Human papillomavirus (HPV) has the highest mortality rate due to cervical cancer in Northeastern Argentina. The aim of this work was to detect and characterize HPV in samples from the Province of Corrientes, Argentina. HPV detection and typing was performed using PCR-RFLP on samples with different cervical lesions (n=255). Seventeen viruses typified as HPV-58 were sequenced (E6 and E7 genes) and mutations were analyzed. HPV DNA was detected in 56.1% of the cervical lesions (143/255). Twenty-two different HPV types were detected. The type most frequently found among the total number of samples and HPV-positive samples was HPV-16 (14.5% and 25.9%, respectively), followed by HPV-58 (8.2%/14.7%, respectively), which is also considered a high-risk viral type. Increased severity of the cytological status was associated with greater rates of HPV detection and, especially, with the detection of greater rates of high-risk types. In addition, the evolutionary dynamics of the alpha-9 species group and HPV-58 was studied. All HPV-58 viruses reported in this work belonged to lineage A, sublineage A2. The phylodynamic analysis indicated that diversification of main groups within lineage A might have accompanied or preceded human migrations across the globe. Given that the most prevalent viruses found belonged to high-risk HPV types, some concerns might arise about the extent of cross protection of the vaccines against the types not included in their design.
El virus del papiloma humano (Human papillomavirus [HPV]) tiene la mayor tasa de mortalidad por cáncer de cuello uterino en el noreste de Argentina. El objetivo de este trabajo fue detectar y caracterizar el HPV en muestras de la provincia de Corrientes, Argentina. La detección y la tipificación se realizó mediante PCR-RFLP en muestras con diferentes lesiones cervicales (n=255). Se secuenciaron 17 virus tipificados como HPV-58 (genes E6 y E7) y se analizaron sus mutaciones. Además, se estudió la dinámica evolutiva de los virus del grupo alfa-9 y, en particular, del HPV-58. Se detectó ADN viral en el 56,1% de las lesiones cervicales (143/255) y se detectaron 22 tipos del HPV. El tipo encontrado con mayor frecuencia entre el total de muestras y entre las HPV-positivas fue el HPV-16 (14,5%/25,9%, respectivamente), seguido por el HPV-58 (8,2%/14,7%, respectivamente), también considerado como de alto riesgo. El aumento de la gravedad de las lesiones se asoció a mayores tasas de detección del HPV y, en especial, con mayores tasas de detección de tipos de alto riesgo. Todos los HPV-58 encontrados pertenecieron al linaje A, sublinaje A2. El análisis filodinámico indicó que la diversificación de los grupos principales dentro del linaje A podría haber acompañado o precedido las migraciones humanas en todo el mundo. Dado que los virus más prevalentes pertenecieron a los tipos del HPV de alto riesgo, podrían surgir interrogantes sobre el alcance de la protección cruzada de las vacunas contra los tipos no incluidos en su diseño.
The surveillance of Human papillomavirus (HPV) infection is epidemiologically relevant considering the incorporation of HPV prophylactic vaccines in different countries. In Argentina, where its general prevalence in 2010 was estimated in 20.1%54, vaccination was introduced into the immunization schedule for females aged 11 years in 201137.
Among the over 150 HPV types described, HPV-16 predominates, whereas the prevalence of other HPV types varies according to the geographic region5. It has been reported that about 70% of cervical cancers worldwide are due to HPV-16 and -1820,32, which are the goals of commercial vaccines. However, there are still other prevalent high-risk HPV types, such as HPV-31, -33, -45 and -5853. In some provinces of Argentina (Chaco, Corrientes, Córdoba and Misiones), an important detection of HPV-33 and HPV-58 has been reported2,17,22,23,51.
Although there have been several studies on the genetic diversity and phylogenetics of HPV4,14,19,21,42, most of them focused on the characterization of HPV-16 and HPV-18 variants and their associations with pathogenic potential and immunogenicity18,19.
Within HPV-58, several authors have identified four distinct clusters (A, B, C and D), being lineage A the most prevalent worldwide14,19. Reports about the important detections of this high-risk type in regions of Argentina such as the Northeast22,23,25, which has the highest mortality rates due to cervical cancer1, require a deeper characterization of viruses of HPV-58.
The aim of this work was to study HPV sequences from the Province of Corrientes, Argentina, especially those belonging to HPV-58. We attempted to characterize the viruses from samples taken from different cervical lesions, their nucleotide changes, phylogenetic relationships and the evolutionary dynamics of HPV-58 in the context of the alpha-9 papillomavirus species.
Materials and methodsPatientsSamples belonged to sexually active women who attended the Services of Gynecology and Obstetrics at hospitals “Angela Iglesia Llano” and “José Ramón Vidal” in the city of Corrientes, Province of Corrientes, Argentina, during 2005–2007 (n=255), and who had colposcopic or cytohistologic findings suggestive of HPV infection (according to the Bethesda System 2001). Epidemiological data were collected through a standardized questionnaire.
All patients included in this study signed a written informed consent and the study was approved by the Ethics Committee of the Instituto de Medicina Regional of the Universidad Nacional del Nordeste, Corrientes, Argentina (Resolution No.: 435/05).
Sample collection, HPV detection and typingEcto- and endocervical samples were obtained and DNA was extracted by the digestion method with cetyltrimethyl ammonium bromide (CTAB) and purification with isoamyl alcohol (24:1). DNA quality was evaluated by PCR targeted to 268 base pairs (bp) of the human beta-globin gene using primers GH20 (5′-GAAGAGCCAAGGACAGGTAC-3′) and PG04 (5′-CAACTTCATCCACGTTCACC-3′) under conditions previously described35. Negative samples for this test were discarded. Viral DNA was detected by PCR using consensus primers MY09/1135. Positive samples were typed by RFLP analysis, as previously described3,40.
E6/E7 gene amplification and sequencingSeventeen samples typified as HPV-58 were amplified and sequenced in the E6 (nt. 110–559) and E7 (nt. 574–870) genes. They were amplified by PCR using primers E6-P1/E6-P2 and E7-P1/E7-P2 as previously described12. Amplicons were purified using the Wizard SV gel and PCR Clean-up System extraction kits (Promega Corporation, USA) and sequenced in both directions (3500xl Genetic Analyzer, Applied Biosystems).
Statistical analysisStatistical associations were evaluated with the chi-square test using the Epi-Info v3.5.1 program8. Logistic regression analyses were used to determine the odd ratios for HPV detection or high-risk HPV detection according to the cytological status of infections, using the Infostat v2004 program26. p-values≤0.05 were considered significant.
Mutations and phylogenetic analysis of HPV-58Sequences from this work (n=17) were aligned with ClustalX v2.1 and edited with Bioedit v7.0. Mutations in the E6 and E7 genes were determined by comparison with the prototype genome of HPV-58 (GenBank accession number: NC_001443).
To classify sequences into HPV-58 lineages, a Bayesian phylogenetic analysis was performed using E6 and E7 concatenated sequences. Analysis was performed using an appropriate substitution model estimated with the jModeltest v2.1 software, the uncorrelated lognormal (UCLN) molecular clock model and a Bayesian Skyline Plot model implemented in BEAST v1.8. The Maximum Clade Credibility Tree (MCCT) was obtained using TreeAnnotator v1.8 from the BEAST package.
Phylodynamic analysesThe phylodynamic patterns of the HPV type 58 and the alpha-9 species group of the genus Alphapapillomavirus (HPV types 16, 31, 33, 35, 52, 58 and 67) were studied using a Bayesian coalescent analysis. Estimations included the demographic reconstruction and the time to the most recent common ancestors (tMRCAs) for the different groups.
Datasets included sequences from all groups of each type (dataset HPV-58: 154 sequences; dataset HPV-Alpha-9: 221 sequences) (available upon request). Analyses were performed on the E6 and E7 genes, using an appropriate substitution model and calibration for each gene. Analyses were carried out using the UCLN molecular clock model and the Skygrid model implemented in BEAST v1.8. A substitution rate of 1.0×10−7substitutions per site per year (s/s/y)29 or previously proposed substitution rates for the E6 and E7 genes (2.39×10−8 and 1.44×10−8(s/s/y), respectively) were used for calibration41. The uncertainty in parameter estimates was evaluated in the 95% highest posterior density (HPD95%) interval.
GenBank accession numbersSequences reported in this work have been deposited in GenBank under accession numbers KP161621 and KP161654.
ResultsHPV detection and typingA total of 255 samples belonging to women with a median age of 29 years (range=15–62) and mode of 25 years (confidence interval 95%=16–34) were collected for this study. These samples were classified as having reactive cellular changes caused by inflammation (RCC; n=63, 24.7%), atypical squamous cells of undetermined significance (ASCUS; n=11, 4.3%), low-grade squamous intraepithelial lesions (LSIL; n=152, 59.6%), high-grade squamous intraepithelial lesions (HSIL; n=18, 7.1%) and cervical cancer (n=11, 4.3%).
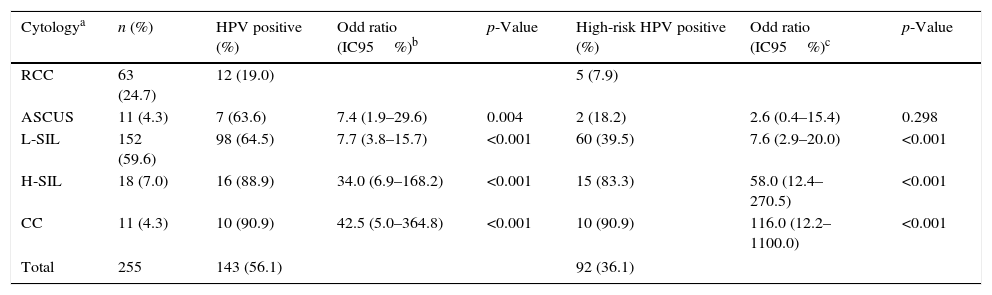
HPV DNA was detected in 56.1% of the cervical lesions (143/255). An increased severity of the cytological status was associated with a greater rate of HPV detection and especially, with a greater rate of high-risk HPV types detection (p<0.01) (Table 1).
Human papillomavirus infections stratified by cytological status
| Cytologya | n (%) | HPV positive (%) | Odd ratio (IC95%)b | p-Value | High-risk HPV positive (%) | Odd ratio (IC95%)c | p-Value |
|---|---|---|---|---|---|---|---|
| RCC | 63 (24.7) | 12 (19.0) | 5 (7.9) | ||||
| ASCUS | 11 (4.3) | 7 (63.6) | 7.4 (1.9–29.6) | 0.004 | 2 (18.2) | 2.6 (0.4–15.4) | 0.298 |
| L-SIL | 152 (59.6) | 98 (64.5) | 7.7 (3.8–15.7) | <0.001 | 60 (39.5) | 7.6 (2.9–20.0) | <0.001 |
| H-SIL | 18 (7.0) | 16 (88.9) | 34.0 (6.9–168.2) | <0.001 | 15 (83.3) | 58.0 (12.4–270.5) | <0.001 |
| CC | 11 (4.3) | 10 (90.9) | 42.5 (5.0–364.8) | <0.001 | 10 (90.9) | 116.0 (12.2–1100.0) | <0.001 |
| Total | 255 | 143 (56.1) | 92 (36.1) | ||||
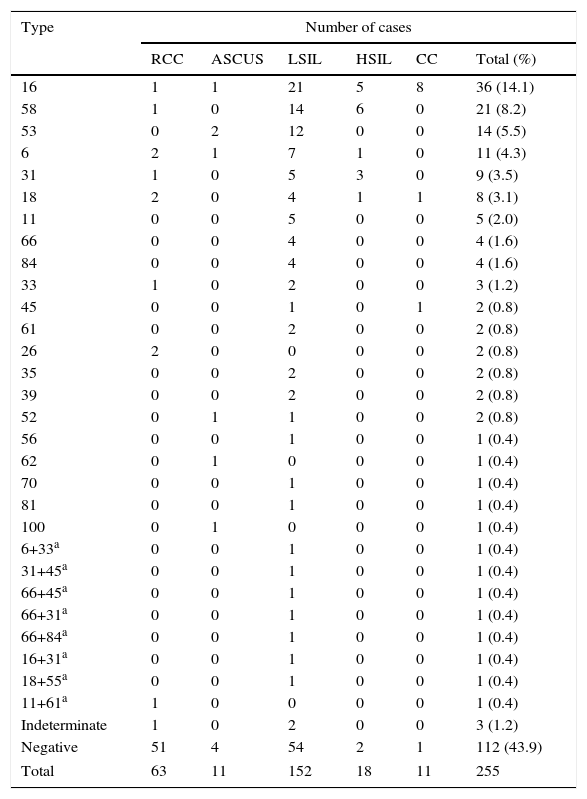
Twenty-two different HPV types were detected. The decreasing order of the main types detected, including their presence in multiple infections, were HPV-16, -58, -53, -31, -6 and -18 (Table 2). Multiple infections were detected in 8 samples (3.1%). The frequency of the current vaccine viral types (-16, -18, -6, -11) was 44.8%, while the high-risk and probable high-risk HPV types not included in the vaccine reached 33.6%.
Distribution of types by cytological classification of cases
| Type | Number of cases | |||||
|---|---|---|---|---|---|---|
| RCC | ASCUS | LSIL | HSIL | CC | Total (%) | |
| 16 | 1 | 1 | 21 | 5 | 8 | 36 (14.1) |
| 58 | 1 | 0 | 14 | 6 | 0 | 21 (8.2) |
| 53 | 0 | 2 | 12 | 0 | 0 | 14 (5.5) |
| 6 | 2 | 1 | 7 | 1 | 0 | 11 (4.3) |
| 31 | 1 | 0 | 5 | 3 | 0 | 9 (3.5) |
| 18 | 2 | 0 | 4 | 1 | 1 | 8 (3.1) |
| 11 | 0 | 0 | 5 | 0 | 0 | 5 (2.0) |
| 66 | 0 | 0 | 4 | 0 | 0 | 4 (1.6) |
| 84 | 0 | 0 | 4 | 0 | 0 | 4 (1.6) |
| 33 | 1 | 0 | 2 | 0 | 0 | 3 (1.2) |
| 45 | 0 | 0 | 1 | 0 | 1 | 2 (0.8) |
| 61 | 0 | 0 | 2 | 0 | 0 | 2 (0.8) |
| 26 | 2 | 0 | 0 | 0 | 0 | 2 (0.8) |
| 35 | 0 | 0 | 2 | 0 | 0 | 2 (0.8) |
| 39 | 0 | 0 | 2 | 0 | 0 | 2 (0.8) |
| 52 | 0 | 1 | 1 | 0 | 0 | 2 (0.8) |
| 56 | 0 | 0 | 1 | 0 | 0 | 1 (0.4) |
| 62 | 0 | 1 | 0 | 0 | 0 | 1 (0.4) |
| 70 | 0 | 0 | 1 | 0 | 0 | 1 (0.4) |
| 81 | 0 | 0 | 1 | 0 | 0 | 1 (0.4) |
| 100 | 0 | 1 | 0 | 0 | 0 | 1 (0.4) |
| 6+33a | 0 | 0 | 1 | 0 | 0 | 1 (0.4) |
| 31+45a | 0 | 0 | 1 | 0 | 0 | 1 (0.4) |
| 66+45a | 0 | 0 | 1 | 0 | 0 | 1 (0.4) |
| 66+31a | 0 | 0 | 1 | 0 | 0 | 1 (0.4) |
| 66+84a | 0 | 0 | 1 | 0 | 0 | 1 (0.4) |
| 16+31a | 0 | 0 | 1 | 0 | 0 | 1 (0.4) |
| 18+55a | 0 | 0 | 1 | 0 | 0 | 1 (0.4) |
| 11+61a | 1 | 0 | 0 | 0 | 0 | 1 (0.4) |
| Indeterminate | 1 | 0 | 2 | 0 | 0 | 3 (1.2) |
| Negative | 51 | 4 | 54 | 2 | 1 | 112 (43.9) |
| Total | 63 | 11 | 152 | 18 | 11 | 255 |
The most prevalent types -16 and -58 represented 21.4 and 14.3% of the LSIL cases in which HPV was detected, respectively. HPV-58 predominated among the HSIL cases with HPV detection, reaching 37.5%, followed by HPV-16 in the 33.3%. In contrast, HPV-58 was not detected among cervical cancers, while HPV-16 was present in 80% of them.
HPV DNA was not found in 43.9% of samples, possibly owing to low viral load or sample degradation, while indeterminate cases (n=3) presented an electrophoretic RFLP pattern that did not match any of the patterns currently taken as reference, according to Bernard et al3.
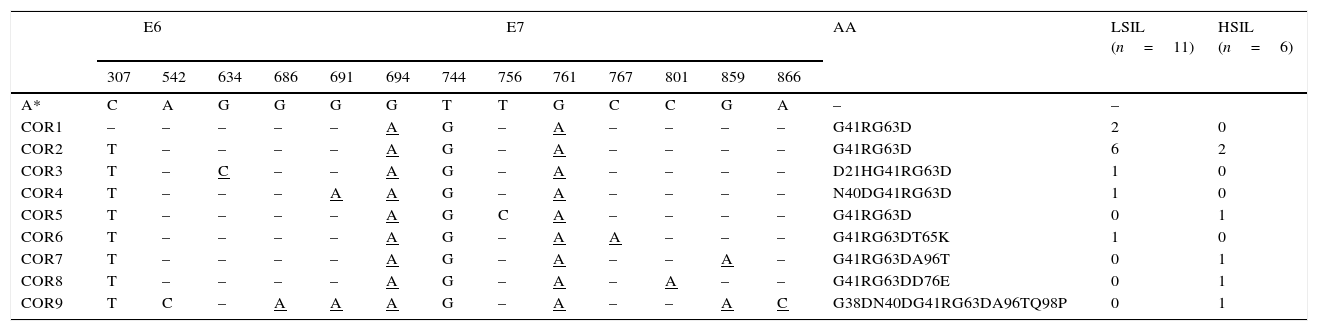
Analysis of mutations in HPV-58A deeper molecular characterization of the HPV-58, the second most prevalent type in this study, was performed to analyze changes with respect to the prototype virus and to look for mutations previously associated with cervical lesions. Seventeen samples from women (22–33 years) with viruses typed as HPV-58 were sequenced along the E6 and E7 genes.
The results showed substitutions in two nucleotide positions in the 450bp of the E6 gene (0.4%) and 11 in the 297bp of the E7 gene (3.7%). The genomes grouped into nine different variants named COR1 to COR9, according to the substitution patterns, compared with the prototype virus (Table 3). Amino acid changes were observed only in the deduced E7 protein sequence.
HPV-58 distribution of variants in LSIL and HSILa
| E6 | E7 | AA | LSIL (n=11) | HSIL (n=6) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 307 | 542 | 634 | 686 | 691 | 694 | 744 | 756 | 761 | 767 | 801 | 859 | 866 | ||||
| A* | C | A | G | G | G | G | T | T | G | C | C | G | A | – | – | |
| COR1 | – | – | – | – | – | A | G | – | A | – | – | – | – | G41RG63D | 2 | 0 |
| COR2 | T | – | – | – | – | A | G | – | A | – | – | – | – | G41RG63D | 6 | 2 |
| COR3 | T | – | C | – | – | A | G | – | A | – | – | – | – | D21HG41RG63D | 1 | 0 |
| COR4 | T | – | – | – | A | A | G | – | A | – | – | – | – | N40DG41RG63D | 1 | 0 |
| COR5 | T | – | – | – | – | A | G | C | A | – | – | – | – | G41RG63D | 0 | 1 |
| COR6 | T | – | – | – | – | A | G | – | A | A | – | – | – | G41RG63DT65K | 1 | 0 |
| COR7 | T | – | – | – | – | A | G | – | A | – | – | A | – | G41RG63DA96T | 0 | 1 |
| COR8 | T | – | – | – | – | A | G | – | A | – | A | – | – | G41RG63DD76E | 0 | 1 |
| COR9 | T | C | – | A | A | A | G | – | A | – | – | A | C | G38DN40DG41RG63DA96TQ98P | 0 | 1 |
Nucleotide sequence alignment showing the mutations in variants named COR1 to COR9. AA, amino acid changes. A*, HPV-58 reference genome (accession number NC_001443). Underlined letters indicate substitutions that result in an amino acid change.
The most frequent mutations were C307T (E6 gene, in 90% of the samples) and G694A (G41R), T744G, and A761G (G63D), found in 100% of the E7 sequences. Other seven mutations (A542C in the E6 gene and G634C, G686A, G691A, C767A, G859A, A866C in the E7 gene) were also found; however, their oncogenic potential has not been described or evaluated yet.
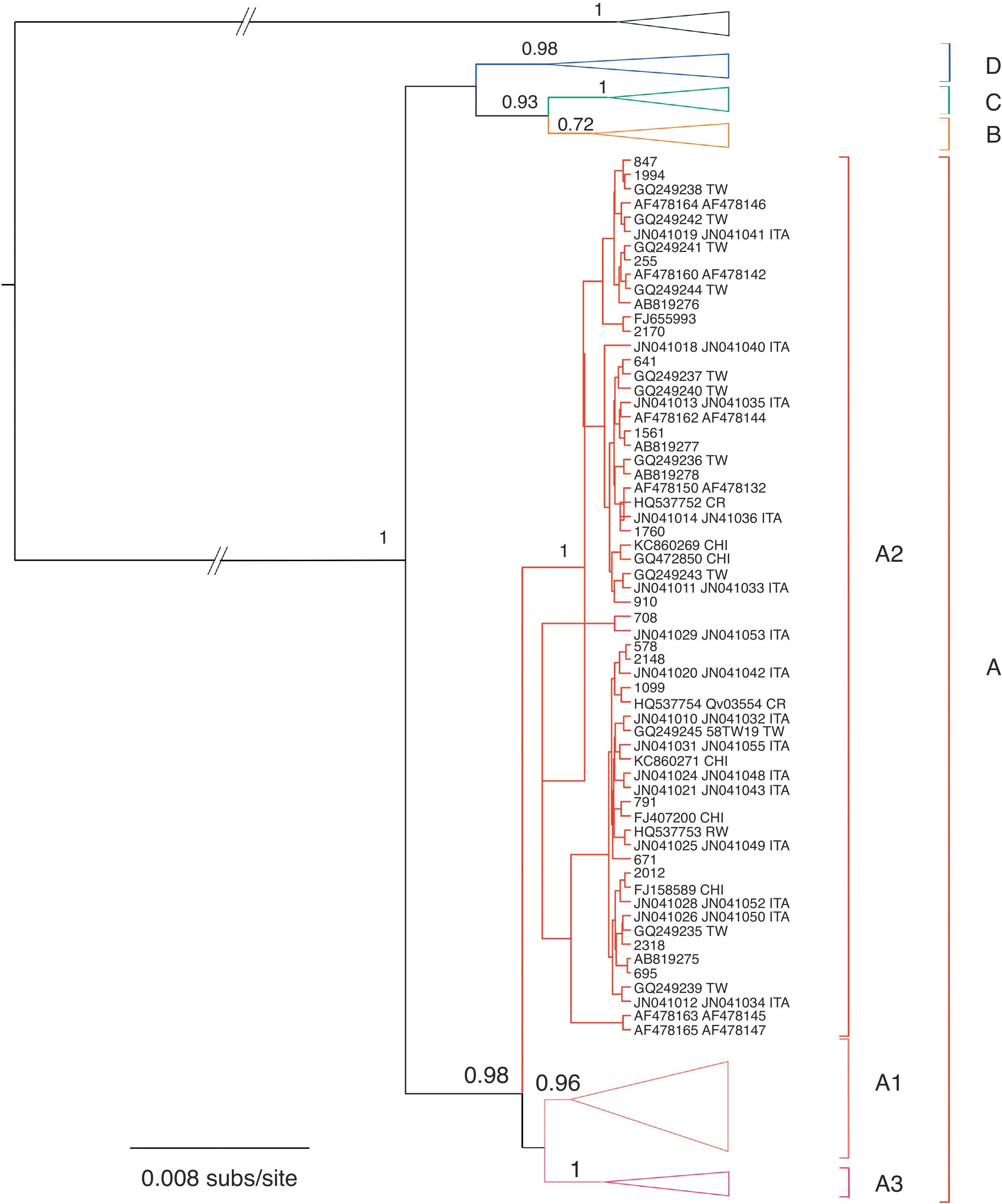
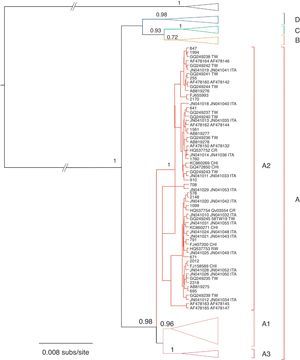
Phylogenetic and phylodynamic analysesThe phylogenetic analysis showed that samples from Corrientes city belonged to lineage A, sublineage 2 (A2), according to the proposed intratypic taxonomy of HPV-5814,19. Moreover, sequences from Corrientes were intermingled with sequences from all around the world: China, Japan, Taiwan, the USA, Scotland and Costa Rica (Fig. 1).
Bayesian phylogenetic tree of the E6 and E7 sequences of HPV-58. Posterior probability values higher than 0.5 are shown at nodes for relevant groups. Isolates reported in this work are shown in bold. Type HPV-33 was used as outgroup. The country of origin is indicated when available (abbreviated in uppercase). BF, Burkina Faso; CHI, China; CR, Costa Rica; ITA, Italy; RW, Rwanda; TW, Taiwan; ZAM, Zambia.
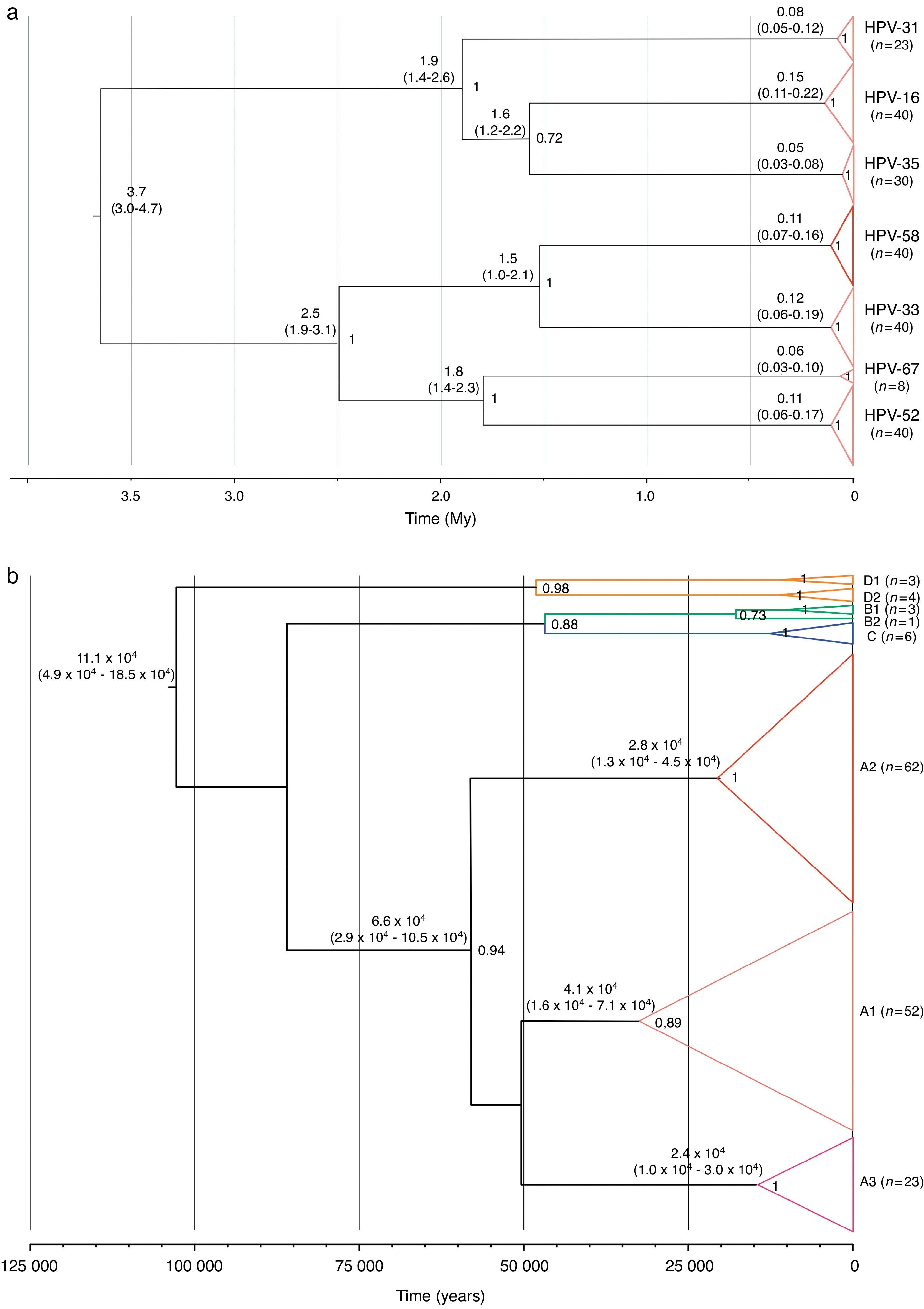
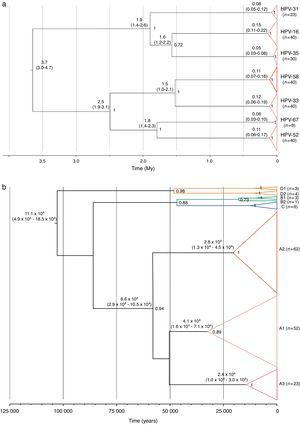
Two substitution rates were used for calibrating the analyses of evolutionary dynamics of the alpha-9 species group and the HPV-58. These alternative hypotheses estimated that the tMRCA for the alpha-9 group originated 20.5 million years (My) ago (HPD95%=16.3–25.8My) or 3.7My (HPD95%=3.0–4.7My) for the slowest and the fastest substitution rates, respectively, whereas, the split up of HPV-58 and its closest cluster, type HPV-33, was dated 8.5My ago (HPD95%=5.4–11.5My) or 1.5My ago (HPD95%=1.0–2.1My). Moreover, type 16 resulted as the most ancient type (∼0.8 or 0.15My), followed by types 58, 33 and 52 (∼0.6 or ∼0.11My) (Fig. 2a).
A deeper analysis on type HPV-58 indicated that its diversification might have started ∼0.54 or 0.11My years ago, while the tMRCA for lineage A was estimated ∼300,000 or ∼65,800 years ago (Fig. 2b). Finally, diversification of main groups within the lineage A (sublineages A1–A3) might have started ∼70,000–200,000 or ∼23,900–41,000 years ago (Fig. 2b).
DiscussionHPV is an important cofactor for cervical neoplasia, and in Argentina it is the most frequent neoplasia (20.9/100,000 women) after mammary cancer (71.2/100,000 women)33. Provinces in the North and Northeastern regions of the country exhibit the highest rates for cervical cancer mortality1, e.g. the Province of Corrientes shows a mortality rate due to cervical cancer of 14/100,000 women, which is three-fold higher than the rate for Buenos Aires, the Capital city of the country, and even exceeds the value of the mortality rate due to mammary cancer estimated by screening studies in Corrientes1,36.
The population under study gathered women with a low socioeconomic status, who are particularly vulnerable to this disease due to reduced access to prevention and control programs.
Despite the studies performed in this region, the number of reported cases is still scarce to evaluate the role of high-risk types2,22–24,47,48. However, it should be noted that we found a high diversity of oncogenic types, with an increased proportion of high-risk and probable high-risk types not included in the current vaccines. This is of concern since there are reports suggesting a limited cross-protection of the vaccines against non vaccine types43,50,53. Therefore, it is necessary to strengthen the monitoring of regional circulation of these types in cases of intraepithelial lesions, contributing to impact assessment of current vaccines against HPV54.
The description of the molecular epidemiology of HPV before the vaccine introduction in 2011 will lead to a better description of the dynamics of the circulation of different genotypes and their variants and also to a better understanding of the relationship between HPV types and cancer in our country, which is useful information for the evaluation of new vaccine formulations. Further studies in post-vaccinal stages will allow to evaluate the dynamics of high-risk HPV types and cross protection among different types10,54. It is worth noting that HPV-58 is included in the nonavalent vaccine V503 formulation, which is still under evaluation16.
The most frequently found type in Corrientes was HPV-16, as also observed all around the world in populations both with normal cytology and cervical abnormalities5,9,34,46. However, the secondary types vary depending on the geographic region under study. In this work, the most important secondary type was HPV-58, as also observed in other studies in the cities of Corrientes and Resistencia22,23. This result differs from the reference meta-analysis conducted by Bruni et al.5 on samples with normal cytology, which showed that HPV-18 was the most important secondary type both globally and in Latin America and the Caribbean. In different regions of the world, and even in other regions of Argentina36,39,45, HPV-58 was found to have low frequency (<2%) when samples with different grade of lesions were considered9,34,46. However, HPV-58 displays a high prevalence in specific regions of the world, especially in Northeast Asia (4–15%), Mexico (24–28%), Costa Rica (12%) and Brazil (12–14%)7,11,28,30,38,52.
Four different lineages (A–D) have been identified within HPV-5814,19. All the viruses reported in this work belonged to lineage A, sublineage A2, which is the most prevalent in the Americas14; none of them belonged to lineage D, which was previously reported in some samples from Argentina14.
HPV-58 is considered a high-risk viral type. However, its disease impact shows geographic variation, since cervical cancer and HSIL are more prevalent in Asian women with HPV-58 than in women from other regions46.
The association between pathogenicity and geographic location could be a consequence of specific genetic backgrounds of the host populations but it could also be related to a differential geographic distribution of distinct HPV variants. For other types it has been proposed that different variant lineages might display different pathogenic potentials31,44. In line with this fact, subgroups within lineage A of HPV-58, which display a differential distribution worldwide, could be related to different clinical progression.
Chan et al.14 proposed to further investigate the possible association of oncogenicity with sublineage A1 of HPV-58 in Asia. Indeed, some mutations in the E7 gene showed a trend of association with the severity of the neoplasia12. In our work, we found no association between high pathogenicity and HPV-58 infections, neither with the mutations reported as involved in this phenotype; however, it should be noted that only sublineage A2 was detected and that the samples belonged to women under 35 years old, and therefore not included in the age range (35–64 years old) strongly associated with progression to cervical cancer32.
The analysis of genome sequences showed that E7 mutations (the only ones rendering amino acid changes) were more frequent than E6 mutations. Neither mutations previously associated with the severity of neoplasia12,15,27, nor cervical cancer associated to HPV-58 were found. However, other mutations not reported previously (such as C767A [T65K] and G859A [A96T]) were found among the samples and deserve further attention given that they localize in a highly reactive epitope among patients with cervical cancer13. Additional studies in the E6 protein assessing the presence of mutations in the protein binding motifs PDZ might help to study their impact on the low oncogenicity of HPV-58 in this population49.
The evolutionary dynamics of the alpha-9 species group and particularly, of type HPV-58 was analyzed. The alpha-9 species separated into two highly supported groups, one formed by types 16, 31, 35 and, the other, by types 33, 52, 58, 67.
Different substitutions rates – needed for phylodynamic analysis calibration – have been proposed for papillomaviruses, such as 1.0×10−7s/s/y (originally proposed for the L1 gene of mucosal HPV)29, 2.4×10−8 and 1.4×10−8s/s/y (from the Feline papillomavirus (FPV) for E6 and E7 genes, respectively)41. The use of these rates resulted in different diversification times of the viral types that belong to the alpha-9 species group, including HPV-58. For instance, at type level, the tMRCA of HPV-16, -33, -52 and -58 showed ancestral times within the last million years (∼0.6 to ∼0.8My) using the substitution rates proposed by Rector et al. or about 0.10–0.15My using those proposed by Halpern. Particularly, lineage A within HPV-58 would have diversified ∼65,800 or ∼300,000 years ago (depending on the calibration used), and the common ancestor of sublineages A1–A3 could have been dated ∼40,000 or ∼200,000 years ago, suggesting that their dispersion – even using the fastest substitution rate – accompanied or preceded human migration across the globe6.
This hypothesis could explain the worldwide distribution of sublineage A2, whereas other factors might be involved in the emergence and dispersion of the others groups, as previously proposed18. Is it worth noting that none of the substitutions rates used to calibrate are proposed for the E6 or E7 genes in HPV, thus these estimations should be taken with caution.
Summarizing, this study describes the molecular epidemiology of HPV in a region of Argentina with a high rate for cervical cancer mortality. The secondary type was HPV-58 (after HPV-16), sublineage A2. Although we found no association between this type and high pathogenicity, there are still some concerns about the extent of cross protection of the vaccines against the types not included in their design. In addition, we described genomic variants of HPV-58 and characterized the ancient origin of this type and its diversification, in the context of the alpha-9 papillomavirus species phylodynamics.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors must have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence must be in possession of this document.
Conflict of interestThe authors declare that they have no conflicts of interest.