternative regimes for IE caused by MRSA | ||||
| Linezolid | 600mg/12h iv | ≥6 w | C-III | |
| Or fosfomycin | 2g/6h iv | ≥6 w | B-II | |
| +Imipenem | 1g/6h iv | ≥6 w | ||
| Or ceftaroline | 600mg/8h iv | ≥6 w | C-III | |
MSSA, methicillin-susceptible S. aureus; MRSA, methicillin-resistant S. aureus; MIC, minimal inhibitory concentration; iv, intravenous rout; vo, oral route; w, weeks.
The studies proving a higher efficacy of cloxacillin, nafcillin and cefazolin over other β-lactams have already been referred.85,149 Clinical evidence of daptomycin's efficacy for IE caused by MSSA is also scarce, results being non-conclusive.94 However, we know that patients with left valve IE caused by MSSA with vancomycin MIC≥1.5mg/L (measured by E-test) have higher mortality, this leading to the search for alternative treatments.171 In IVDUs with right valve IE, with no pulmonary emboli and with negative blood cultures after 72h of treatment, a two-week course of cloxacillin may be enough, gentamicin adding no benefits.114 The standard length of therapy recommended for left valve non-complicated IE is 4 weeks, or 6 weeks in the case of pulmonary emboli or periannular complications.7,112,226Recommendations:
For native valve left side IE caused by MSSA, cloxacillin for 4–6 weeks is recommended (B-II), and two weeks in non-complicated right valve IE among IVDUs (A-I). Daptomycin may be added to cloxacillin in the case of persistent bacteremia detected by the positivity of subsequent blood cultures, especially in episodes caused by an isolate with vancomycin MIC≥1.5mg/L (measured by E-test) (C-III). Systemic combination with gentamicin is not recommended (D-II). In patients allergic to β-lactams, the combination of daptomycin to fosfomycin is recommended (C-III).
What is the treatment for prosthetic valve IE caused by MSSA?In addition to previous considerations, in prosthetic valve IE caused by MSSA, the length of therapy will be at least 6 weeks, due to the difficulties for eradicating the biofilm on the surface of the prosthesis.112,226-234 Also, according with a recent study on IE caused by Enterococcus faecalis, gentamicin may be added to the treatment on a once-daily dose during the first two weeks.112,235Recommendations:
Cloxacillin is recommended in prosthetic IE caused by MSSA (C-II), in association with rifampin after the first 5 days of treatment (C-III), and gentamicin in a once-daily dose during the first two weeks of therapy.
In the case of allergy to β-lactams, the same combination of antibiotics may be used, with the substitution of cloxacillin by daptomycin (C-III).
What is the treatment for native valve IE caused by MRSA?Some available evidence has proven a bad prognosis for vancomycin-treated complicated bacteremia caused by MRSA, when vancomycin MIC is higher than 1.5mg/, although a recent meta-analysis has failed to prove so.7 Also, some studies have shown a higher efficacy of daptomycin as compared with vancomycin in complicated bacteremia caused by MRSA when vancomycin MIC is higher than 1.5mg/L.103,151 Therefore, daptomycin will be the treatment of choice for these cases. Also, there is scarce but excellent clinical results of the synergistic combination of daptomycin plus β-lactams or fosfomycin against MRSA isolates causing bloodstream infection and IE. These results have been also observed in experimental studies.117,118,183 Overall, this evidence supports the priorization of these combinations in the setting of native valve IE caused by MRSA.Recommendations:
Daptomycin plus cloxacillin is recommended in native valve IE caused by MRSA when vancomycin MIC is≥1.5mg/L (B-II).
The same treatment may be administered when vancomycin MIC is <1.5mg/L (measured by E-test), or vancomycin at doses providing trough levels of 15–20mg/L (B-II).
In patients allergic to β-lactams, the combination of daptomycin plus fosfomycin is recommended (B-II), or the use of vancomycin at doses providing trough levels of 15–20mg/L (B-II).
Neither the addition of rifampin (D-III) or gentamicin (D-III) to the treatment are recommended.
What is the treatment for prosthetic valve IE caused by MRSA?The need for avoiding vancomycin when its MIC is high places daptomycin in the first line. Also, there is no available evidence supporting the use of alternative agents with good activity against biofilm different from rifampin or gentamicin, so its use is still recommended.Recommendations:
In prosthetic valve IE caused by MRSA with vancomycin MIC≥1.5mg/L (measured by E-test), the use of daptomycin, in combination with rifampin after 5 days of treatment, and gentamicin in one single daily dose during the first two weeks of therapy is recommended (C-III). Daptomycin plus fosfomycin could be used alternatively (C-III).
In the case of MIC<1.5mg/L (using E-test), the same combination may be used (C-III), or vancomycin combined with rifampin after 5 days of treatment, plus gentamicin in a one single daily dose during the first two weeks of treatment (B-II).
Are there any alternative treatments for IE caused by MRSA?As previously mentioned, there is very scarce evidence supporting the use of linezolid or ceftaroline for IE caused by MRSA.200,224,225 In a recently published multicentric study, the combination of imipenem plus fosfomycin was used as salvage therapy for MRSA bloodstream infection (12 patients with IE, 2 with vascular graft infection and 2 with complicated bacteremia). Cure rate was 69%, and only 1 in 5 deaths was associated with MRSA infection. All patients had negative blood cultures within the first 72h after starting this combination, which showed to be safe, except for a cirrhotic patient with ascites secondary to fosfomycin-related sodium overload.194
One of daptomycin's drawbacks in the treatment of IE is the emergence of resistance. To avoid this, the combination of imipenem plus fosfomycin, or the use of linezolid seems appropriate. More recently, the combination of daptomycin plus ceftaroline has shown good activity in vitro,223,236 and excellent but scarce clinical results against daptomycin-resistant MRSA isolates.237 In specific clinical scenarios, daptomycin plus linezolid could be a good choice for IE caused by MRSA presenting with pneumonia or meningitis. In the latter, daptomycin plus ceftaroline might also have a better efficacy than either monotherapy.Recommendations:
In patients with IE caused by MRSA presenting clinical failure with previous recommended schedules, the administration of daptomycin plus fosfomycin may be used (B-II). Fosfomycin plus imipenem could be also used (C-II). If this cannot be done, either because of allergy or a high risk of sodium overload, ceftaroline, either alone (B-II) or combined with daptomycin (C-II), or linezolid, alone (C-II) or associated with daptomycin (C-III), may be valid alternatives.
Role for surgery in IE caused by S. aureusIs there any specific indication for surgery in the setting of IE caused by S. aureus?Persistent bacteremia by MRSA is recognized in international guidelines as the only microbiological criteria indicating per se the need for surgery.238 Persistent positive blood cultures must lead to eradication of primary (valves) or metastatic infectious foci in order to ameliorate the prognosis. In the European guidelines, persistent bacteremia is defined as positive blood cultures after 10 days of appropriate antimicrobial treatment.226 However, it has been recently proven that the prognosis of left side IE worsens if blood cultures still yield microorganisms 48–72h after the onset of treatment, mortality rate being twice as high.239 Therefore, a case with persistent bacteremia (positive blood cultures in the third day of appropriate treatment) in the setting of IE caused by MRSA should be considered for surgical management, even if there are no other features indicating so.Recommendations:
Patients suffering from IE caused by S. aureus share the same indications for surgery as other cases due to other microorganisms, with the exception of prolonged MRSA bacteremia. Therefore, international guidelines may be followed (A-II), but if blood cultures after 72h from the onset of appropriate treatment still yield MRSA, complementary tests should be performed in order to rule out metastatic foci, and the cardiac surgeons should contacted (B-II).
How long should be the treatment in patients submitted to cardiac surgery for IE?There are few data regarding this question, and none specific for IE caused by S. aureus. Classically, the guidelines have recommended an arbitrary period of 4–6 weeks after surgery in order to avoid relapse, even if valve cultures were negative.240 Alternatively, a complete course of prosthetic valve IE was recommended for cases of operated native valve IE, subtracting the days of antibiotic treatment received before the surgery.112 However, a study involving 358 cases of IE submitted to valve surgery proved that, in cases with negative valve cultures, two weeks of treatment after surgery, or completing the initial scheduled treatment, were sufficient.241 The most appropriate length of therapy in patients with positive valve cultures is uncertain, current guidelines recommending to complete a whole treatment after the surgery.112,226Recommendations:
In patients with native or prosthetic valve IE caused by S. aureus undergoing valve replacement and cultures being negative, it is recommended to administer two more weeks of therapy or simply finish the initially scheduled treatment (B-II).
In patients with positive valve cultures after surgery, it is recommended to restart the treatment of IE (i.e., ≥4 weeks for native valve IE, and ≥6 weeks for prosthetic IE) (C-III).
Measures for improving the management of SABWhich are the quality-of-care indicators to evaluate the management of SAB?It is well known that the outcome of patients with SAB is influenced by the particularities of the patient and the microorganism. As an example, a recent international retrospective cohort study including more than 3000 cases found that age, nosocomial SAB, MRSA etiology and some bacteremia sources were independently associated with an increased risk of death.160 Beyond that, the clinical management of bloodstream infection in general, and of SAB in particular have also been shown to have impact in the outcome.34,159,242 Therefore, it is necessary to identify which aspects of clinical management are the most important.
A recent study included a systematic review of the literature in order to identify the aspects related to the clinical management of SAB which had a significant influence in prognosis.34 The authors identified 6 management-related activities which were found to have impact on the outcome in at least 2 of 21 selected articles31,33,36,68,88,115,134,138,140,161,169,243–248 (Table 4); an intervention aimed at improving adherence to these 6 activities was associated with reduction in mortality among patients with SAB in 12 Spanish hospitals,34 thus providing additional validation of their importance.
Key aspects of the clinical management of Staphylococcus aureus bacteremia to be considered for quality-of-care interventions.
| • Performance of follow-up blood cultures 2–3 days after start of antimicrobial therapy. |
| • Evaluate clinical response at day 3. |
| • Early source control (e.g., surgical drainage of abscess if feasible, removal of infected device). |
| • Performance of echocardiography in patients with specific criteria. |
| • Early use of intravenous cloxacillin in cases of methicillin-susceptible S. aureus (or cefazolin in patients under hemodialysis) as definitive therapy in non-allergic patients. |
| • Adjustment of vancomycin dose according to trough levels in order to achieve the PK/PD target. |
| • Provide an appropriate duration of therapy according to the complexity of infection. |
| • Provide vancomycin MIC and consider an alternative agent to vancomycin when MIC by E-test is ≥1.5mg/L. |
We have reviewed again the literature until March 22, 2014 using the same methods; in summary, the PubMed database was searched using the terms: “Staphylococcus aureus” or “S. aureus”, and “bacteremia” or “bloodstream infection” or “sepsis”, and “outcome” or “complication” or “mortality” or “death” or “recurrence”. Studies were selected if the following criteria were fulfilled: the predictors for outcome were investigated, and control of confounding factors was appropriately performed. We have found 3 additional studies supporting early achievement of the vancomycin pharmacokinetic–pharmacodynamic target by determining trough levels in patients with MRSA bacteremia.249–251 One of the studies was performed in patients with septic shock.249 These data would support the need to adjust vancomycin dosage according to trough levels. Also, another study on MRSA bacteremia found that the lack of response at day 3 was a predictor for a bad outcome252; this adds to previous studies also identifying early clinical response (e.g., disappearance of fever) in SAB as a predictor of outcome.142 In addition, two recently published meta-analysis found that a higher vancomycin MIC (even in the susceptible range) is associated with higher mortality in MRSA bacteremia.43,253 As previously mentioned, another recent meta-analysis contradicts these results,7 but overall they suggest that vancomycin MIC should be reported to clinicians as soon as possible. Finally, three additional studies103,151,180 found that therapy with daptomycin (at doses higher than 6mg/kg/day) for treating bacteremia caused by MRSA with vancomycin MIC>1.5mg/L (measured by E-test) was associated with lower mortality; two of these studies were retrospective.103,151 A meta-analysis found similar results.180Recommendation:
At least the quality-of-care indicators included inTable 4should be considered in all patients with SAB (BII).
What interventions should be implemented to improve the management of SAB?Adherence to quality-of-care indicators in the management of SAB is frequently lower than desired.31,34,246 General interventions performed in any patients with bacteremia usually include early reporting of preliminary results of blood cultures (i.e., results of Gram stain), management advice including antimicrobial therapy, and control of the source of infection.242
Apart from those, interventions specifically addressed at improving the management of SAB have been based on infectious disease specialist (IDS) consultation, either only when solicited or routinely offered to physicians in charge of the patients.31,33,36,243,245,246 These studies included comparative parallel cohorts31,33,244–246 or a pre-post desing,36,243 but they all showed improvement after IDS consultation and even lower mortality, too.
However, the recommendations provided by IDS were not structured. A recent study investigated the impact of a protocolized intervention based on IDS active (non-solicited) consultation comprising 6 quality-of-care indicators (see above).34 The intervention consisted on an combined activity performed by clinical microbiologists and IDS who provided specific recommendations in a structured form with a bundle of measures; the form was included in the patients’ chart so the physician in charge could consult it, and it was updated 3 days per week; this activity had been previously informed to all hospital departments, and was associated with improved adherence to quality-of-care indicators and reduced mortality.Recommendations:
Active, unsolicited IDS consultation for management and follow-up should be provided to physicians in charge of all patients with SAB (BII). The specialized recommendations to physicians in charge of the patients with SAB should be provided in a structured manner so all quality-of-care indicators of the management are considered (BII).
Conflicts of interestFrancesc Gudiol has received academic grants from Novartis, Astellas and AstraZeneca.
José María Aguado has been a consultant to and on the speakers’ bureau for Astellas Pharma, Astra Zeneca, Pfizer, Gilead Sciences, Novartis, Merck Sharp and Dohme, and Roche.
Benito Almirante has carried out consultancy work or received monetary payments for giving talks from Astellas, Astra Zeneca, Gilead Sciences, Janssen-Cilag, Merck Sharp & Dhome, Novartis and Pfizer.
Jesús Rodríguez-Baño has been consultant and speaker for Pfizer, Novartis, Merck, AstraZeneca and Astellas, and has recevied research grants from Novartis and Gilead.
Jose M. Miro has received consulting honoraria from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Merck, Novartis y Sanofi, research and academic grants from Cubist, Gilead, ViiV, Novartis, Merck, Fondo de Investigaciones Sanitarias (FIS) del Instituto de Salud Carlos III (Madrid), Fundación para la Investigación y Prevención del Sida en España (FIPSE, Madrid), Ministerio de Sanidad, Servicios Sociales e Igualdad (MSSSI, Madrid), National Institutes of Health (NIH, Bethesda, MA, USA) y NEAT and honoraria for lectures from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Merck, Novartis y ViiV Healthcare.
Alex Soriano has been speaker for Pfizer and Novartis.
We are indebted to Dr. Rafael San Juan for his invaluable help in the reference management of this article. REIPI is supported by the Plan Nacional de I+D+i 2008–2011 and the Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI RD12/0015) – co-financed by European Development Regional Fund “A way to achieve Europe” ERDF.
| Level of scientific evidence | |
| I | Evidence obtained from ≥1 randomized clinical trial |
| II | Evidence obtained from ≥1 well-designed non-randomized clinical trial, or cohort studies, or case–control-studies, especially if they have been performed in more than one center. |
| III | Evidence obtained from documents or opinions of experts, based in clinical experience or case series |
| Grades of recommendation | |
| A | Good evidence to recommend the use of a measure or practice |
| B | Moderate evidence to recommend the use of a measure or practice |
| C | Poor evidence to recommend the use of a measure or practice |
| D | Moderate evidence to discourage the use of a measure or practice |
| E | Good evidence to discourage the use of a measure or practice |