Candida species, in conditions of microbiota imbalance or decreased immune defenses, may be one of the main human fungal pathogens. Virulence factors constitute the mechanisms used by the fungus to avoid host defenses.
AimsThis study aimed to investigate the in vitro production of virulence factors, such as hemolytic activity, and deoxyribonuclease (DNase), proteinase, and phospholipase activities in Candida spp.
MethodsFifty clinical isolates were analyzed for virulence factors: Candida albicans (15), Candida tropicalis (15), Candida parapsilosis (10), Candida glabrata (5), and Candida krusei (5). Hemolytic activity was determined in Sabouraud dextrose agar plates containing 3% glucose and 7% sheep red cells. Culture media containing, respectively, agar-base DNA, egg yolk, and bovine albumin were used to determine DNase, phospholipase and proteinase activities, respectively.
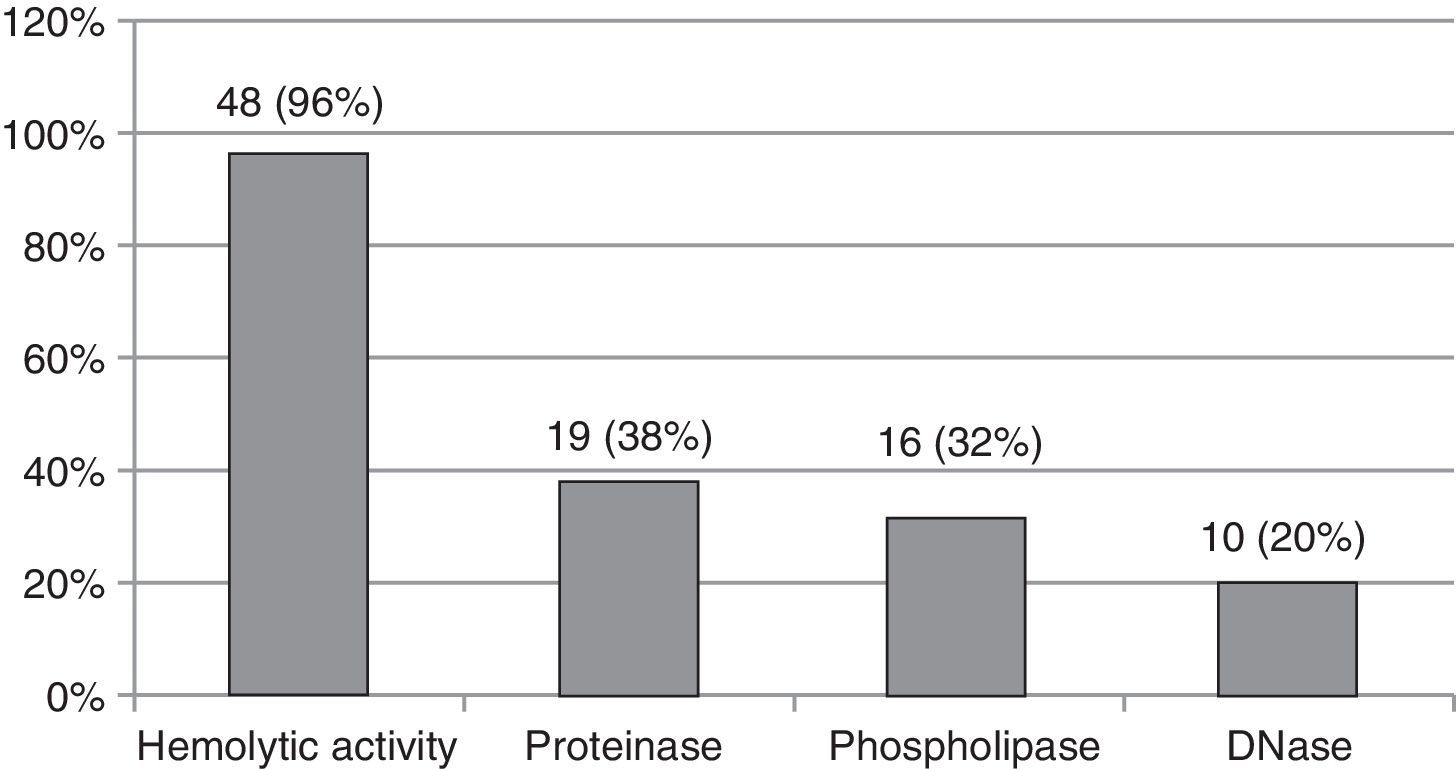
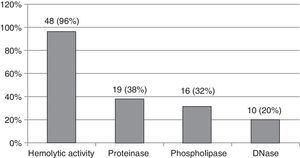
ResultsForty-eight (96%) of 50 isolates showed hemolytic activity, with 10 (20%) positive for DNase, 19 (38%) for proteinase, and 16 (32%) for phospholipase. Statistically significant differences were observed between species for phospholipase (p<0.0001) and proteinase (p<0.05) production.
ConclusionsIt is concluded that all species had hemolytic activity. DNase activity was detected in all species except in C. glabrata; proteinase activity was detected in C. albicans, C. tropicalis, and C. parapsilosis; and phospholipase activity was observed in C. albicans and C. tropicalis.
Las levaduras del género Candida, en condiciones de desequilibrio de la microbiota o de disminución de las defensas inmunológicas, pueden ser uno de los principales patógenos fúngicos del hombre. Los factores de virulencia constituyen los mecanismos utilizados por el hongo para evadir las defensas del huésped.
ObjetivosEste estudio tiene como objetivo investigar la producción in vitro de algunos factores de virulencia, como la actividad hemolítica, y las actividades desoxirribonucleasa (DNasa), proteinasa y fosfolipasa en Candida spp.
MétodosSe analizaron 50 aislamientos clínicos: Candida albicans (15), Candida tropicalis (15), Candida parapsilosis (10), Candida glabrata (5), y Candida krusei (5). La actividad hemolítica fue determinada en placas de agar glucosado de Sabouraud, con glucosa al 3% y un 7% de hematíes de oveja. Los medios de cultivo de agar-ADN, yema de huevo y albúmina bovina fueron utilizados para determinar las actividades DNasa, fosfolipasa y proteinasa, respectivamente.
ResultadosDe los 50 aislamientos, 48 (96%) presentaron actividad hemolítica, 10 (20%) fueron positivos para DNasa, 19 (38%) para proteinasa y 16 (32%) para fosfolipasa. Se observaron diferencias estadísticamente significativas entre las especies para las actividades fosfolipasa (p<0,0001) y proteasa (p<0,05).
ConclusionesSe concluye que todas las especies estudiadas poseen actividad hemolítica. La actividad DNasa fue detectada en todas las especies, excepto en Candida glabrata; la actividad proteinasa fue detectada en C. albicans, C. tropicalis y C. parapsilosis, y la actividad fosfolipasa se observó en C. albicans y C. tropicalis.
Recent increases in the incidence of fungal infections worldwide are a matter of great concern in public health. Among yeasts, Candida species remain the main cause of hospital infections, particularly in adult or pediatric intensive care units.10,23 The fungal infection is debilitating and recurrent in immunocompromised patients and the co-existence of other disorders or diseases may retard or aggravate diagnosis and inclusively cause death.1,2,18
Commensal Candida species may become pathogenic for humans if microbiota balance is disturbed. Pathogenicity will depend on factors such as host predisposition (immunodepression), parasitic load and fungal virulence that facilitate tissue invasion and evasion of host defense mechanisms.2,4,23
The pathogenicity of fungi has been investigated in an attempt to identify the differences in clinical presentation and severity of the infection. Among the properties of Candida species that may explain pathogenicity, different virulence factors are present, which include adhesion to substrates, formation of the germ tube, phenotypic and genotypic variability, toxin production and extracellular enzymes. These constitute important factors in the launching and maintenance of infections. Proteinases and phospholipases are among the hydrolytic enzymes produced. Hemolytic activity and DNase are considered by some authors to be important factors in virulence, as well as the capacity to multiply at high temperatures, for example 39°C and 42°C.6,8,12,15
Characterization of virulence factors contributes to the understanding of Candida infections, including the differences between different species. The aim of this study was to investigate the in vitro hemolytic activity, and DNase, phospholipase and proteinases activities in five species of Candida: Candida albicans, Candida tropicalis, Candida parapsilosis, Candida glabrata and Candida krusei.
Materials and methodsFifty Candida spp. strains distributed as C. albicans (15), C. tropicalis (15), C. parapsilosis (10), C. glabrata (5) and C. krusei (5) were isolated from different clinical materials (blood, synovial and peritoneal liquids), and stored at −20°C in BHI-glycerol at the Laboratory of the Technical Course in Clinical Analysis, Technical Health School, Federal University of Uberlandia, Minas Gerais, Brazil. Subcultivation for tests was carried out in Sabouraud dextrose (ASD)-chloramphenicol media by incubation at 30°C for 24h.
Experimental samples of each isolate were prepared by suspension in saline solution to a turbidity corresponding to tube 2 of the McFarland scale (1×108 to 5×108cells/ml). Petri dishes (90mm×15mm) containing, respectively, ASD-blood,14 DNase agar,19 phospholipase agar22 and proteinase agar16 were each inoculated with 5μL aliquots of five different isolates at equidistant points.
Results were described and interpreted according to the characteristics of each test. Hemolytic activity was evidenced by the translucent halo with defined borders around colonies, and expressed by an index, hemolytic index (Hi), calculated by dividing the colony diameter by the diameter of colony plus the hemolysis zone. DNase activity was expressed as positive or negative by the detection of a clearing halo without defined borders around the colonies. The activities of phospholipase and proteinase were identified by an opaque zone (precipitation of a calcium complex) and a clear halo around the yeast colonies, respectively, and were expressed by using the Pz value (phospholipase zone) for phospholipase and Prz (proteinase zone) for proteinase. Both Pz and Prz values were calculated by dividing the colony diameter by the colony diameter plus the precipitation or clearing, respectively, according to Price et al.12 Values of 1.0 for Hi, Pz and Prz mean the isolate did not exhibit the tested activity (score 1); values between 0.64 and 0.99 (score 2) indicate moderate activity, and those values equal to or below 0.63 indicate strong enzymatic activity (score 3).
The Mann–Whitney test was used to calculate statistic differences between enzymatic activities in isolates of C. albicans, C. parapsilosis, C. krusei, C. glabrata and C. tropicalis. In all tests, p<0.05 was considered significant.21
ResultsForty-eight (96%) of the fifty isolates included in the study had hemolytic activity, 19 (38%) proteinase activity, 16 (32%) phospholipase activity and 10 (20%) DNase activity (Fig. 1).
Hemolytic activity was characterized as moderate in 45 isolates (90%), with indexes varying between 0.64 and 0.99 and without statistically significant differences between the species. The moderate hemolytic index was shown by all isolates of C. albicans, C. glabrata, and C. krusei, and by 74% of C. tropicalis and 90% of C. parapsilosis.
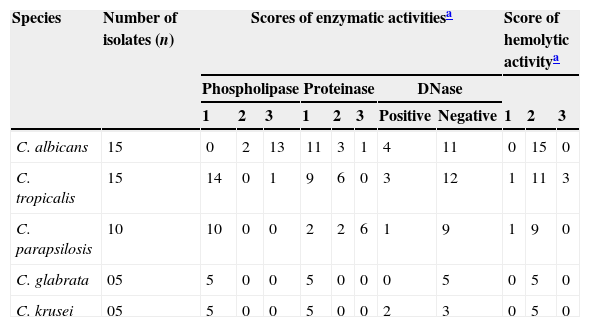
Twenty percent of the isolates had DNase activity distributed as follows: 26% in C. albicans, 20% in C. tropicalis, 10% in C. parapsilosis and 40% in C. krusei (Table 1). Statistical differences among species were not significant.
Frequency of Candida spp. isolates showing hemolytic activity and activities of phospholipase, proteinase and DNase enzymes according to the respective scores.
| Species | Number of isolates (n) | Scores of enzymatic activitiesa | Score of hemolytic activitya | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phospholipase | Proteinase | DNase | ||||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | Positive | Negative | 1 | 2 | 3 | ||
| C. albicans | 15 | 0 | 2 | 13 | 11 | 3 | 1 | 4 | 11 | 0 | 15 | 0 |
| C. tropicalis | 15 | 14 | 0 | 1 | 9 | 6 | 0 | 3 | 12 | 1 | 11 | 3 |
| C. parapsilosis | 10 | 10 | 0 | 0 | 2 | 2 | 6 | 1 | 9 | 1 | 9 | 0 |
| C. glabrata | 05 | 5 | 0 | 0 | 5 | 0 | 0 | 0 | 5 | 0 | 5 | 0 |
| C. krusei | 05 | 5 | 0 | 0 | 5 | 0 | 0 | 2 | 3 | 0 | 5 | 0 |
Proteolytic activity was observed in 19 (38%) isolates, distributed in eight (80%) C. parapsilosis, seven (46%) C. tropicalis and four (26%) C. albicans. Statistically significant differences were found between C. albicans and C. parapsilosis (p=0.004), and C. tropicalis and C. parapsilosis (p=0.003).
Phospholipase activity was present in all C. albicans isolates and one C. tropicalis. Statistical differences were significant (p<0.0001) for C. albicans and each of the other species. No significant differences were observed between the other species when compared in groups of two.
DiscussionInfective processes are dependent on host defense mechanisms, general and local conditions and upon virulence factors characterizing the causative microorganisms. Virulence factors are widely investigated in an attempt to explain differences in pathogenicity of several fungal isolates including Candida spp., as well as to understand the parasite–host relationship.
Candida isolates included in this study showed hemolytic activity with the exception of only two strains: one C. tropicalis and one C. parapsilosis that did not show hemolysis in blood agar in the test conditions. Rorig et al.14 reported hemolytic activity only in C. parapsilosis and C. albicans and negative results for C. glabrata, C. krusei, Candida guilliermondii and Candida dubliniensis. However, other authors observed in vitro hemolytic activity in isolates of C. albicans, C. glabrata and C. tropicalis from blood or other sources, as central venous catheters and urine.5,11,20
Some of the discrepancies in the results may be due to difficulties in the quantification of hemolytic activity conducted in different test conditions, definitions and interpretations of results. Thus, the use of suggested models to characterize activities, although empirical, will permit comparison of results from different laboratories. The model proposed by Price et al.,13 which utilizes a practical, rapid and easy-to-determine score, was used in indexes expressing activity of phospholipases.
Results concerning DNase activity in Candida species were not found in the literature. Some strains of all the species, except C. glabrata, were able to produce DNase. This lack of information may be due again to experimental problems of the in vitro detection of the activity. The method requires prolonged incubation periods, and halos surrounding colonies do not have well-defined borders, suggesting the use of qualitative interpretations as positive or negative. DNase in fungi was reported by Cazin et al.3 and Sanchez et al.17 The last authors demonstrated that all clinical and environmental isolates of Cryptococcus neoformans and Cryptococcus gattii had DNase activity, but it is more frequent in clinical isolates of C. neoformans.
The implication of extracellular DNase as a virulence factor for fungi has not yet been defined. Hypotheses are that DNase contributes to the evasion of the immune system, preventing the death of the yeast that can be caused by neutrophils.17 DNase may be important in combination with other exoenzymes to degrade DNA of other microorganisms in microenvironment, especially bacteria, facilitating the colonization site by reduction of microbial competition. However recent studies have shown the role of extracellular DNase produced by C. albicans in maintaining the integrity of the biofilm and in decreasing susceptibility to antifungal drugs9. However, future studies should be conducted in order to verify the importance of DNase for Candida spp., its association with other virulence factors, especially with adhesins, and its implication in virulence, pathogenicity and antifungal resistance.
Phospholipase activity was detected in all C. albicans isolates, confirming the results by Mahmoudabadi et al.,8 who found this activity in all urogenital isolates of this species. Among the other species, only one isolate of C. tropicalis was positive, while Junqueira et al.7 observed that isolates of C. albicans, C. dubliniensis, C. tropicalis and C. krusei from oral cavity were phospholipase producers. However D’Eça Junior et al.4 analyzed clinical isolates from several sources and concluded that among the different species in the study, C. tropicalis was the most frequent producer of phospholipase, while the activity was not present in C. parapsilosis, C. glabrata and C. krusei.
Proteinase activity was present in C. tropicalis, C. parapsilosis and C. albicans, confirming observations by Junqueira et al.,7 who detected the activity in isolates of C. albicans, C. parapsilosis and C. dubliniensis from oral cavity and by Seneviratine et al.20 in blood isolates of C. albicans, C. parapsilosis, C. tropicalis and C. dubliniensis.
It is concluded that all the species tested had hemolytic activity; DNase activity was detected in all the species except in C. glabrata. Proteinase was detected in C. albicans, C. tropicalis and C. parapsilosis and phospholipase only in C. albicans and C. tropicalis. It is suggested that DNase activity observed in clinical isolates of Candida spp. may become an interesting attribute to be considered in pathogenicity studies in this genus.
Conflict of interestThe authors declare no conflict of interest.
The authors are grateful to PROPP-UFU, FAPEMIG and CNPq for Scientific Initiation scholarships awarded to Érika B. M. Riceto and Ralciane P. Menezes. They thank Adriano Gonçalves Martins and Lorraine Cristina Ribeiro Silva for their collaboration in some of the experiments.