Neospora caninum is a protozoan parasite that causes abortion and important economic losses in cattle worldwide. The accurate diagnosis of neosporosis is essential for management and control measures. The aims of this study were: i) to evaluate the performance of an in-house enzyme-linked immunosorbent assay based on the 38kDa native antigen (p38-ELISA) to diagnose bovine neosporosis in Argentina using a well- characterized local sera panel from experimentally infected and naturally exposed cattle and ii) to compare the diagnostic performance and agreement of three N. caninum serological tests: p38-ELISA, indirect fluorescence antibody test (IFAT) and immunoblotting (IB) using the same sera panel. Serum samples testing either positive or negative by IFAT and IB were considered “Relative Standards of Comparison” (RSC) and used for p38-ELISA evaluation. Receiver operating characteristics analysis revealed that p38-ELISA was highly accurate (area under the curve= 0.982) according to RSC with a cut-off index of 0.0905. Relative sensitivity and specificity of p38-ELISA were 97.8% and 99.5%, respectively and agreement between RSC and p38-ELISA was almost perfect (k= 0.97). The evaluation and performance comparison of serological tests were performed according to the definition of gold standard based on the decision of the “majority of tests”. All tests displayed high sensitivity and specificity values (greater than 95%); and excellent agreement. This study describes the accurate performance of p38-ELISA evaluated locally and the highly accurate diagnostic performance of the studied tests for the detection of anti-N. caninum antibodies in cattle from Argentina.
Neospora caninum es un parásito protozoo responsable de abortos y pérdidas económicas en bovinos. La realización de un diagnóstico serológico preciso y con resultados comparables obtenidos por diferentes pruebas contribuye al manejo de este problema y a encarar medidas de control. Los objetivos del presente trabajo fueron los siguientes: 1) evaluar en Argentina una prueba de enzimoinmunoensayo in-house con el antígeno nativo de 38kDa de N. caninum (ELISA-p38) para el diagnóstico de la neosporosis bovina, utilizando un panel de sueros locales bien caracterizados, procedentes de bovinos infectados de modo experimental o naturalmente expuestos; 2) comparar el desempeño y establecer el nivel de concordancia de tres pruebas serológicas para la detección de N. caninum, ELISA-p38, inmunofluorescencia indirecta (IFI) e inmunoblot (IB), con el mismo panel de sueros. Los sueros que resultaron positivos o negativos a IFI e IB fueron considerados como estándares relativos de comparación (ERC) para evaluar la prueba de ELISA-p38. El análisis de característica operativa del receptor determinó que la prueba de ELISA-p38 fue altamente precisa (área bajo la curva= 0,982) usando el punto de corte 0,0905. La sensibilidad y especificidad relativa del ELISA-p38 fue 97,8% y 99,5%, respectivamente, con una concordancia casi perfecta (k= 0,97) respecto del ERC. La comparación del desempeño de las pruebas se realizó usando como gold standard el criterio de la decisión de la “mayoría de las pruebas”. Las pruebas exhibieron altos valores de sensibilidad y especificidad (mayores del 95%) y excelente concordancia. Este trabajo describe un buen desempeño de la prueba de ELISA-p38 evaluada localmente y adecuada performance diagnóstica de las pruebas serológicas analizadas para la detección de anticuerpos anti N. caninum en bovinos de Argentina.
Neospora caninum is a protozoan parasite from the phylum Apicomplexa7, which is responsible for abortions in dairy and beef cattle worldwide8. Neosporosis causes important economic losses in the cattle industry, a situation that is of utmost importance in many South American countries. A recent study conducted in the Humid Pampa region of Argentina estimated annual economic losses of approximately US$ 44 million and US$ 13 million in the dairy and beef industry, respectively14, due to N. caninum abortions. The objective interpretation of data from different countries and laboratories depends on the reliability of serological diagnostic tests. Many serological techniques are available for N. caninum diagnosis in cattle. The indirect fluorescence antibody test (IFAT) is commonly used for this purpose, despite a possible subjective interpretation of results depending on the operator's experience. In addition, many commercial ELISA tests are available, which are more objective in terms of result interpretation16. However, in Argentina, commercial kits must be purchased abroad, implying high costs and long waiting periods. When considering the implementation of a new technique validated elsewhere, similar diagnostic characteristics should not be necessarily assumed, since local conditions, the target population and the epidemiological situation may vary from the original validation. Therefore, if the assay is to be applied in a different geographical region and/or population, revalidation of the assay under the new conditions is recommended, since sensitivity and specificity may vary within and among animal populations9,15,23. Furthermore, cattle are presumably infected with diverse isolates of N. caninum and each region has a particular epidemiological situation.
Schares et al.17 evaluated an in-house ELISA based on the native p38 antigen of N. caninum having good diagnostic performance and different recommended cut-offs depending on the abortion pattern. In a multi-centered standardization study performed in Europe for several bovine neosporosis serological tests, a lower cut-off was recommended to obtain higher sensitivity values for p38-ELISA21. The p38-ELISA method is a low cost, practical and quick diagnostic assay; therefore its implementation for the diagnosis of bovine neosporosis in our country would be very beneficial. However, p38-ELISA has not been evaluated locally. In addition, to our knowledge, the diagnostic performance of available serological tests for bovine neosporosis in Argentina has not been thoroughly evaluated recently. Therefore, the aims of this study were: i) to evaluate the performance of p38-ELISA to diagnose bovine neosporosis in Argentina using a well-characterized local sera panel from experimentally infected (EI) and naturally exposed (NE) cattle and ii) to compare the diagnostic performance and agreement of three N. caninum serological tests: p38-ELISA, IFAT and immunoblotting (IB) using the same sera panel.
Materials and methodsSerum samplesAll serum samples used in this work were kindly provided by the coauthors and belong to other research projects. Experimental sera (n= 36) provided by Dr. Hecker11 came from 3 heifers intravenously inoculated with cell culture-derived tachyzoites from N. caninum NC-6 Argentina isolate4, and from 3 heifers subcutaneously inoculated with PBS. All 6 heifers were sampled at 0, 2, 3, 5, 9, 13 weeks post-inoculation (wpi), as described by Hecker et al11. All animals used in the above study were handled in strict accordance with good animal practice and the conditions defined by the Ethical Committee of Animal Welfare (CICUAE) at INTA Balcarce.
Serum samples from NE cattle, raised in dairy herds located in Santa Fe (n= 218) and beef herds located in Buenos Aires province (n= 44) were supplied by Dr. Campero CM. Additionally, sera from 37 mother-precolostrum calf pairs (n= 74), belonging to a dairy herd in Buenos Aires, were provided by Dr. Moré12.
IFAT and immunoblotDetection of specific N. caninum antibodies was carried out by IFAT, as previously described7. A fluorescein isothiocyanate (FITC) labelled affinity-purified rabbit anti-bovine IgG antibody (Sigma-Aldrich, St. Louis, USA) conjugate was used. Reading was performed with an epifluorescence microscope (Leica). Unbroken fluorescence of the tachyzoite membrane and titer ≥1:25 was considered positive13,17.
Detection of specific N. caninum antibodies was carried out by IB as described by Schares et al18. Briefly, 4×107 tachyzoites of NC-1 isolate were used for antigen preparation under non-reducing conditions, separated by electrophoresis in each SDS-polyacrylamide gel and electroblotted onto a PVDF membrane. Bovine serum samples were diluted 1:100. A conjugate anti-bovine IgG [H+L] peroxidase (1:1000) (Jackson Immunoresearch Lab. Inc., PA, USA) was used. The substrate solution consisted of 30mg 4-chloro-1-naphthol (Sigma-Aldrich, St. Louis, USA), 10ml methanol, 30ml PBS and 40μl 30% H2O2. The reaction against five immunodominant antigens (IDAs) with relative molecular masses of 19, 29, 30, 33, 37kDa was recorded. A sample was considered positive when two or more IDAs were recognized18,19.
Both techniques (IFAT and IB) used positive and negative control sera obtained from experimentally-infected and uninfected cows, respectively.
p38-ELISAThe 38kDa antigen of N. caninum was obtained by affinity chromatography using monoclonal antibody 4.15.15 (IgG2a). Prior to affinity purification, 2×109 cell culture- derived tachyzoites from NC-1 isolate in 10ml of PBS-0.5% Triton X-100 were sonicated on ice for 90 s. The suspension was centrifuged at 13000g for 30min at 4°C and the supernatant was used for p38 affinity purification. The indirect ELISA was performed as described by Schares et al17. Briefly, the antigen (1.5ng/well) was diluted in coating buffer (0.1M sodium bicarbonate, pH 8.3) and used to sensitize ELISA plates (Nunc-Immuno-Polysorb). A blocking solution with PBS-0.05% Tween 20-20% horse serum was applied; and the plate was incubated for 30min at 37°C. A serum dilution of 1:200 was used and samples were analyzed in duplicate. A biotinylated monoclonal anti-bovine IgG (Sigma-Aldrich, St. Louis, USA) diluted 1:2000 and an extravidin-peroxidase conjugate (Sigma-Aldrich, St. Louis, USA) diluted 1:4000 were used. Antibodies were detected by incubation with substrate solution containing 100μg/ml 3,3′,5,5′-tetramethylbenzidine and 0.004% hydrogen peroxide in 0.2M sodium acetate and 0.2M citric acid for 15min at 37°C. Optical density (OD) values were measured at 492nm on a microplate reader (Labsystems MS Multiskan). Sample index values were recorded as the arithmetic mean of two index values, SI1 and SI2. These index values were calculated by the formula SIn= (Sn-N)/(P-N) where SIn is one of the two individual index values, and Sn one of the two individual OD values obtained for a single sample. N is the arithmetic mean of two OD values obtained for the negative serum, and P represents the arithmetic mean of two OD values obtained for the positive serum. Monoclonal antibody 4.15.15 (IgG2a) and positive and negative control sera were kindly provided by Dr. Schares. The optimal cut-off index value for maximal sensitivity and specificity for p38-ELISA was determined by receiver operating characteristic (ROC) analysis considering a 95% confidence interval relative to Relative Standards of Comparison (RSC).
Statistical analysisSerum samples testing either positive or negative by both IFAT and IB were considered as RSC for p38-ELISA evaluation2. ROC analysis (Medcalc 13.0) was performed to determine the optimal cut-off value and relative sensitivity and specificity considering a 95% confidence interval for the p38-ELISA relative to RSC. According to an arbitrary guideline by ROC analysis, the area under the curve (AUC) was evaluated as: non-informative (AUC= 0.5), less accurate (0.5< AUC≤ 0.7), moderately accurate (0.7< AUC≤ 0.9), highly accurate (0.9<AUC≤1) and perfect tests (AUC= 1)20.
The diagnostic characteristics of the tests were calculated according to the result of the “majority of tests” (Majority) since there is no gold standard test available for bovine neosporosis diagnosis1,21.
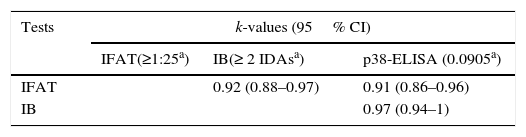
Agreement between serological tests was evaluated using Epidat 3.1 software (Organización Panamericana de la Salud y Xunta de Galicia, Consellería de Sanidade). Kappa values (k) were considered as follows: poor agreement (k= 0.00), slight agreement (k= 0.00–0.20), fair agreement (k= 0.21–0.40), moderate agreement (k= 0.41–0.60); substantial agreement (k= 0.61–0.80); almost perfect agreement (k> 0.81)6.
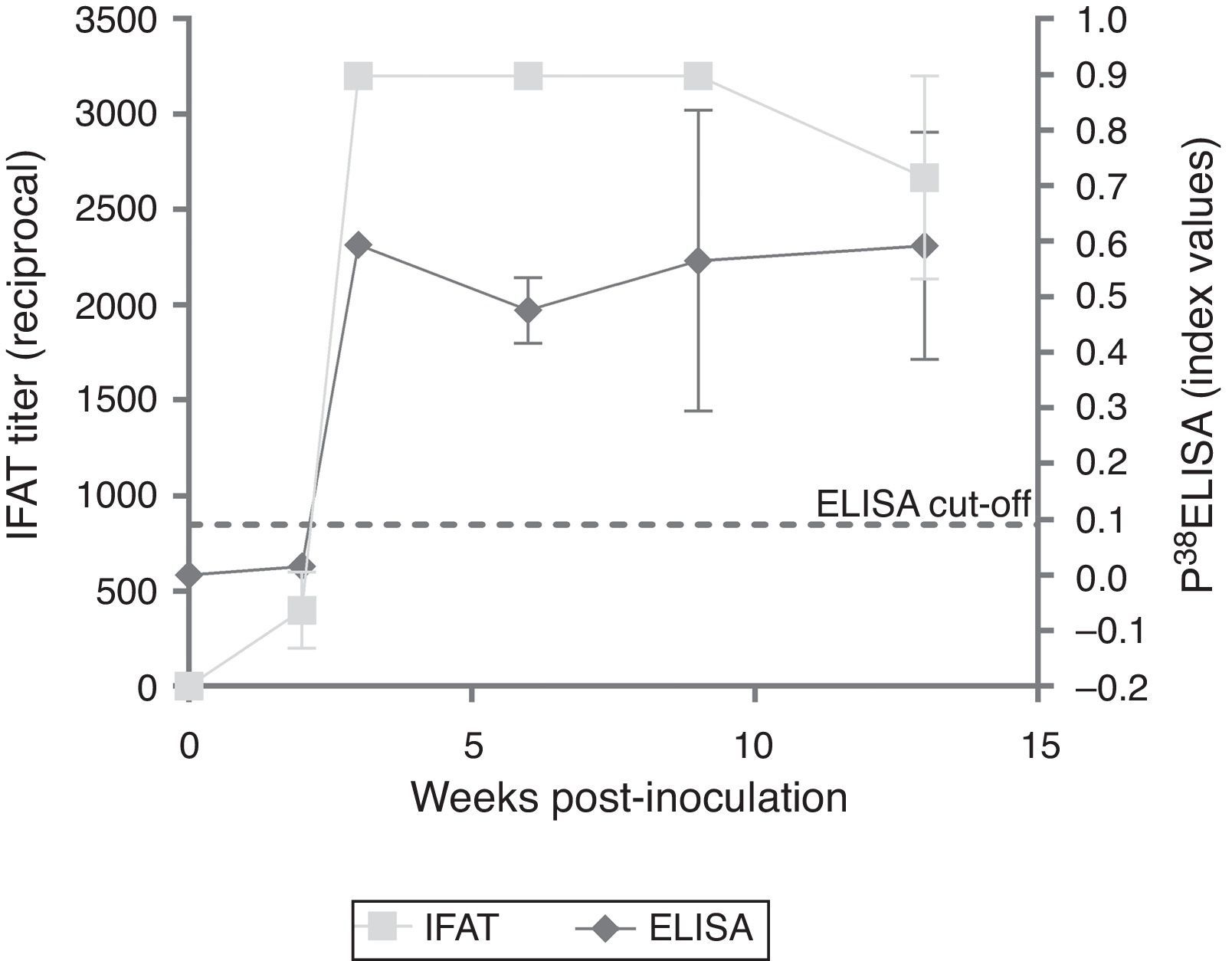
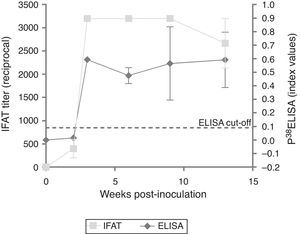
ResultsExperimentally-infected heifersThe three EI heifers seroconverted at 2 wpi, showing an average titer of 1:400 determined by IFAT. An increase in the titer was observed in the third wpi that was maintained through 9 wpi and decreased at 13 wpi (Fig. 1). The non-infected control group showed no positive IFAT reactions on all sampling dates.
Testing of sera from EI heifers by p38-ELISA detected a peak at 3 wpi; however, 2 wpi index values were below the cut-off (= 0.0905, determined by the ROC analysis). Additionally, a slight decrease was observed from 3 to 5 wpi (Fig. 1). The non-infected control group showed no positive ELISA reactions at all sampling dates.
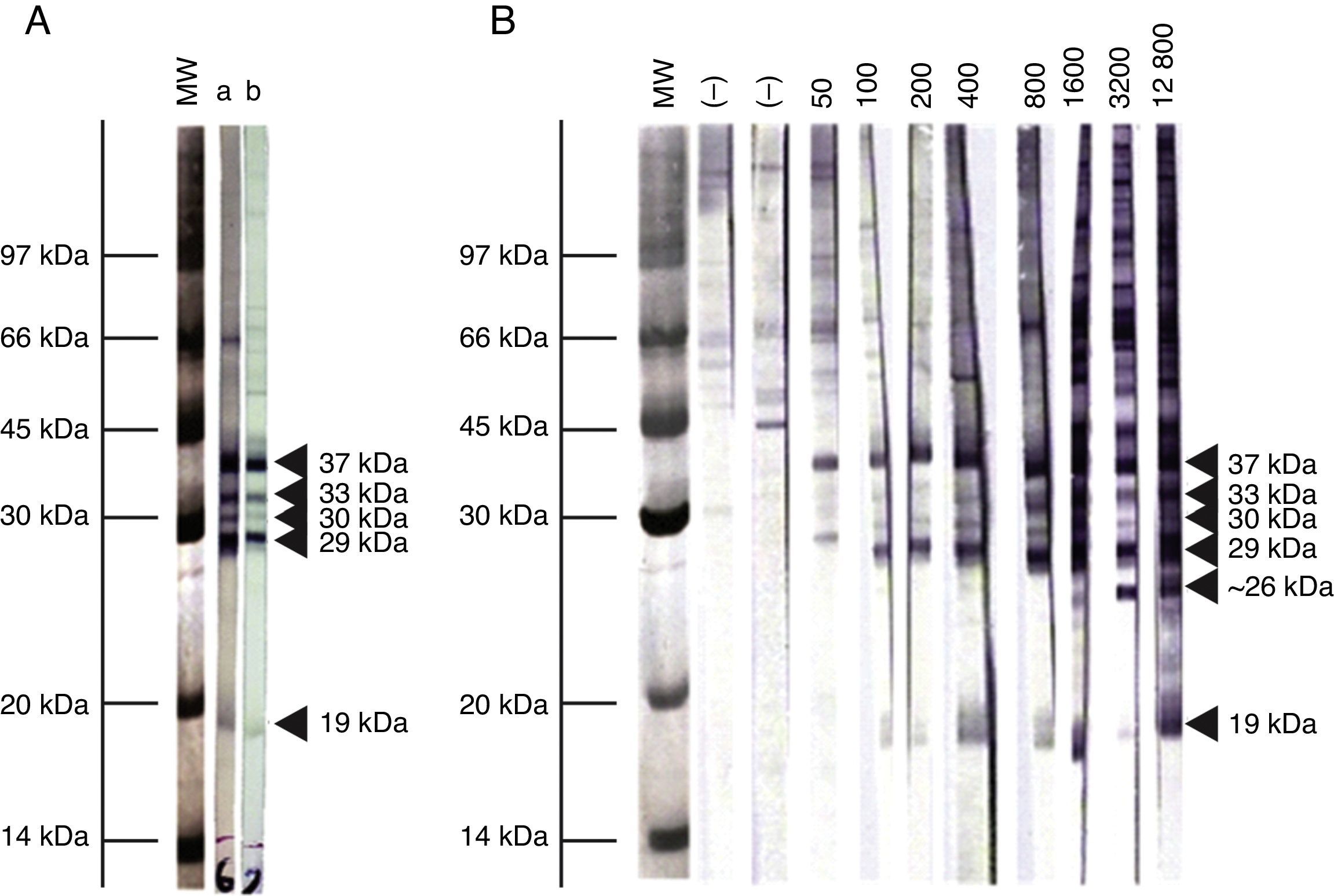
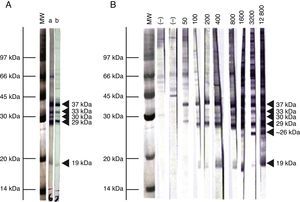
Immunoblot analysis of sera from the 3 EI heifers evidenced a positive reaction to the 5 IDAs at 2, 3, 5, 9, and 13 wpi (Fig. 2A). Sera collected at 0 wpi as well as sera from the non-infected control group showed no positive reactions by IB.
Representative pattern of N. caninum immunodominant antigen recognition in A) naturally and experimentally infected cattle and B) naturally exposed cattle at different IFAT titers. MW= molecular weight pattern; a= sera from a naturally infected cow; b= sera from an experimentally infected heifer; (–)= seronegative cow; IFAT titers are expressed as the reciprocal of the highest serum dilution showing positive fluorescence.
Based on the frequency and intensity of recognition by sera from NE cattle, the 37kDa antigen and a 29kDa antigen were detected by 100% and 98% of seropositive animals, respectively. The 30 and 33kDa antigens were recognized with higher frequency and intensity in samples with IFAT titer ≥1:400 and were detected by 78% and 68% of seropositive animals, respectively. The 19kDa protein was recognized in 70% of sera from seropositive animals, mostly with IFAT titer ≥ 1:100. Finally, a protein ∼26kDa was only detected by 13% of samples, having IFAT titer ≥ 1:3200. When EI sera were analyzed by IB, similar immunoblotting patterns were observed compared to NE cattle (Fig. 2A). A clear relationship between an increasing IFAT titer and a more intense and diverse IDA recognition was observed (Fig. 2B) among NE cattle.
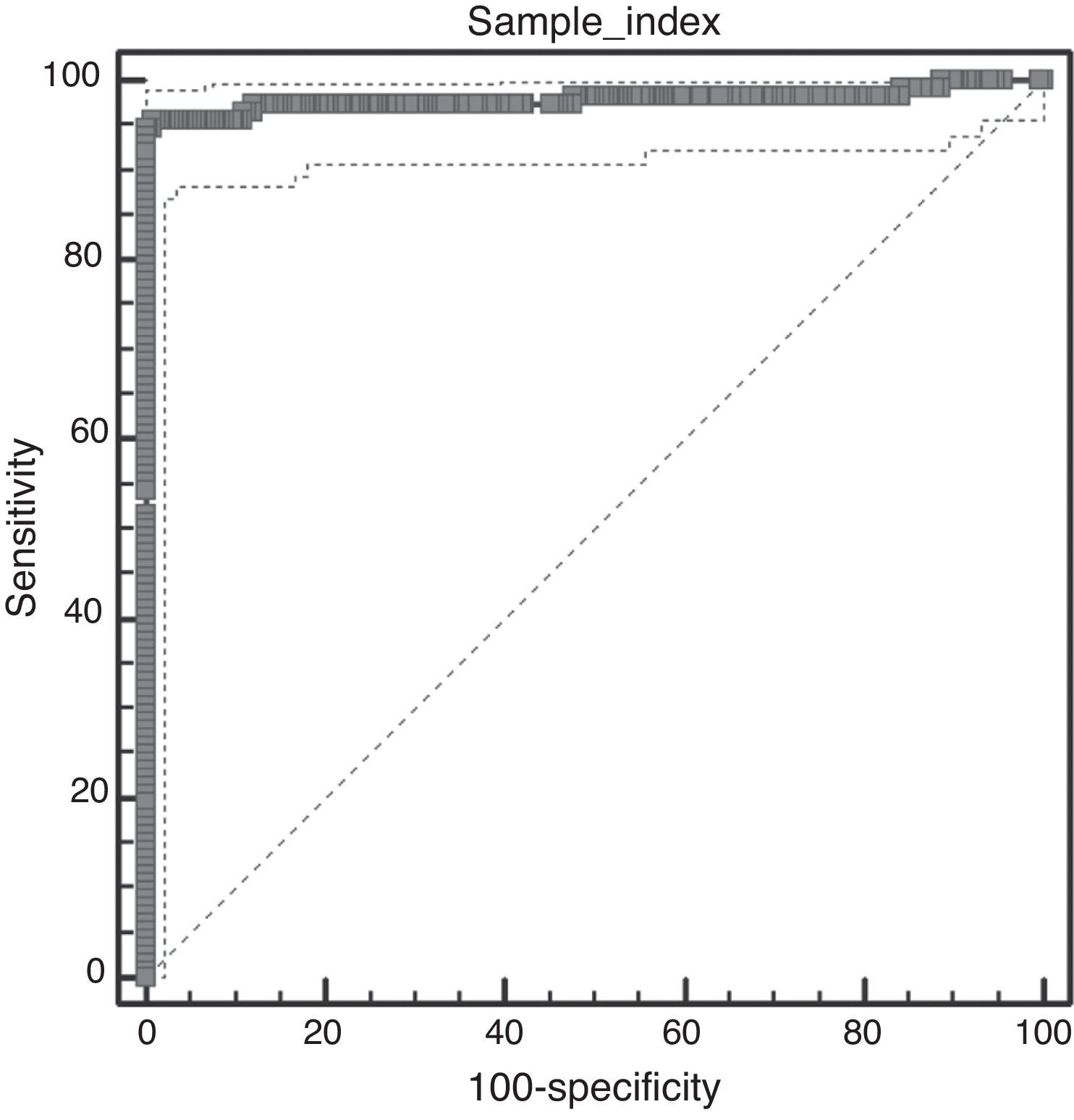
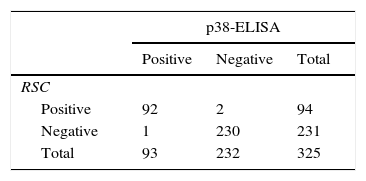
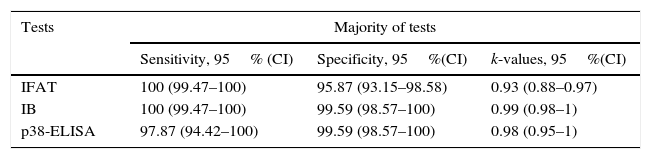
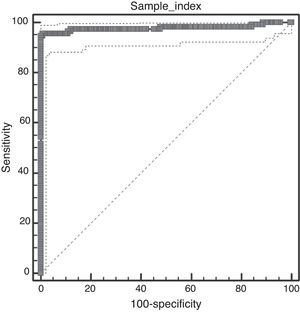
Techniques performance comparisonAntibodies to N. caninum were detected in 31% (104/336), 28% (95/336), and 27% (93/336) of samples from NE cattle by IFAT, IB and p38-ELISA, respectively. RSC consisted of 94 sera that tested positive and 231 sera that tested negative by both IFAT and IB (n= 325) and were used for the evaluation of p38-ELISA. Ninety-two out of 94 positive RSC sera tested positive by p38-ELISA and 230 of 231 negative RSC sera tested negative by p38-ELISA (Table 1). The negative sample by RSC that tested positive by p38-ELISA, did not show any reaction to the 38kDa IDAs in IB. Agreement between RSC and p38-ELISA was almost perfect (k= 0.97). Relative sensitivity and specificity with a cut-off index value of 0.0905 were 97.8% and 99.5%, respectively. ROC analysis revealed that p38-ELISA was highly accurate (AUC= 0.982) relative to the RSC (Fig. 3).
Sensitivity and specificity values were calculated for each test in relation to the Majority criterion (Table 2). The Agreement of each test with respect to the Majority criterion, as well as agreement between tests, was almost perfect (Tables 2 and 3).
Sensitivity, specificity and agreement (k-values) relative to the “majority of tests” decision
| Tests | Majority of tests | ||
|---|---|---|---|
| Sensitivity, 95% (CI) | Specificity, 95%(CI) | k-values, 95%(CI) | |
| IFAT | 100 (99.47–100) | 95.87 (93.15–98.58) | 0.93 (0.88–0.97) |
| IB | 100 (99.47–100) | 99.59 (98.57–100) | 0.99 (0.98–1) |
| p38-ELISA | 97.87 (94.42–100) | 99.59 (98.57–100) | 0.98 (0.95–1) |
Serological tests are the most suitable tool to evaluate N. caninum infection in animals, and contribute important information to control measures. Despite their importance, there is scarce information regarding the evaluation and comparison of serological diagnostic tests using a well-characterized sera panel. Such studies are highly recommended prior to considering a certain diagnostic technique as a routine diagnostic tool1,10. Even though IFAT is considered subjective, time-consuming and limited for large-scale investigations, it is still the most frequently used test for neosporosis detection in many South American countries. In addition, different cut-off values are commonly used, making comparisons more difficult22. The present study aimed to evaluate an in-house ELISA using local sera and to compare the diagnostic performance of serological tests available in Argentina for the detection of antibodies to N. caninum. Since a true gold standard is not available for bovine neosporosis, RSC are necessary when evaluating new techniques and ideally include results from more than one technique2.
Evaluation of EI sera with p38-ELISA detected a peak of antibodies at 3 wpi, however, at 2 wpi, index values were below the cut-off. Nonetheless, IFAT titers at 2 wpi were 1:400. Potentially the cut-off value applied in the ELISA could have underestimated low avidity IgG produced in early or recent infections5. A lower cut-off value was evaluated to detect seropositive animals at 2 wpi; however, under such conditions, the specificity of the test was considerably lower in the ROC analysis (data not shown). A slight decrease in the antibody response was detected by IFAT between 9 and 13 wpi, but not by the p38-ELISA.
We also determined the immunoreactivity of sera by IB. Cattle are presumably infected with a diverse population of N. caninum isolates, with potential immunogenic differences. In our study, though, the IB banding pattern found for EI and NE sera was similar. This fact is highly relevant for the selection of target antigens for diagnostic purposes, especially for the development and validation of serological tests in different geographical regions where cattle could be exposed to different N. caninum isolates. The reaction to the 38kDa IDA in 100% of positive serum samples is in agreement with the high relative sensitivity and specificity obtained in this work for the p38-ELISA method. Additionally, a ∼26kDa antigen was only found in samples having IFAT titers ≥1:3200. Immunoreaction to this antigen could be indicative of high serological titers developed in different situations: cows with recrudescence of infection, cows that aborted as a result of N. caninum infection, and cows or calves evidencing vertical transmission3. Further studies should be performed in order to clarify the role of this antigen in the outcome of the disease.
Ideally, laboratories should perform internal evaluation and validation studies to analyze the diagnostic performance of serological tests with local sera and everyday routine conditions. Using the decision of the “majority of tests” as gold standard, high sensitivity, specificity and agreement values were demonstrated for IFAT, IB and p38-ELISA. Based on our results, IFAT was very sensitive and was able to detect early stages of infection in EI heifers. However, a greater number of samples should be analyzed in order to determine the capacity of IFAT to detect recent infections, since only 3 EI animals were analyzed in our study. In addition, IFAT can be a useful tool to study serological profiles in infected cattle. Moreover, p38-ELISA displayed accurate diagnostic performance and is a practical and quick diagnostic tool for large-scale studies. In our country, commercial ELISAs are purchased abroad, implying high costs and delay. Therefore, a local in-house ELISA would be a low-cost and time-saving alternative.
The IB method can be used as a confirmatory technique for samples yielding inconclusive serological results from other tests8. It allows an unequivocal serological diagnosis with the highest sensitivity and specificity values among the tests analyzed, as shown in this work. However, only a few reference laboratories are performing this technique, since it is laborious, time-consuming and requires specialized equipment and training.
In conclusion, this study describes the accurate performance of p38-ELISA and the almost perfect diagnostic performance of the studied tests for the detection of anti-N. caninum antibodies, evaluated with a well-characterized sera panel of cattle from Argentina. The selection of the technique and cut-off applied will depend on the purpose of diagnosis as well as on feasibility in each laboratory. Results from the present work emphasize the importance of comparative studies and provide useful information for future serological diagnostic standardization programs among different laboratories in South America.
FundingEl presente trabajo fue financiado con fondos del PIP 2012-2014 N° 112-20110100488 otorgado por CONICET y recursos del Laboratorio de Inmunoparasitología, FCV, UNLP.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the conditions defined by the Code of Ethics of the World Medical Association (Declaration of Helsinki), and approved by the Ethical Committee of Animal Welfare (CICUAE) of INTA Balcarce, Argentina.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors state that they have no conflicts of interest to declare.
We thank I. Ercoli and S. Peñaloza for their excellent technical assistance. Financial support for this study was provided by CONICET, through PIP 2012-2014 N° 112-20110100488 and by resources from Laboratorio de Inmunoparasitología, FCV, UNLP.