Salmonella is a worldwide-distributed pathogen that affects both humans and animals and is usually associated with intensive animal production such as poultry and swine. This bacterium carries different virulence genes, whose expression favors its interaction with the host and may influence the course of the infection. Extended usage of antibiotics for metaphylaxis or prophylaxis and as growth promoters favors the emergence of multiresistant Salmonella strains. The aim of this work was to assess the association between the presence of virulence-associated genes and the antimicrobial resistance phenotype in Salmonella isolates obtained from swine intensive and backyard farms in Argentina during 2012–2018. A total of 59 Salmonella strains belonging to several serotypes were studied. All the strains carried the sopB and ssaQ genes, whereas more than 90% of the isolates carried the mgtC, avrA, and siiD genes. Some isolates also carried the bcfC, sodC1, gipA, sopE1 and spvC genes; however, their presence varied among them. Susceptibility to the antibiotics tested was diverse. Isolates from intensive farms were resistant to a larger number of antimicrobials than those from backyard farms and some of the strains showed high virulence potential and extensive antimicrobial resistance profiles. Continuous surveillance is essential to detect the emergence of strains that may represent a significant risk not only for animal production but also for the human population.
Salmonella es un patógeno de distribución mundial, que afecta tanto a humanos como a animales, y generalmente está asociado a la producción animal intensiva, entre ellas, la aviar y la porcina. Esta bacteria contiene diferentes genes de virulencia, cuya expresión favorece su interacción con el huésped y puede influir en el curso de la infección. El uso extendido de antibióticos en forma metafiláctica o profiláctica, o como promotores del crecimiento, favorece la aparición de cepas de Salmonella multirresistentes. El objetivo de este trabajo fue determinar la presencia de genes de virulencia y la sensibilidad a antimicrobianos en cepas de Salmonella aisladas en producciones porcinas intensivas y de traspatio de la República Argentina entre los años 2012 y 2018. Se estudiaron un total de 59 aislamientos de Salmonella pertenecientes a distintos serotipos. Todos los aislamientos presentaron los genes sopB y ssaQ, mientras que más del 90% portaron los genes mgtC, avrA y siiD. Algunos aislamientos también fueron positivos para los genes bcfC, sodC1, gipA, sopE1 y spvC, pero su presencia fue variable entre las cepas analizadas. La sensibilidad a los antibióticos evaluados fue diversa. Los aislamientos de granjas intensivas fueron resistentes a un mayor número de antimicrobianos que los de granjas de traspatio y algunas de las cepas mostraron perfiles con un alto potencial de virulencia, así como resistencia extendida a antimicrobianos. La vigilancia continua es esencial para detectar la aparición de este tipo de cepas, que pueden representar un riesgo significativo no solo para la producción animal, sino también para la población humana.
Salmonella is a worldwide-distributed pathogen that affects both humans and animals13. This microorganism is usually associated with intensive animal production, such as swine, since the confinement of animals facilitates the transmission and persistence of the pathogen in farms. Salmonellosis may cause significant economic losses due to mortality, morbidity, stunted growth, increased feed conversion rates and costs of non-specific treatments. Some animals, such as pigs and chickens, may be infected without manifesting any clinical signs of the disease and, thus, are relevant in the dissemination of the pathogen within farms and usually serve as sources of contamination of food26. In Argentina, swine production is heterogeneus, comprising large-scale industrial farms and backyard productions6. Intensive farms are usually highly technified with high density of animals, which favors the transmission of infectious diseases30. Furthermore, the use of antimicrobials as therapeutics or growth promoters that may promote the emergence of resistant strains is common. On the other hand, semi-intensive and backyard farms are less technified and pigs may be in close contact with other farm animals such as poultry or wild species1.
S. enterica serovar Choleraesuis (S. Choleraesuis) is the serotype usually associated with the disease in pigs, which can also affect humans10. Likewise, pigs may be infected or carry a large number of serotypes with zoonotic potential, S.enterica serovar Typhimurium (S. Typhimurium) being the most commonly isolated serovar in swine production in Argentina12 and worldwide3. This serotype is also among the most commonly isolated from human samples in Argentina (personal communication ANLIS-Malbran) and worldwide16.
Salmonella can carry different virulence genes, whose expression may determine the course of the infection by favoring the interaction of the microorganism with the host. Thus, in recent years, the analysis of these virulence genes and their involvement in the mechanisms of pathogenicity has been introduced as a tool to characterize Salmonella isolates17. This analysis usually includes the detection of representative genes from the pathogenicity islands 1–5 (avrA, ssaQ, mgtC, siiD and sopB), which are common in all serotypes, prophages (gipA, sodC1 and sopE1), the virulence plasmid (spvC) associated with the systemic dissemination of Salmonella, and the fimbrial cluster (bcfC)22,28. This approach complements the information and knowledge on circulating Salmonella strains and allows to typify them not only by their antigenic properties but also by their pathogenic potential. Furthermore, the virulence of bacteria is influenced not only by the presence of virulence genes but also by their antimicrobial resistance22. Currently, antimicrobials are widely used in animal production as therapeutics or growth promoters. However, the irrational use of these antimicrobials favors the emergence of multiresistant Salmonella strains and their resistance can be spread to other microorganisms in the gut. Many studies have reported high levels of resistance against several antibiotic families in strains isolated from different sources8,9.
The aim of this work was to study the presence of virulence-associated genes and the antimicrobial resistance phenotype in Salmonella isolates obtained from swine intensive and backyard farms in different areas of Argentina during the 2012–2018 period.
Materials and methodsBacterial strainsVirulotyping and susceptibility to antimicrobials were analyzed in 59 isolates of Salmonellaenterica obtained from Argentinean swine production. Strains were isolated at the DILAB (Senasa) and the Instituto de Patobiologia (INTA) laboratories during the 2012–2018 period. Intensive swine production strains were isolated from gallbladder and mesenteric lymph nodes in the slaughterhouse (21/59) or from fecal samples in intensive production farms (28/59), whereas strains from backyard production (10/59) were obtained from fecal samples in the farms (Supplementary Table). The following serotypes were included: Agona (5), Anatum (3), Bredeney (1), Choleraesuis (2), Cerro (2), Derby (6), Enteritidis (1), Lille (1), Lindenburg (1), Liverpool (3), Mbandaka (1), Newport (2), Ohio (2), Rissen (4), Schwarzengrund (5), Stanleyville (1), Thompson (1), Typhimurium (12), monophasic variant of S. Typhimurium 1,4,[5],12:i:- (MST) (3), Westhampton (1) and Worthington (2). Samples were incubated in tetrathionate broth for selective enrichment and sub-cultured on xylose-lysine-deoxycholate agar plates with the addition of tergitol. Isolates were identified by biochemical tests and serotyped according to the Kauffman-White-LeMinor Scheme18.
Virulence genotypingThe presence or absence of ten virulence genes was investigated in all the 59 Salmonella isolates by PCR amplification using specific primer pairs. The technique was carried out according to Huehn et al.22 and Osman et al.27,28 The ten virulence genes analyzed were: avrA, ssaQ, mgtC, siiD, sopB, gipA, sodC1, sopE1, spvC and bcfC. Five of these genes (avrA, ssaQ, mgtC, siiD and sopB) are located in the SPIs, three (gipA, sodC1 and sopE1) are located in prophages, the spvC and the bcfC genes are located, respectively, in the virulence plasmid and the fimbrial cluster. These virulence determinants represent regions that are known to be either highly conserved (SPIs) or variable (prophages, plasmid) within the Salmonella genome25. Forward and reverse primers were used according to Huehn et al.22 DNA from the isolated colonies was extracted by thermal cell lysis. A single PCR reaction was done for each gene. Each PCR was performed in a final volume of 25μl containing 2.5μl of 10× Reaction Buffer TAS (Inbio Highway, Buenos Aires, Argentina), 1.5μl of 25mmol/l MgCl2 (Inbio Highway, Buenos Aires, Argentina), 2μl of 10mmol/l deoxynucleotide triphosphate (Inbio Highway, Buenos Aires, Argentina), 1μl of 10pmol/μl each primer (Biodynamics, Buenos Aires, Argentina), 0.25μl of 5000U/μl Taq DNA polymerase (Inbio Highway, Buenos Aires, Argentina), 13.8μl of DNAse/RNase-free water and 4μl of DNA template. PCR was carried out using a first step of 95°C for one minute followed by 30 cycles of 95°C for 30s, 58°C for 30s and 72°C for 30s and finally 72°C for 4min. Those conditions were used for all targets except for bcfC PCR, whose annealing temperature was 53°C22. After electrophoresis at 90V for 40min, amplification products were stained with ethidium bromide and observed in 1.5% agarose gel (Inbio Highway, Buenos Aires, Argentina). A sample was considered positive when a band located approximately at the height of the desired molecular weight for each gene was observed, and coincided with the band amplified in the positive control. The virulence index (VI) was calculated as the ratio between the number of positive genes found in each isolate and the 10 genes analyzed.
Antimicrobial susceptibility testingEach isolate was tested for susceptibility to 15 antibiotics commonly used in veterinary or human medicine. The profile of antimicrobial susceptibility of each isolate was determined by the agar diffusion method based on the Kirby-Bauer technique4. The following antimicrobials were tested: ampicillin (10μg), aztreonam (30μg), imipenem (10μg), cefalotin (30μg), cephalexin (30μg), cefoxitin (30μg), ceftiofur (30μg), cefepime (30μg), trimethoprim-sulfamethoxazole (1.25/23.75μg), gentamicin (10μg), chloramphenicol (30μg), tetracycline (30μg), nalidixic acid (30μg), ciprofloxacin (5μg) and enrofloxacin (5μg). The reference strain used was Escherichia coli ATCC 25922. The diameters of the zones of inhibition were interpreted according to the tables recommended by the Clinical Laboratory Standards Institute11. The extensively drug-resistant (XDR) index was calculated as “a/b,” where “a” was the number of antibiotics for which a particular isolate was resistant and “b” the total number of antibiotics tested29.
Analysis of dataThe association between the presence of virulence genes and antimicrobial resistance patterns was analyzed by the Chi square contingency test. Differences of XDR indexes of the strains from intensive and backyard productions were analyzed by the Student's t-test. p values <0.05 were considered significant. All analyses were performed using the Graphpad Prism 5 commercial software.
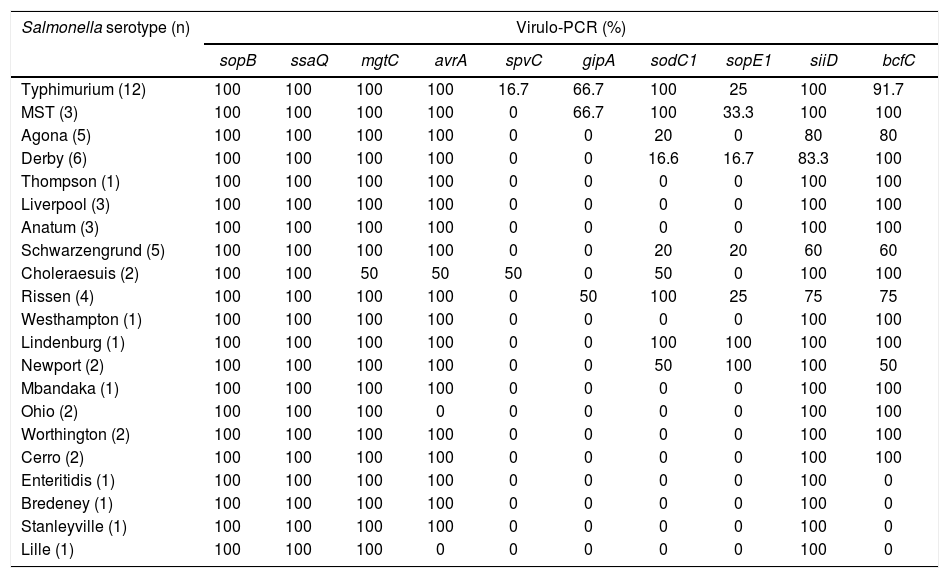
ResultsVirulence genotypingThe sopB, ssaQ, mgtC, avrA and siiD genes were selected as markers of SPI-1 to SPI-5 respectively. All the isolates carried the sopB and ssaQ genes (59/59). The mgtC, avrA and siiD genes were present in 98.31% (58/59), 93.22% (55/59), and 91.53% (54/59) of the isolates respectively, while the prophage-associated sodC1, gipA and sopE1 genes were detected in 42.37% (25/59), 20.34% (12/59), and 18.64% (11/59) of the isolates respectively. The bcfC fimbrial gene was present in 83.05% (49/59) of the isolates and the spvC gene -used as an indicator of the virulence plasmid- was found in 5.08% (3/59) of the isolates analyzed. Table 1 shows the different percentages of presentation of the virulence genes studied in relation to Salmonella serotypes. The average VI was 0.6; the maximum VI (0.9) was found in serotypes Typhimurium and MST, whereas the minimum VI (0.4) was observed in serotype Lille (Supplementary Table). The virulence profiles of the strains isolated from intensive and backyard production were similar and no significant differences were found between their average virulence index (p=0.1886).
Presence of the virulence genes for each serotype.
| Salmonella serotype (n) | Virulo-PCR (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| sopB | ssaQ | mgtC | avrA | spvC | gipA | sodC1 | sopE1 | siiD | bcfC | |
| Typhimurium (12) | 100 | 100 | 100 | 100 | 16.7 | 66.7 | 100 | 25 | 100 | 91.7 |
| MST (3) | 100 | 100 | 100 | 100 | 0 | 66.7 | 100 | 33.3 | 100 | 100 |
| Agona (5) | 100 | 100 | 100 | 100 | 0 | 0 | 20 | 0 | 80 | 80 |
| Derby (6) | 100 | 100 | 100 | 100 | 0 | 0 | 16.6 | 16.7 | 83.3 | 100 |
| Thompson (1) | 100 | 100 | 100 | 100 | 0 | 0 | 0 | 0 | 100 | 100 |
| Liverpool (3) | 100 | 100 | 100 | 100 | 0 | 0 | 0 | 0 | 100 | 100 |
| Anatum (3) | 100 | 100 | 100 | 100 | 0 | 0 | 0 | 0 | 100 | 100 |
| Schwarzengrund (5) | 100 | 100 | 100 | 100 | 0 | 0 | 20 | 20 | 60 | 60 |
| Choleraesuis (2) | 100 | 100 | 50 | 50 | 50 | 0 | 50 | 0 | 100 | 100 |
| Rissen (4) | 100 | 100 | 100 | 100 | 0 | 50 | 100 | 25 | 75 | 75 |
| Westhampton (1) | 100 | 100 | 100 | 100 | 0 | 0 | 0 | 0 | 100 | 100 |
| Lindenburg (1) | 100 | 100 | 100 | 100 | 0 | 0 | 100 | 100 | 100 | 100 |
| Newport (2) | 100 | 100 | 100 | 100 | 0 | 0 | 50 | 100 | 100 | 50 |
| Mbandaka (1) | 100 | 100 | 100 | 100 | 0 | 0 | 0 | 0 | 100 | 100 |
| Ohio (2) | 100 | 100 | 100 | 0 | 0 | 0 | 0 | 0 | 100 | 100 |
| Worthington (2) | 100 | 100 | 100 | 100 | 0 | 0 | 0 | 0 | 100 | 100 |
| Cerro (2) | 100 | 100 | 100 | 100 | 0 | 0 | 0 | 0 | 100 | 100 |
| Enteritidis (1) | 100 | 100 | 100 | 100 | 0 | 0 | 0 | 0 | 100 | 0 |
| Bredeney (1) | 100 | 100 | 100 | 100 | 0 | 0 | 0 | 0 | 100 | 0 |
| Stanleyville (1) | 100 | 100 | 100 | 100 | 0 | 0 | 0 | 0 | 100 | 0 |
| Lille (1) | 100 | 100 | 100 | 0 | 0 | 0 | 0 | 0 | 100 | 0 |
MST: Salmonella 4,[5],12:i:-.
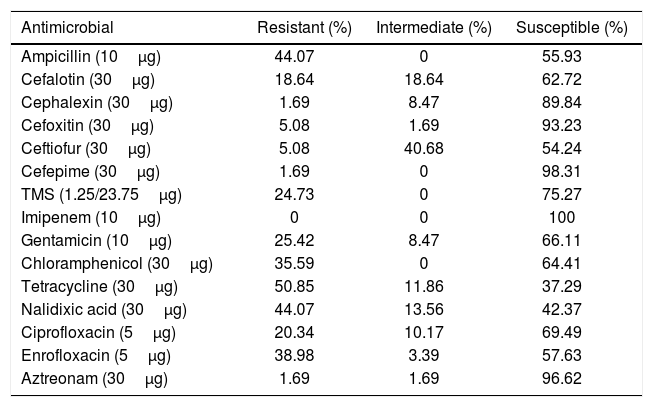
Table 2 shows the percentage of susceptibility to the antimicrobials in all strains. Some isolates belonging to serotypes Agona, Anatum, Choleraesuis, Derby, Enteritidis, Lille, Liverpool, Newport, Schwarzengrund, Stanleyville and Westhampton were susceptible to all the antimicrobials evaluated (Supplementary Table). Extended resistance was frequently observed in strains belonging to serotypes Typhimurium (with a maximum XDR index of 0.60) and MST (with a maximum XDR index of 0.80). The average XDR value of strains from backyard production was 0.08 (SD=0.15) whereas for the isolates from intensive production was 0.24 (SD=0.21), and this difference was significant (p=0.0231).
Percentage of susceptibility of Salmonella isolates to the different antimicrobials evaluated.
| Antimicrobial | Resistant (%) | Intermediate (%) | Susceptible (%) |
|---|---|---|---|
| Ampicillin (10μg) | 44.07 | 0 | 55.93 |
| Cefalotin (30μg) | 18.64 | 18.64 | 62.72 |
| Cephalexin (30μg) | 1.69 | 8.47 | 89.84 |
| Cefoxitin (30μg) | 5.08 | 1.69 | 93.23 |
| Ceftiofur (30μg) | 5.08 | 40.68 | 54.24 |
| Cefepime (30μg) | 1.69 | 0 | 98.31 |
| TMS (1.25/23.75μg) | 24.73 | 0 | 75.27 |
| Imipenem (10μg) | 0 | 0 | 100 |
| Gentamicin (10μg) | 25.42 | 8.47 | 66.11 |
| Chloramphenicol (30μg) | 35.59 | 0 | 64.41 |
| Tetracycline (30μg) | 50.85 | 11.86 | 37.29 |
| Nalidixic acid (30μg) | 44.07 | 13.56 | 42.37 |
| Ciprofloxacin (5μg) | 20.34 | 10.17 | 69.49 |
| Enrofloxacin (5μg) | 38.98 | 3.39 | 57.63 |
| Aztreonam (30μg) | 1.69 | 1.69 | 96.62 |
The association between the presence of virulence genes and resistance to antimicrobials was diverse. With regard to the genes from the SPIs, only siiD was found to be related to resistance to enrofloxacin (p=0.0420), whereas no statistical association was detected between the presence of the mgtC, avrA, ssaQ, and sopB genes and the antimicrobials evaluated. The virulence genes gipA and sodC1, located in the prophage, were associated with resistance to ampicillin (p=0.0067 and p=0.0040), cefalotin (p=0.0022 and p=0.0018), TMS (p=0.0184 and p=0.0093), gentamicin (p=0.0002 and p<0.0001), and ciprofloxacin (p=0.0016 and p=0.0260). gipA was also associated with resistance to nalidixic acid (p=0.0002). No association was found between the presence of prophage gene SopE1 and resistance to any of the antibiotics. Plasmid gene spvC was associated with resistance to tetracycline (p=0.0247), nalidixic acid (p=0.0066) and aztreonam (p=0.0402), and e fimbrial cluster gene bcfC was associated with resistance to ampicillin (p=0.0007) and cefalotin (p=0.0020).
DiscussionIn this work, we analyzed 59 strains of Salmonella isolated from intensive and backyard swine production in Argentina. Among them, we included 12 strains of S. Typhimurium, which is the most prevalent serotype in the Argentinean swine industry23, comprising 25% of Salmonella isolates in the human population from Argentina (ANLIS Malbran, personal communication). Strains were characterized by the presence of two relevant indicators for the pathogenicity of Salmonella: virulence genes and susceptibility to antimicrobials, which may help to understand the potential risk of the strains circulating in swine production.
Most of the strains carried the genes from SPIs 1 to 5, supporting previous studies that suggest that virulence determinants located in these SPIs are highly conserved among serotypes5. These genes may be essential for the early infection of the animals since they regulate intestinal epithelial cell invasion, toxin production, intracellular survival, and also control of inflammation induced by the microorganism7. In contrast, the genes located in the prophages, the fimbrial cluster and the virulence plasmid showed variability. Only the strains of S. Typhimurium and S. Choleraesuis analyzed in this work carried the spvC gene, which is strongly associated with strains that cause bacteremia20 and with systemic dissemination of the microorganism21. However, Astolfi-Ferreira et al.2 proposed that the spvC gene may not be essential for virulence, since they found that spvC-negative strains were still able to cause invasive infections in chickens.
Virulence gene gipA, which is related to colonization and invasion of the small intestine31, was found in only 50% of the strains of S. Rissen and in 66.7% of the strains of S. Typhimurium and its monophasic variant; similar percentages were observed by Huehn et al.22,31 The presence of sopE1, which is associated to disruption of tight junctions25 varied among the serotypes. On the other hand, in our work superoxide dismutase-encoding sodC1 gene was detected in all the S. Typhimurium isolates, as also reported by De Toro et al.14, Osman et al.27, and Bertelloni et al.5 To understand its relevance, the association between the presence of virulence genes and the pathogenicity of circulating strains should be considered in a broader gene context. In fact, if a certain gene is essential or not for pathogenicity is still controversial. For example, some authors consider that the presence of the gipA and sopE1 genes in non-typhoidal Salmonella may be irrelevant for invasiveness33.
Overall, in the present study, the strains from intensive farms were resistant to a larger number of antimicrobials than those from backyard farms independently of the serotype. The highest XDR index value was found for a monophasic S. Typhimurium strain isolated from an intensive farm located in the Province of Buenos Aires. This fact may be explained by the extended usage of antibiotics for metaphylaxis or prophylaxis and as growth promoters in industrial production that may favor the emergence of multiresistant strains as proposed by Esperbent and Migliorati15. In fact, the increasing spread of resistance to different drugs through horizontal transfer among the gut microorganisms is an imperative Public Health concern worldwide14.
Susceptibility to other antimicrobials varied among the strains. We found that 44.07% of the strains were resistant to ampicillin; similar levels of resistance were reported by Campos et al.9 in swine farms from Portugal in 2019, but higher levels were reported by Cameron-Veas et al.8 in Spain in 2018 with around 58% of the isolates resistant to this antimicrobial. In contrast, although practices in swine production in Brazil are similar to those in Argentina, Guerra Filho et al.19, reported only 30% of resistance to ampicillin in strains from the former country. In our work, resistance to cefepime to cefalotin was relatively low, which agrees with reports from other countries14,19. With regard to fluoroquinolones, the levels of resistance of Salmonella are diverse worldwide, ranging from 14.4%32 to 90%19. We found that 20.34% percent of the isolates were resistant to ciprofloxacin and 38.98% of the strains were resistant to enrofloxacin. The emergence of mechanisms of resistance to beta-lactams, which are commonly used to treat severe Salmonella infections, may limit their administration in animal production and thus other alternatives should be explored and implemented14. Susceptibility to third and fourth-generation cephalosporins in strains from livestock is critical since these antibiotics are the main therapeutic option for infections caused by enterobacteria in human medicine8.
The presence of the virulence genes analyzed in this work does not seem to be strictly related to antimicrobial resistance as shown by the dissimilar association between carriage of virulence genes and resistance to antimicrobials. Anyhow, it should be considered that the association between the carriage of genes encoding virulence and resistance to antimicrobials should be focused on plasmid genes. Prophage genes gipA and sodC1 showed the highest association with resistance to most of the antimicrobials tested, whereas we found that genes from SPI 1 to 5 had a relatively low association. In contrast, Osman et al.27 reported 100% association between the presence of genes from these SPIs and resistance to ampicillin, chloramphenicol, gentamicin, nalidixic acid, and TMS. Nevertheless these authors only included strains isolated from poultry, most of them being S. Enteritidis, while in our work a broader spectrum of serotypes was analyzed.
In our study strains with extensive antimicrobial resistance profiles and virulence potential were detected, which may represent a serious threat for both animal production and public health. In the context of the One Health approach, continuous surveillance is essential to detect the emergence and circulation of such strains in order to minimize their impact on animal and human medicine. Thus, efforts to control the pathogen throughout the entire production chain and its environment must be done in order to ensure food safety for consumers. Control of Salmonella in swine production requires tailored-made programs adapted to each farm. Together with strict biosecurity measures, vaccination is one of the main tools to control the microorganism in the long-term, although this strategy is still not common in pigs since there is no commercial vaccine available in the Argentinean market. Moreover, the use of alternative products such as probiotics or phytochemicals that promote gut health may be effective24.
Conflict of interestThe authors declare that they have no conflicts of interest.
We thank Instituto Nacional de Tecnología Agropecuaria and Servicio Nacional de Sanidad y Calidad Agroalimentaria, Argentina for their financial support. We thank Dr. Carla Bustos for her help with molecular biology methodologies, and Pablo Barbano, Laura Magri, Gustavo Orella and Fernando Bessone for providing samples from farms.