There is a lack of standardized protocols for assessing the presence of indoor fungi. It is thus difficult to compare results from different studies or to measure the effect of indoor fungal presence on occupants.
AimsThe aim of the present work was to evaluate the presence of airborne fungal propagules within a hospital taking into account the influence of environmental factors.
MethodsThe study was conducted in a hospital over a period of two years. Two portable aerobiological samplers were used: one capturing propagules onto a sticky surface, and the other onto a culture medium consisting of Sabouraud dextrose agar in Petri dishes, supplemented with chloramphenicol. Sampling was performed indoors at four sites (two on the ground floor and two on the third floor, each consisting of an open ward and a closed room). Samples were also taken outdoors. The following factors were considered for fungus occurrence: season, weather conditions, number of people present in the wards, the insulation of the indoor sites and the existence of construction works on the two floors. We carried out 60 ten-minute samples, weekly during the spring (24 samples), and fortnightly for the rest of the year (36 samples).
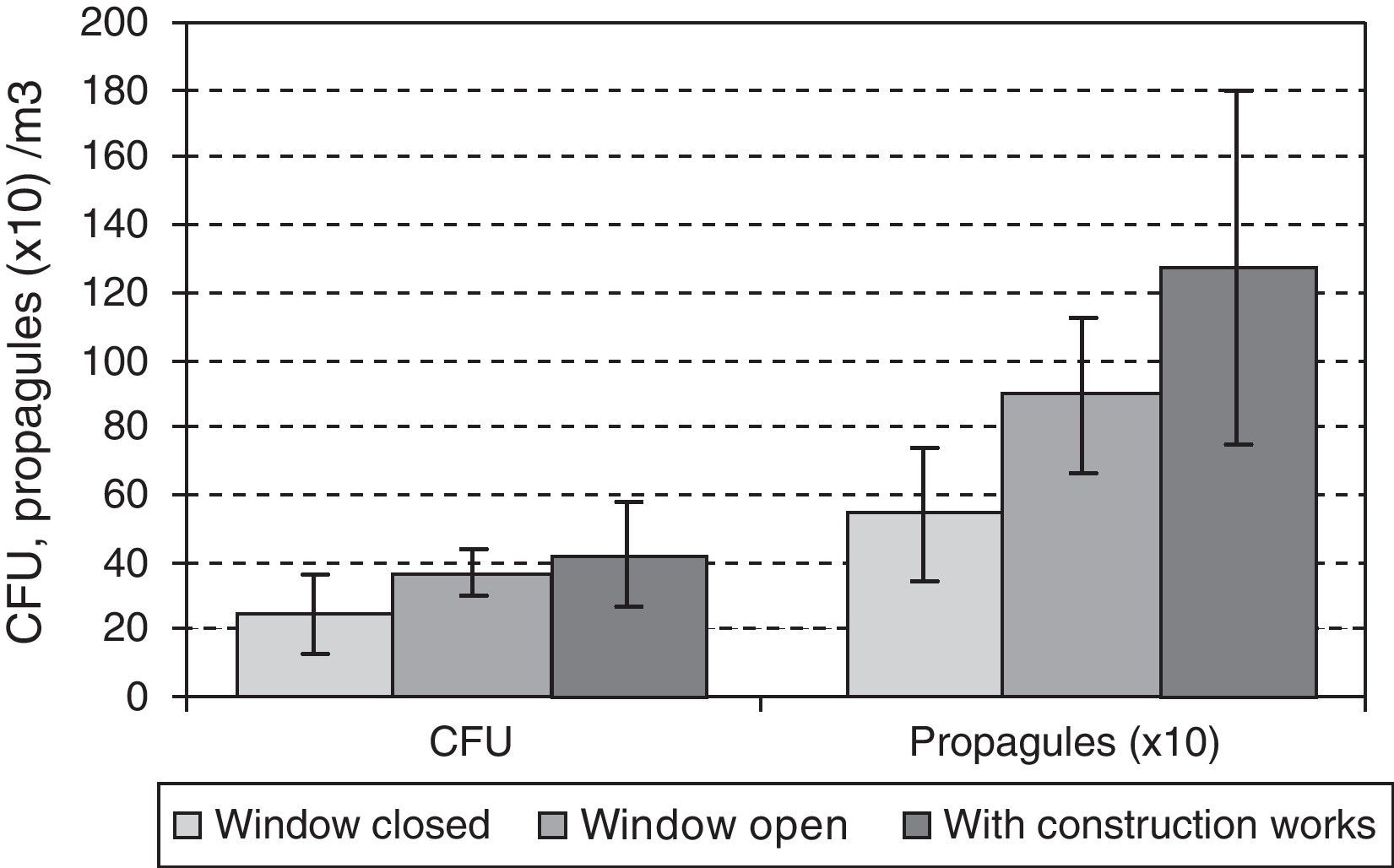
ResultsA total of 2456 colony forming units (CFU) were obtained, with mean propagule concentrations of 107CFU/m3 outdoors and 24CFU/m3 indoors. 35330 counts were recorded for propagules. The mean concentrations were 2473 propagules/m3 outdoors and 790 indoors. A statistically significant positive correlation was found between the number of people in one of the wards and fungus occurrence, and the occurrence in both ground floor and third floor rooms was positively correlated with outdoor levels. These showed a seasonal pattern with peaks in summer. Indoors, however, the peaks appeared in spring and autumn. Outdoor construction activities affected the propagule loads but not the number of CFU.
ConclusionsThe indoor fungus occurrence in the hospital was independent of meteorological conditions and of insulation from outside of the indoor sites selected, but was correlated with the season and number of people in the third floor ward. Outdoor construction activities affected values of indoor propagules, although seasonality could mask their effect.
No existen protocolos estandarizados para el muestreo de hongos en interiores. Por esta causa es complicada la comparación de resultados a partir de diferentes estudios o la evaluación de los efectos de la presencia de hongos en los interiores de los edificios y en sus ocupantes.
ObjetivosEl objetivo del presente trabajo ha sido contribuir al conocimiento de la presencia de propágulos fúngicos aerovagantes en un hospital teniendo en cuenta diferentes factores ambientales.
MétodosPara analizar las causas potenciales de la aparición de hongos en el interior de un hospital durante dos años se han utilizado dos captadores aerobiológicos portátiles, uno para la captura y registro de propágulos fúngicos sobre una superficie adhesiva y otro sobre placas de Petri con agar Sabouraud con cloranfenicol como medio de cultivo. El muestreo se realizó en cuatro lugares del interior (dos en la planta baja y dos en la tercera planta, en cada caso se incluyó una habitación cerrada y una sala de espera) y uno en el exterior. Se estudiaron los siguientes factores: estación del año, condiciones meteorológicas, número de personas presentes en las salas de espera, el grado de aislamiento de los lugares de interior y la existencia de obras en el exterior y en las dos plantas. Se realizaron 60 muestreos de 10 minutos de duración, semanalmente en primavera (24 muestreos) y quincenalmente el resto del año (36 muestreos).
ResultadosSe obtuvieron 2456 unidades formadoras de colonias (UFC), con una concentración promedio de 107UFC/m3 en el exterior y 24UFC/m3 en el interior. En el caso de los propágulos se contaron un total de 35330, con una concentración promedio de 2473 propágulos/m3 en el exterior y 790 propágulos/m3 en el interior. Se encontró una correlación positiva estadísticamente significativa entre el número de personas en una de las salas de espera y la presencia de hongos. La aparición de hongos en las habitaciones de ambas plantas apareció positivamente correlacionada con los niveles de hongos en el exterior. La presencia de hongos en el exterior mostró un patrón estacional con valores más elevados en verano; sin embargo, en el interior, los picos de concentración aparecieron en primavera y otoño. Las obras en el exterior afectaron a la presencia de propágulos en el interior pero no de colonias fúngicas.
ConclusionesLa presencia de hongos en el interior del hospital fue independiente de las condiciones meteorológicas y del grado de aislamiento de los lugares seleccionados, pero se apreció una correlación con la estación y el número de personas en la sala de espera de la tercera planta. Los trabajos del exterior parecen haber tenido influencia en la presencia de propágulos del interior, aunque la estacionalidad podría enmascarar estos efectos.
Fungi are among the most successful organisms in their adaptations to different ecological conditions, mainly due to their diverse reproductive capacity. Indoor environments do not escape the presence of their propagules (i.e. as spores, hyphae and/or sporangia). The positive correlation between spore levels and the risk of infection is widely accepted, and the most widely used method for determining this risk is by air sampling.5,7,16,24 Two methods provide qualitative and quantitative information on the presence of airborne fungi. Both involve sampling a known volume of air per unit time: (1) counting propagules impacted onto a sticky surface and identifying them according to morphological features, and (2) exposing the propagules to a culture medium and identifying the growing colonies (mostly with sporulating structures). The results of such studies show that counts of airborne fungi change both with the time of day and the season.
Indoor fungal epidemiology involves numerous factors, including moisture, ventilation, temperature, and organic matter present in building materials, but also outdoor meteorological parameters, seasonal variations in climate, and presence of construction activities.
Culture-based analysis is widely used to collect and count fungal propagules – indeed non-viable methods are rarely used. The commonest solid culture media employed are Sabouraud dextrose (SDA),1,9,12,28,30,34,35 and malt extract (MEA) or Czapek agar.22,23,25 Nevertheless, using a medium with a lower water activity, such as dichloran 18% glycerol (DG18),17 would increase the number of records, including the most xerophilic ones.38,42,44
Propagule loads in hospital atmospheres have seldom used the two types of samplers simultaneously.36
Different factors have been studied that might determine the presence of fungi in hospitals, construction activities being among those of greatest concern,6,27,34,39 followed by the varied use of air filtration systems.23,32,37,39 Less account has been taken, however, of the differences between floors in the same hospital, or of the number of people per room, as potential carriers of propagules,2 or even of the season of the year.35
The aim of the present work was to contribute to the understanding of the presence of indoor airborne fungal propagules in hospitals surroundings. To this end, we made a 2-year-long study of the airborne fungal load in our hospital simultaneously using two complementary air sampling methods (CFU analysis and spore counts),20 and taking into consideration the influence of different spatial conditions such as room insulation through doors or windows and floor level, meteorological parameters, seasonal variations, construction activities and the relative number of people during the sampling process.
Material and methodsSample selectionSampling was carried out in the hospital Infanta Cristina, in the capital city of Badajoz (Spain). It is an eight-floor building on the outskirts. The sampling period began in April of 2007 and ended in March of 2009. A total of 60 samples were taken, 24 during the first three months on a weekly basis (April to June) and the remaining 36 fortnightly (July to March). The sampling frequency was higher in spring because a previous study in the area had shown higher concentrations in this season.26
Two portable Burkard samplers were used,41 one using slides with white petrolatum (CAS number 8009-03-8) as an adhesive, and the other using Petri dishes with Sabouraud dextrose agar supplemented with chloramphenicol as a culture medium. The two samplers were placed simultaneously on the floor. (A previous study had shown no statistically significant differences whether they were on the floor or 1m above.)40
Four indoor and one outdoor site were selected. The indoor sites were located on the ground and third floors of the building. The ground floor site was selected near the main entrance, and the third, according to availability and advice of the hospital management, near the middle of the building. On each floor, an open ward (OW) and an insulated with door room, office or closet (CR) were selected. The outdoor site was near the main hospital entrance for the patients. On the ground floor, the area of the OW was 148m2, and that of the CR, which had no windows, 17m2. On the third floor, the OW was 49m2, and the CR 9m2 (with a window closed most of the time). For each OW the number of people seated and in transit during the sampling period was counted.
Sampling was performed in the morning between 10:00h and 12:00h for 10min with Burkard samplers. These have an intake air flux of 10l/min, equivalent to 0.1m3. Slides were covered with glycerogelatin,12 and all the propagules in the 14mm-long deposition line from the inlet orifice counted. Petri dishes were incubated at 27°C, within the range 25–30°C most commonly cited in the literature,10,11,21,22,25,27,28,35,44 although thermophilic fungi may be underestimated by this method. After five days, the CFUs were counted and identified, and after 10 days the identifications were confirmed. Another Petri dish following the same protocol but not exposed to the air was incubated as control. Colonies and propagules were identified using basic reference works.8,14,18,31,33Mycelia sterilia includes colonies that have not developed reproductive structures and consequently they were not identified by morphological characteristics.
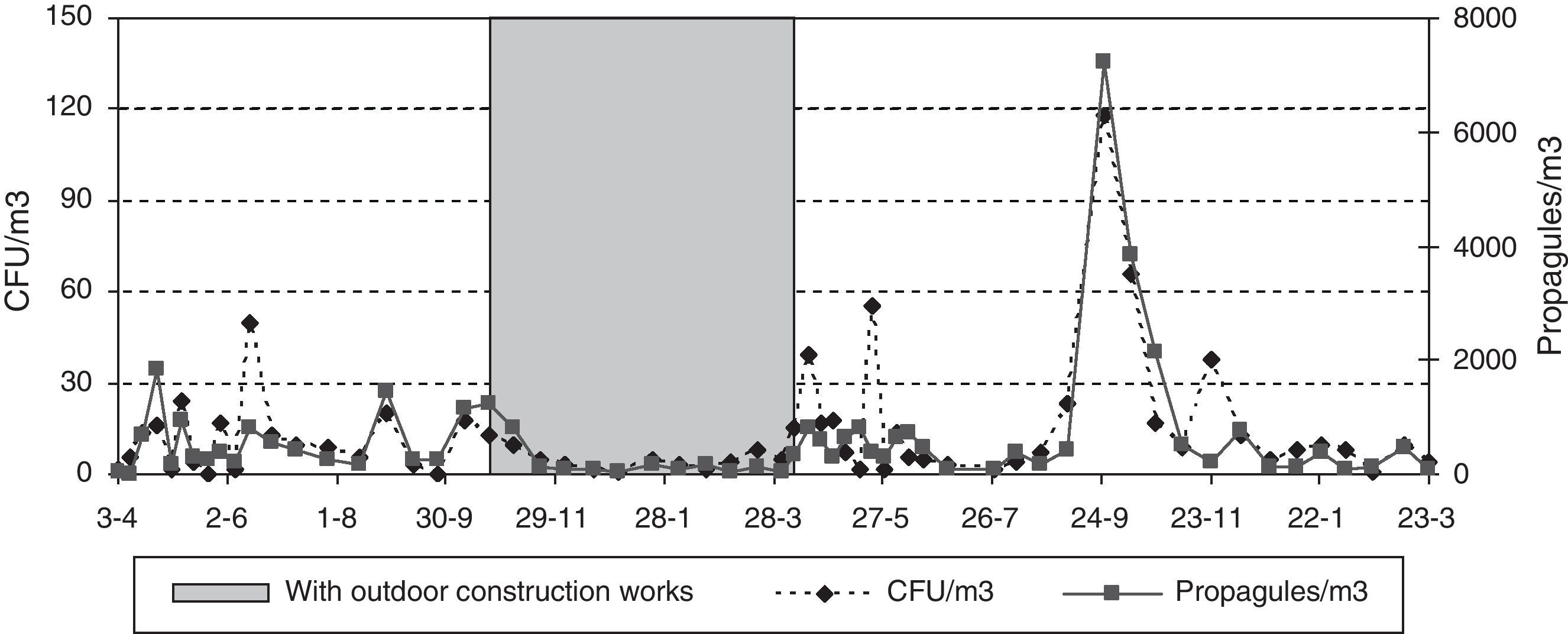
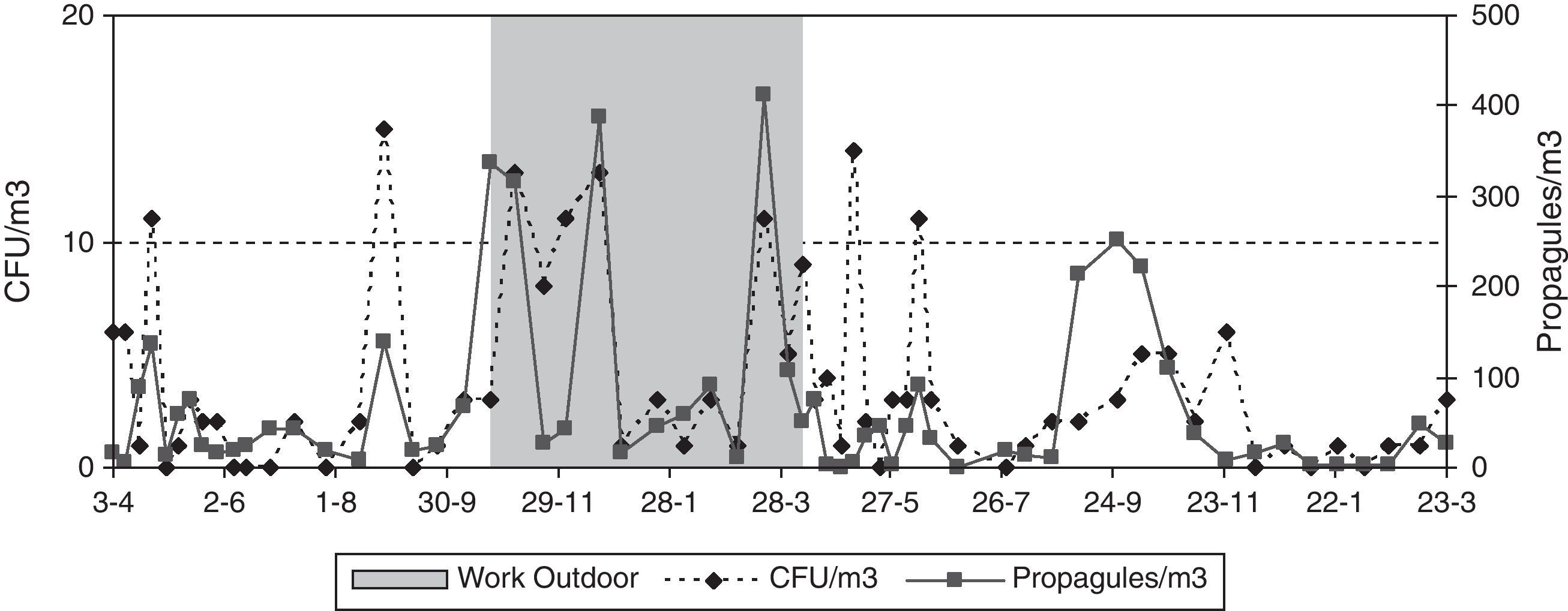
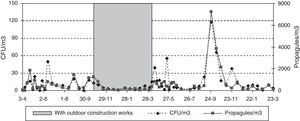
There were outdoor construction works near the main entrance from November 2007 to March 2008, and in the third floor CR from August to early November 2007. Outside, a new 8-floor building was being built on a ca. 20m×50m surface area, and inside, 3–5 workers were renovating two sections in the third floor, including the room sampled. Here the window was found open before sampling on eight occasions, but during the sampling period the window was always shut. To partly prevent exposure to dust, the construction areas inside de building were sealed off with impermeable plastic sheeting during sampling.
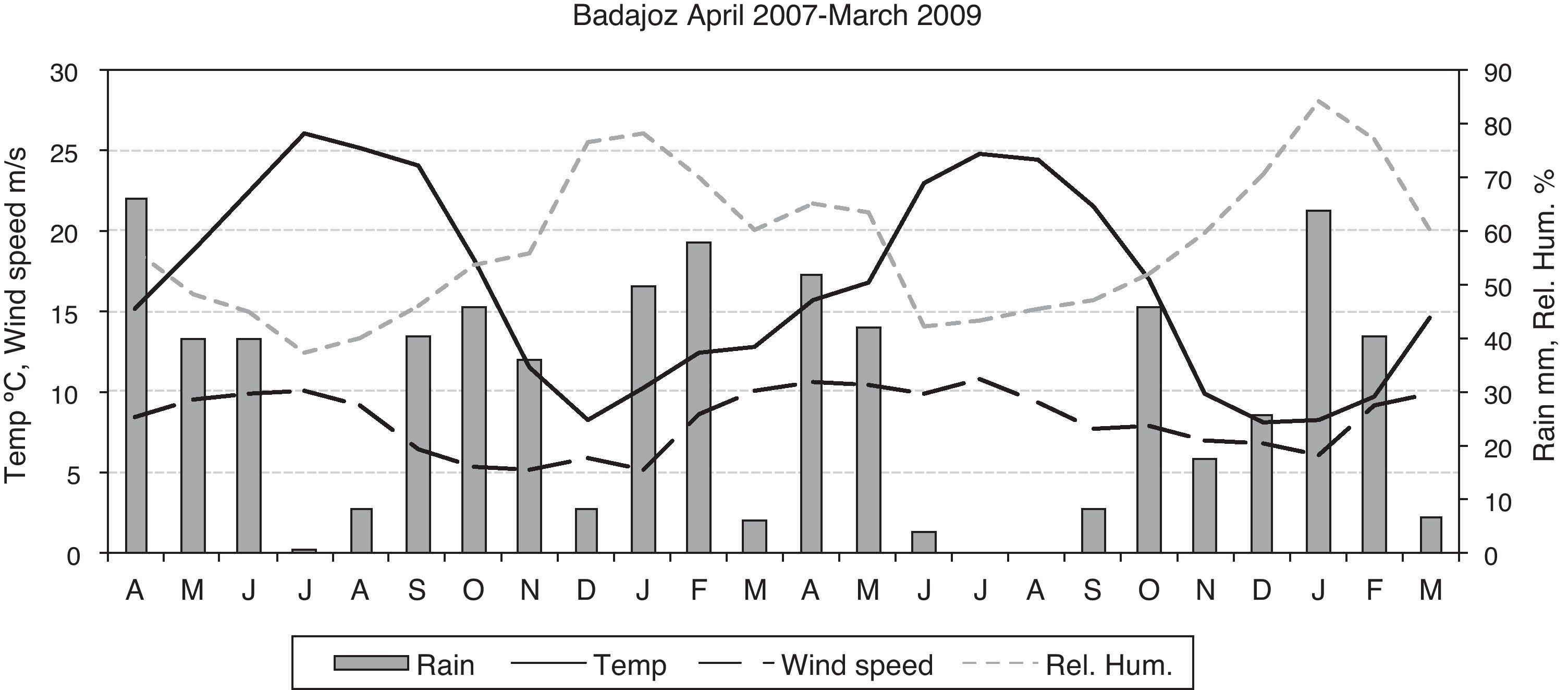
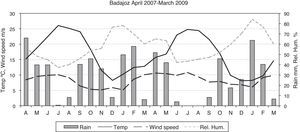
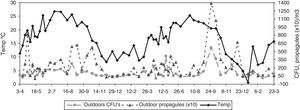
Statistical methodsThe Shapiro–Wilk test was used to evaluate the normality of the data. In spite of a log transformation we had to use non-parametric statistics. To test for seasonality, we applied the non-parametric Friedman test, which permits the comparison of two sources of variation (blocks and ranks). Blocks or groups are the sampling point data and ranks are the seasons. Non-parametric Spearman correlation coefficients (Rho) between the CFU or propagule data and the daily meteorological data were calculated. These were mean temperature, rainfall, wind speed, and relative humidity. Fig. 1 shows the monthly meteorological data for the period studied. To test the influence of other environmental circumstances, such as construction works or the fact of a window having been left open temporarily, the non-parametric Kruskal–Wallis test was used. Seasonal comparisons and their representation in the figures included entire months (winter: J, F, M; spring: A, M, J; summer: J, A, S; autumn: O, N, D).
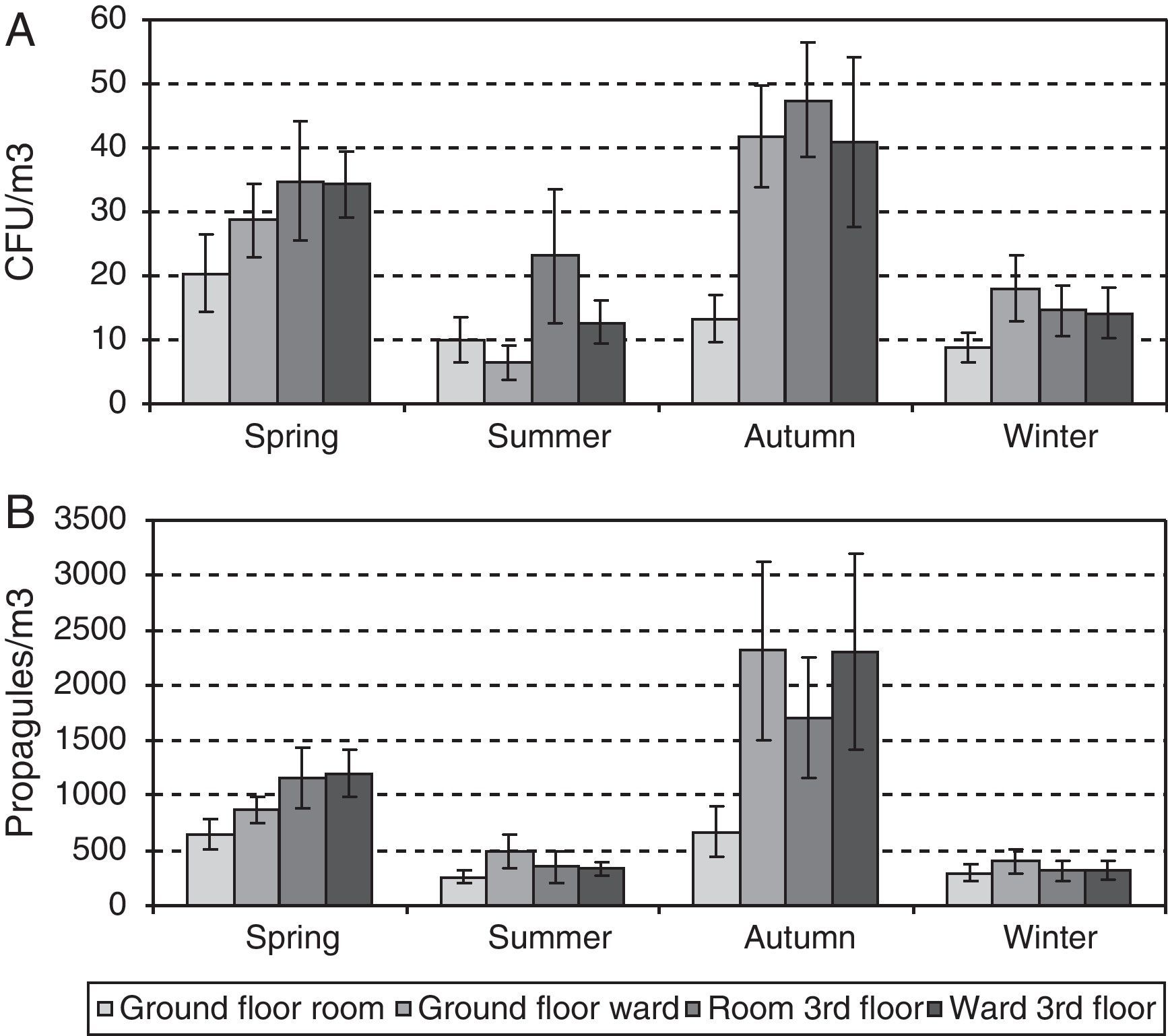
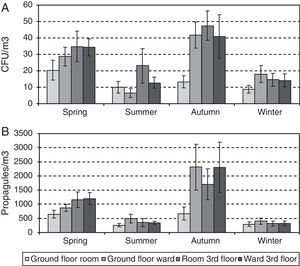
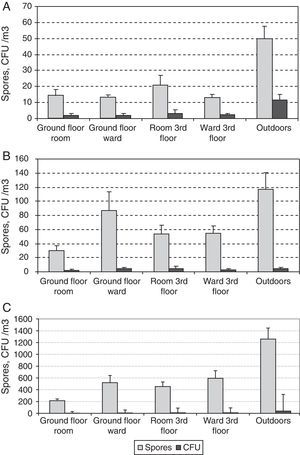
ResultsA total of 2456 CFUs were counted. The outdoor mean concentration was of 107CFU/m3, and that indoors was of 24CFU/m3. The highest indoor mean values were for the third-floor CR (31CFU/m3) and the lowest for the ground-floor CR (14CFU/m3). The mean values for the two OWs were very similar (25 and 27CFU/m3 for the ground and the third floors, respectively).
A total of 35,330 propagules were counted. The outdoor mean was of 2473 propagules/m3 and that indoors of 790 propagules/m3. The highest values: peaks were for the third-floor OW (1053 propagules/m3), and the lowest were again for the ground floor CR (505 propagules/m3). For the two OWs, the means were likewise similar (959 and 1053 propagules/m3 for the ground and the third floors, respectively).
Since neither the CFU nor the propagule data followed a normal distribution [Shapiro–Wilk test (W=0.605, p-value<0.001 for CFU; and W=0.610, p-value<0.001 for propagules)], non-parametric tests were applied. No statistically significant difference was found between the CFU and propagule data when both years were compared. The outdoor sampling results showed the lowest values in winter and the highest in summer, with those of spring and autumn being similar. For the indoor sampling, the differences in the CFU results were greater between the four sampling points than between seasons (Friedman test, p-value 0.0824) (Fig. 2A) but, for propagules, there were statistically significant differences between seasons (p-value 0.0194): the values were lowest in winter, highest in spring and autumn, and intermediate in summer (Fig. 2B). The Kruskal–Wallis test showed statistically significant seasonal differences for the indoor data (CFU K-W 31.2971, value 0.0006E−3 and propagules K-W 48.9005, p-value 0.0001E−6) and for outdoor propagules (K-W 11.9472, p-value 0.0076), but not for outdoor CFUs.
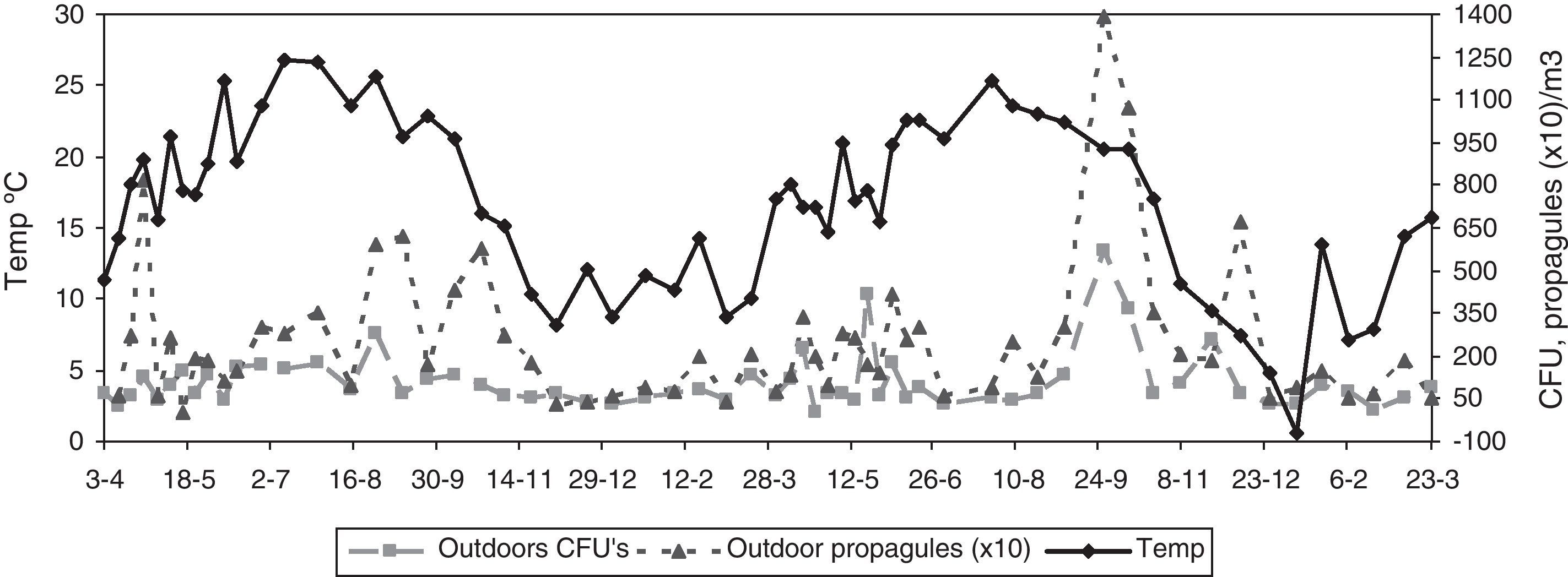
With regard to the meteorological data, only for the outdoor data were there statistically significant correlations for mean temperature (positive) and for relative humidity (negative) (temperature: Rho 0.370, p-value 0.004 for CFU; and Rho 0.486, p-value 0.000 for propagules; relative humidity: Rho −0.281, p-value 0.030 for CFU; and Rho −0.357, p-value 0.005 for propagules) (Fig. 3).
The mean number of people present in the OWs was 25.2 (2–79, s 16.1) for the ground floor and 24.7 (2–57, s 11.2) for the third floor. The Spearman correlation coefficient was only statistically significant (positive) for propagules on the third floor OW (Rho 0.348, p-value 0.011).
Comparing the outdoor results with those indoors, the Spearman correlation coefficients were statistically significant for propagules at the four indoor sampling points (Rho 0.407, p-value 0.001, for the ground floor CR; Rho 0.644, p-value 0.000 for the ground floor OW; Rho 0.422, p-value for the third floor CR; Rho 0.548, p-value 0.000 for the third floor OW) and CFUs at the three indoor sampling points (Rho 0.300, p-value 0.020, for the ground floor CR; Rho 0.258, p-value 0.046 for the ground floor OW; Rho 0.408, p-value 0.001 for the third floor OW).
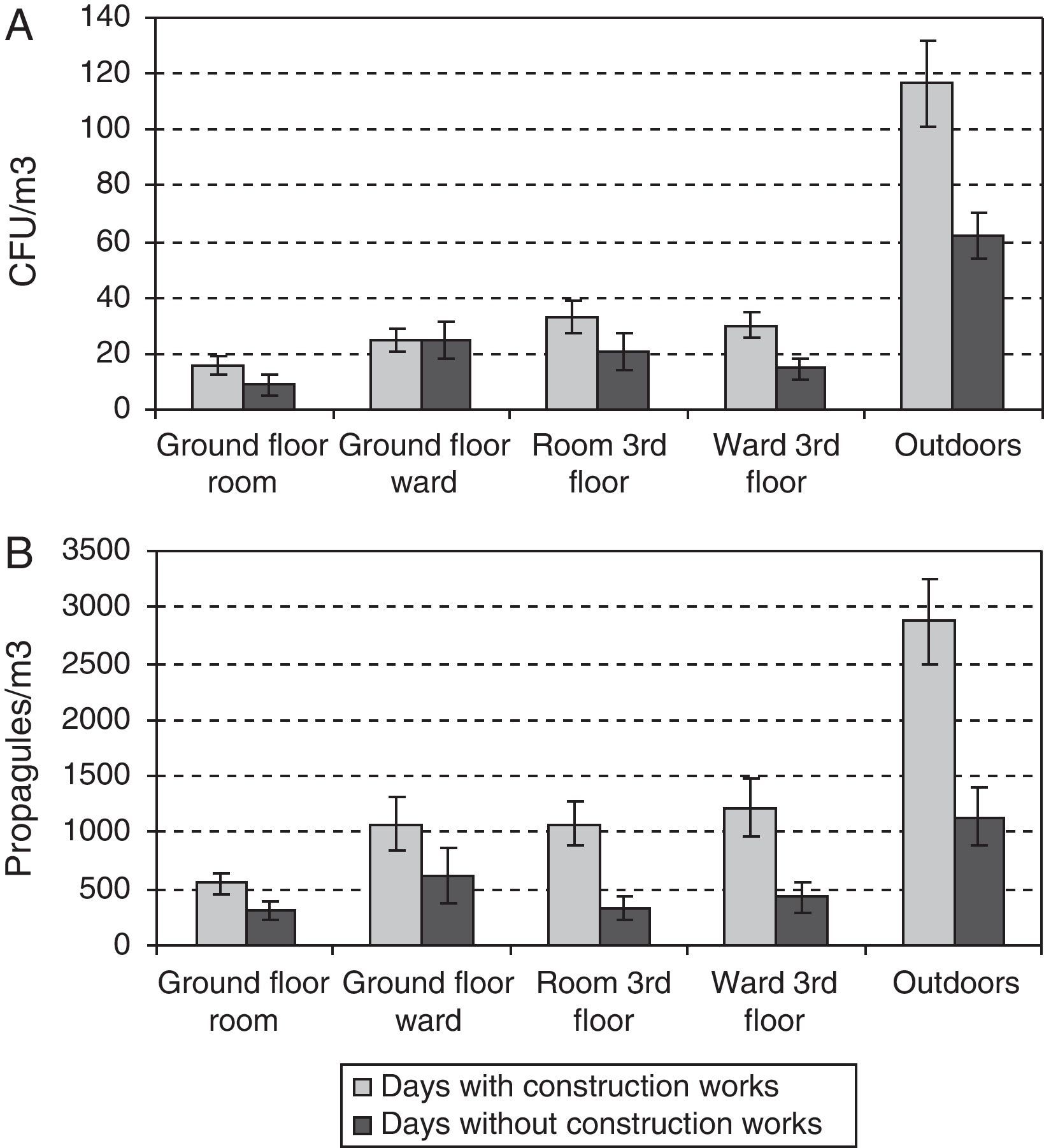
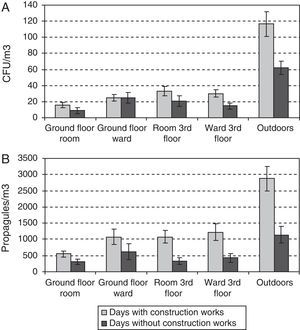
During the outdoor construction period (only studied in the first year), the mean outdoor fungal concentrations (62CFU and 1140 propagules/m3) were lower than for the rest of the sampling period (106CFU/m3 and 3083 propagules/m3). Only for one of the four indoor sampling points and for one set of measurements (CFU counts on the ground-floor OW) was the concentration higher during this period. The Friedman test applied to the data for the five sampling points comparing days with and days without construction works showed no difference for CFU values (p-value 0.1797), but there was a significant difference for propagules (p-value 0.0253). These differences apply to data from outdoors and from the ground floor (Fig. 4A and B).
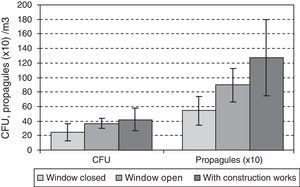
The data for the third-floor CR relating to the eight days of the first year studied, when the window found open before the sampling period gave means of 32CFUs and 894 propagules/m3, and for the days when the window was closed, 25CFU/m3 and 544 propagules/m3. During the renovation activities on this floor, the means were higher (42 CFUs and 1271 propagules/m3). In both cases there were no statistically significant differences using the Kruskal–Wallis test (Fig. 5).
Comparing results for the same floor, we found statistically significant differences only for the CFU data between the CR and OW on the ground floor, even if the data corresponding to the renovation period in the third floor were ignored.
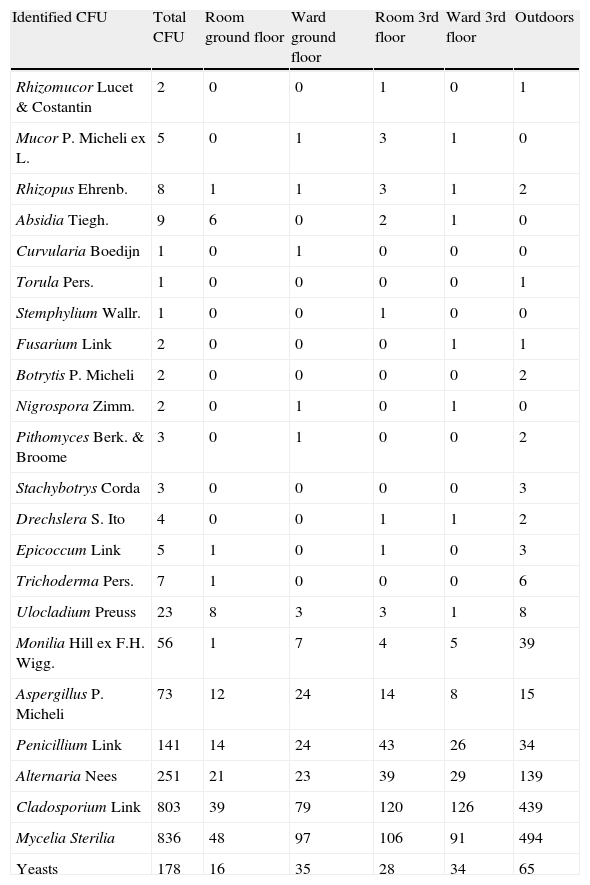
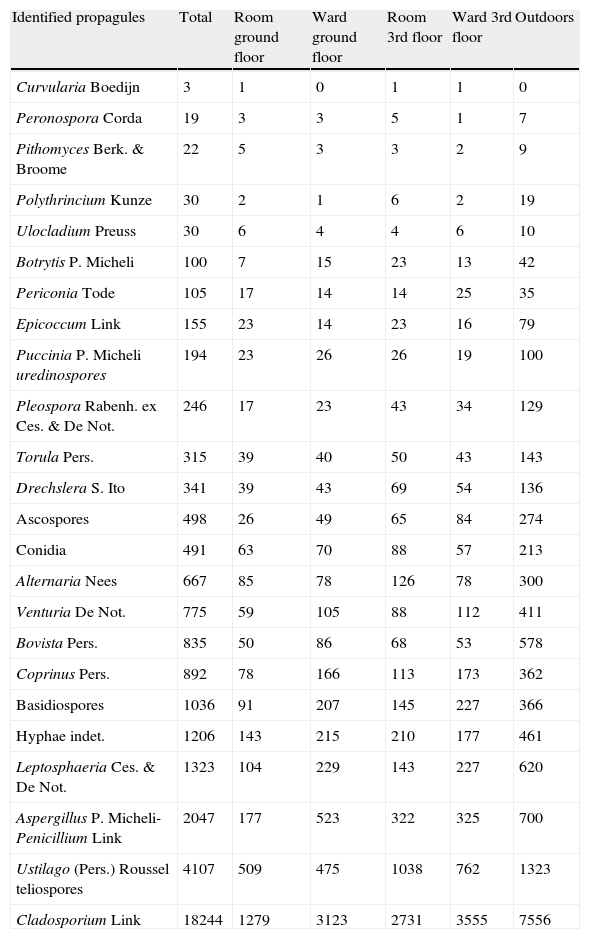
Total colonies and propagules found appear in Tables 1 and 2. The five most frequent fungal taxa identified appear in Table 3.
Number of total CFU.
| Identified CFU | Total CFU | Room ground floor | Ward ground floor | Room 3rd floor | Ward 3rd floor | Outdoors |
| Rhizomucor Lucet & Costantin | 2 | 0 | 0 | 1 | 0 | 1 |
| Mucor P. Micheli ex L. | 5 | 0 | 1 | 3 | 1 | 0 |
| Rhizopus Ehrenb. | 8 | 1 | 1 | 3 | 1 | 2 |
| Absidia Tiegh. | 9 | 6 | 0 | 2 | 1 | 0 |
| Curvularia Boedijn | 1 | 0 | 1 | 0 | 0 | 0 |
| Torula Pers. | 1 | 0 | 0 | 0 | 0 | 1 |
| Stemphylium Wallr. | 1 | 0 | 0 | 1 | 0 | 0 |
| Fusarium Link | 2 | 0 | 0 | 0 | 1 | 1 |
| Botrytis P. Micheli | 2 | 0 | 0 | 0 | 0 | 2 |
| Nigrospora Zimm. | 2 | 0 | 1 | 0 | 1 | 0 |
| Pithomyces Berk. & Broome | 3 | 0 | 1 | 0 | 0 | 2 |
| Stachybotrys Corda | 3 | 0 | 0 | 0 | 0 | 3 |
| Drechslera S. Ito | 4 | 0 | 0 | 1 | 1 | 2 |
| Epicoccum Link | 5 | 1 | 0 | 1 | 0 | 3 |
| Trichoderma Pers. | 7 | 1 | 0 | 0 | 0 | 6 |
| Ulocladium Preuss | 23 | 8 | 3 | 3 | 1 | 8 |
| Monilia Hill ex F.H. Wigg. | 56 | 1 | 7 | 4 | 5 | 39 |
| Aspergillus P. Micheli | 73 | 12 | 24 | 14 | 8 | 15 |
| Penicillium Link | 141 | 14 | 24 | 43 | 26 | 34 |
| Alternaria Nees | 251 | 21 | 23 | 39 | 29 | 139 |
| Cladosporium Link | 803 | 39 | 79 | 120 | 126 | 439 |
| Mycelia Sterilia | 836 | 48 | 97 | 106 | 91 | 494 |
| Yeasts | 178 | 16 | 35 | 28 | 34 | 65 |
Number of total propagules identified. Ascospores, Conidia and Basidiospores include other kind of these propagules not identified.
| Identified propagules | Total | Room ground floor | Ward ground floor | Room 3rd floor | Ward 3rd floor | Outdoors |
| Curvularia Boedijn | 3 | 1 | 0 | 1 | 1 | 0 |
| Peronospora Corda | 19 | 3 | 3 | 5 | 1 | 7 |
| Pithomyces Berk. & Broome | 22 | 5 | 3 | 3 | 2 | 9 |
| Polythrincium Kunze | 30 | 2 | 1 | 6 | 2 | 19 |
| Ulocladium Preuss | 30 | 6 | 4 | 4 | 6 | 10 |
| Botrytis P. Micheli | 100 | 7 | 15 | 23 | 13 | 42 |
| Periconia Tode | 105 | 17 | 14 | 14 | 25 | 35 |
| Epicoccum Link | 155 | 23 | 14 | 23 | 16 | 79 |
| Puccinia P. Micheli uredinospores | 194 | 23 | 26 | 26 | 19 | 100 |
| Pleospora Rabenh. ex Ces. & De Not. | 246 | 17 | 23 | 43 | 34 | 129 |
| Torula Pers. | 315 | 39 | 40 | 50 | 43 | 143 |
| Drechslera S. Ito | 341 | 39 | 43 | 69 | 54 | 136 |
| Ascospores | 498 | 26 | 49 | 65 | 84 | 274 |
| Conidia | 491 | 63 | 70 | 88 | 57 | 213 |
| Alternaria Nees | 667 | 85 | 78 | 126 | 78 | 300 |
| Venturia De Not. | 775 | 59 | 105 | 88 | 112 | 411 |
| Bovista Pers. | 835 | 50 | 86 | 68 | 53 | 578 |
| Coprinus Pers. | 892 | 78 | 166 | 113 | 173 | 362 |
| Basidiospores | 1036 | 91 | 207 | 145 | 227 | 366 |
| Hyphae indet. | 1206 | 143 | 215 | 210 | 177 | 461 |
| Leptosphaeria Ces. & De Not. | 1323 | 104 | 229 | 143 | 227 | 620 |
| Aspergillus P. Micheli-Penicillium Link | 2047 | 177 | 523 | 322 | 325 | 700 |
| Ustilago (Pers.) Roussel teliospores | 4107 | 509 | 475 | 1038 | 762 | 1323 |
| Cladosporium Link | 18244 | 1279 | 3123 | 2731 | 3555 | 7556 |
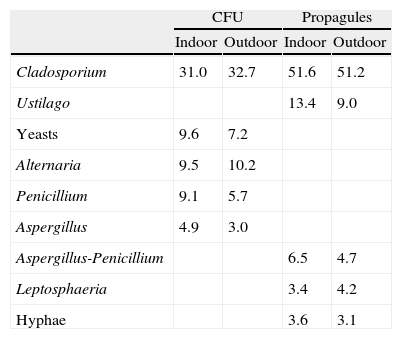
Percentage of representation for the most important airborne fungi of the study. Aspergillus-Penicillium type includes all the spores from both genera as they cannot be separated by light microscope.
| CFU | Propagules | |||
| Indoor | Outdoor | Indoor | Outdoor | |
| Cladosporium | 31.0 | 32.7 | 51.6 | 51.2 |
| Ustilago | 13.4 | 9.0 | ||
| Yeasts | 9.6 | 7.2 | ||
| Alternaria | 9.5 | 10.2 | ||
| Penicillium | 9.1 | 5.7 | ||
| Aspergillus | 4.9 | 3.0 | ||
| Aspergillus-Penicillium | 6.5 | 4.7 | ||
| Leptosphaeria | 3.4 | 4.2 | ||
| Hyphae | 3.6 | 3.1 | ||
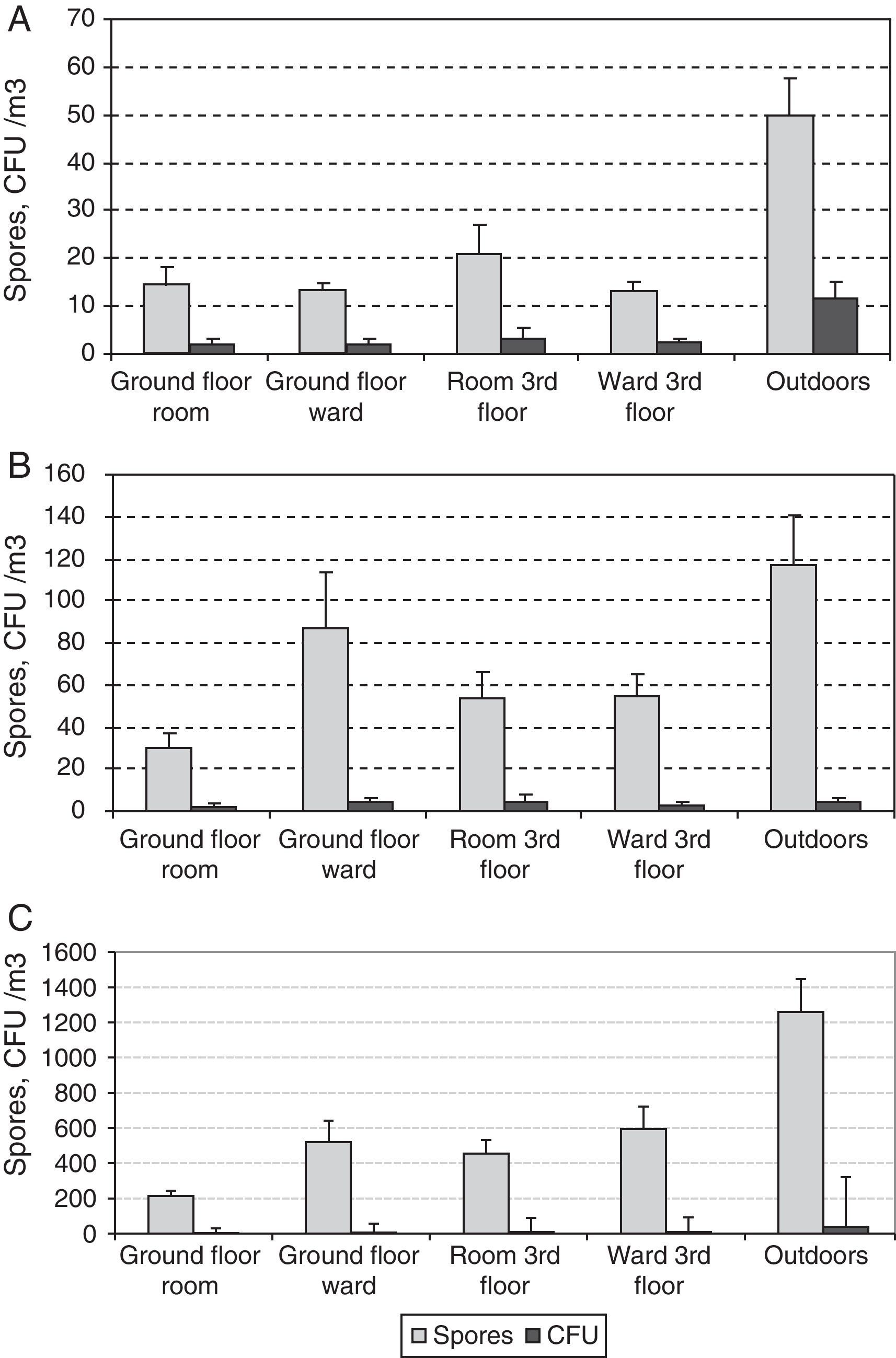
The presence of Cladosporium showed the same seasonality as that of the total airborne fungi, with lowest values in winter (both indoors and outdoors), and peaks in summer for outdoors and in spring and autumn for indoors (Fig. 6). The mean loads during the outdoor construction works were lower than during the rest of the period, for both the indoor and the outdoor sampling. For indoors, differences between seasons were higher (Friedman p-value for CFU 0.0192 and propagules 0.0169) than between sites (Friedman p-value for CFU 0.0314 and propagules 0.1272).
For either CFUs or propagules of Aspergillus and Penicillium, the differences between seasons were greater than between sites when using the Friedman test, although none of them was statistically significant (Fig. 7). In the third-floor CR, the Kruskal–Wallis test showed no statistically significant differences when the window was open or when renovations were taking place.
The two sampling methods could distinguish presumably between viable and non-viable fungi. Another useful index is culturability, which has been defined as the rate between colonies and spores (C/S).19Fig. 8 shows average concentrations for both colonies and spores of the three main fungal types, Alternaria, Aspergillus-Penicillium and Cladosporium at the five sites studied. The highest values of C/S were for Alternaria (0.12–0.23), followed successively by Aspergillus-Penicillium (0.04–0.07) and Cladosporium (0.01–0.03).
DiscussionOverall, our mean concentration of indoor airborne fungi, with less than 25U/m3, is similar to values reported in other sampling areas accessed by many people.2,22,27,28,30 The lower values found in other studies27,28 can be explained because they were performed in special, restricted-access areas, such as haematology units.
The seasonal presence of airborne fungi outdoors was evident, temperature being the main meteorological factor involved. Thus, the highest values corresponded to summer, and the lowest to winter. But for the indoor data, the values in summer were lower than in spring and autumn. Indoor summer increases have been detected in some studies,9,11,27,29,35,36 while others have not detected seasonality.28 In the present study, the decrease observed in summer could be because main doors or even some windows are kept closed for more time in the air-conditioned rooms, thus reducing the access of fungal propagules.
The presence of people during the sampling period seems to affect propagule levels, although the evidence is not very strong. This could mean that people act as vectors of propagules. Nevertheless, we only counted the people present during the sampling period, not those present beforehand, who could have also affected the results. We cannot discard this potential influence as it has been previously adduced.21
Propagule data from the four indoor sampling points were correlated with those from outdoors. The same was found for the CFUs, except those from the third floor CR. This is congruent with the idea that the main door is one of the main avenues of propagule entry, as observed by other workers,32 although other sources have been suggested, as water.43 The insulation of the ground floor closed room was only reflected in the CFU values. This could be attributed to the fact that many propagules may be of fungi that do not usually grow on the media supplied, for example most Basidiomycetes and Myxomycetes, and many Ascomycetes as teleomorphs.
Studies on the presence of airborne fungi during construction works have had contradictory results. While some workers find an increase,4,23,24,34 others do not.6,15,39 In many cases, the increase was tested outdoors but not indoors because the insulation measures applied were sufficiently effective as it seems that occurred in the present study. Nevertheless seasonal differences could be strong enough to mask the effects of construction works.
When using culture media as traps,10,28,32Cladosporium conidia are considered to be the most frequent airborne spores in nearly all environments, but some workers have found Aspergillus more so. This would support that only using culture media is insufficient for estimating the overall presence of fungi in indoor air, as many airborne micro-organisms cannot be cultured, with the result that they are not detected.3
In conclusion, culturing fungus spores only detects a small fraction of the total airborne spora. It is thus necessary to also count and identify spores on glass slides for a good estimate of the indoor spora concentration.13 The five most frequent groups of CFU identified were Cladosporium, yeasts, Alternaria, Penicillium and Aspergillus. For the propagules, they were Cladosporium, Ustilago, Aspergillus-Penicillium, Leptosphaeria, and hyphae. The indoor fungus occurrence in our hospital was independent of meteorological conditions and of the insulation of the indoor sites selected, but was correlated with the season and number of people in one of the wards. Outdoor construction works affected the propagule data although seasonal differences could mask this evidence.
Conflict of interestThe authors have no conflict of interest to declare.
This study was supported in part by a grant from the Junta de Extremadura, Consejería de Sanidad y Consumo, SCSS0536, and PRE 08058 by European Social Fund. Special thanks are due to the assistant personnel at the hospital.