Critically ill COVID-19 patients have higher pro-inflammatory (IL-1, IL-2, IL-6, tumor necrosis alpha) and anti-inflammatory (IL-4, IL-10) cytokine levels, less CD4 interferon-gamma expression, and fewer CD4 and CD8 cells. This severe clinical situation increases the risk of serious fungal infections, such as invasive pulmonary aspergillosis, invasive candidiasis or Pneumocystis jirovecii pneumonia. However, few studies have investigated fungal coinfections in this population. We describe an update on published reports on fungal coinfections and our personal experience in three Spanish hospitals. We can conclude that despite the serious disease caused by SARS-CoV-2 in many patients, the scarcity of invasive mycoses is probably due to the few bronchoscopies and necropsies performed in these patients because of the high risk in aerosol generation. However, the presence of fungal markers in clinically relevant specimens, with the exception of bronchopulmonary colonization by Candida, should make it advisable to early implement antifungal therapy.
Los pacientes gravemente enfermos con COVID-19 presentan concentraciones más elevadas de citoquinas pro-inflamatorias (IL-1, IL-2, IL-6 y factor de necrosis tumoral alfa) y anti-inflamatorias (IL-4 e IL-10), menor expresión de interferón-gama y un número más bajo de células CD4 y CD8. Esta grave situación clínica aumenta el riesgo de padecer coinfecciones fúngicas, como la aspergilosis pulmonar invasora, la candidiasis invasora o la neumonía por Pneumocystis jirovecii. Sin embargo, pocos estudios han investigado las coinfecciones fúngicas en esta población. En esta revisión describimos una actualización de las publicaciones sobre coinfecciones fúngicas en esta población de pacientes y nuestra experiencia personal en tres hospitales españoles. Podemos concluir que a pesar de la grave enfermedad causada por el SARS-CoV-2 en muchos pacientes, la baja frecuencia de micosis invasoras se debe probablemente a las pocas broncoscopias y necropsias realizadas en estos pacientes debido al alto riesgo de producción de aerosoles. Sin embargo, la presencia de marcadores fúngicos en muestras clínicas relevantes, con la excepción de la colonización broncopulmonar por Candida, debería aconsejar la instauración precoz de una terapia antifúngica.
In the last months, the world has been suffering the worst health public crisis since the 1918 influenza pandemic. On March 11th, 2020, and due to the rapid global spread of coronavirus disease 2019 (COVID-19), pandemic was officially declared by the World Health Organization (WHO).43 SARS-CoV-2 virus causes the new infectious disease that at the time of writing (July 16, 2020) has caused 13,578,608 confirmed COVID-19 infections and more than 580,000 deaths in 188 countries around the world. The majority (62%) of cases are concentrated in eight countries: USA, Brazil, India, Russia, Peru, Chile, Mexico and South Africa.21
To date, very few studies have been specifically designed to investigate superinfections by bacteria, fungi or other viruses in COVID-19 patients.1,16 In addition, limited data on superinfections are included in published studies. The scarce information on co-infections in these patients may be because most studies are retrospective, with poor quality data, and do not include protocols for additional microbiological studies.14,20 Furthermore, in most health institutions routine diagnostic procedures, such as bronchoscopies, induced sputum collection, necropsy, and microbiological tests, have been cut down to avoid staff exposure to SARS-CoV-2.12
About 5–30% of COVID-19 patients become critically ill and require intensive care unit (ICU) admission.44,18 As it is well known, ICU patients, especially those undergoing mechanical ventilation, are at greater risk to develop bacterial or fungal infections. Severe COVID-19 is associated with immune dysregulation, affecting both T-helper cell 2 (Th2) and Th1 responses, including the cytokine release syndrome, which contribute to lung pathology and promote pulmonary microbial proliferation and a subsequent infection.29 Critically ill COVID-19 patients have higher pro-inflammatory (IL-1, IL-2, IL-6, tumor necrosis alpha) and anti-inflammatory (IL-4, IL-10) cytokine levels, less CD4 interferon-gamma expression, and fewer CD4 and CD8 cells.9,37 This severe clinical situation increases the risk of invasive fungal infections (IFI), such as invasive pulmonary aspergillosis (IPA), invasive candidiasis (IC) or Pneumocystis jirovecii pneumonia (PJP).
COVID-19-associated invasive pulmonary aspergillosis (CAPA)The issue that patients with COVID-19 might be at risk of suffering a CAPA is an increasing medical concern. Although IPA is a well-known complication in immunocompromised patients, half of the cases occur in patients who are often non-neutropenic when admitted to ICU.33 In these patients, severe influenza is a recognized risk factor for developing IPA, and several studies from Asia and Europe have reported up to 28% of IPA in patients with severe influenza.19,39 However, these high incidence rates of influenza-associated pulmonary aspergillosis (IAPA) may not be universal, because in a North American study only 7% of the patients suffered IPA.35 Respiratory epithelium disruption, along with dysfunctional mucociliary clearance and local immune impairment, are important pathophysiological factors in the development of IAPA.33
Most IPA cases affect people without immunodeficiency, so the European Organization for Research and Treatment of Cancer Mycoses Study Group (EORTC-MSG) criteria 13to define invasive aspergillosis cannot be applied. Therefore, it is suggested to apply the AspICU algorithm described by Blot et al.8 This algorithm combines culture from respiratory specimens and galactomannan (GM) detection, both in the broncho-alveolar lavage (BAL) fluid and serum, to distinguish putative IPA from Aspergillus colonization in ICU patients. Although AspICU definition shows a promising diagnostic performance, it can only be applied to patients with positive respiratory cultures.6
While data on pathologic changes in COVID-19 patients with acute respiratory distress syndrome (ARDS) are scarce, diffuse alveolar injury (including hyaline membrane formation) combined with intra-alveolar neutrophilic infiltration and vascular congestion have been reported.38 These histologic damages could clear the way for secondary infections like CAPA, a new described entity affecting ICU patients with severe pulmonary abnormalities.
Recently, a group of 29 international experts reviewed the epidemiology, diagnosis and management of IAPA and proposed a case definition of IAPA.42 The entry criterion was defined as a patient requiring ICU admission for respiratory distress with a positive influenza test. Proven IAPA would require histological evidence of invasive septate hyphae and mycological evidence for Aspergillus. Probable IAPA would require the detection of GM, or positive Aspergillus culture in BAL, or serum with pulmonary infiltrates, or a positive culture in upper respiratory samples with bronchoscopy evidence for tracheobronchitis, or cavitating pulmonary infiltrates of recent onset. These authors suggest that IAPA case definitions may be useful to classify patients with CAPA, while awaiting further studies that provide more insight.
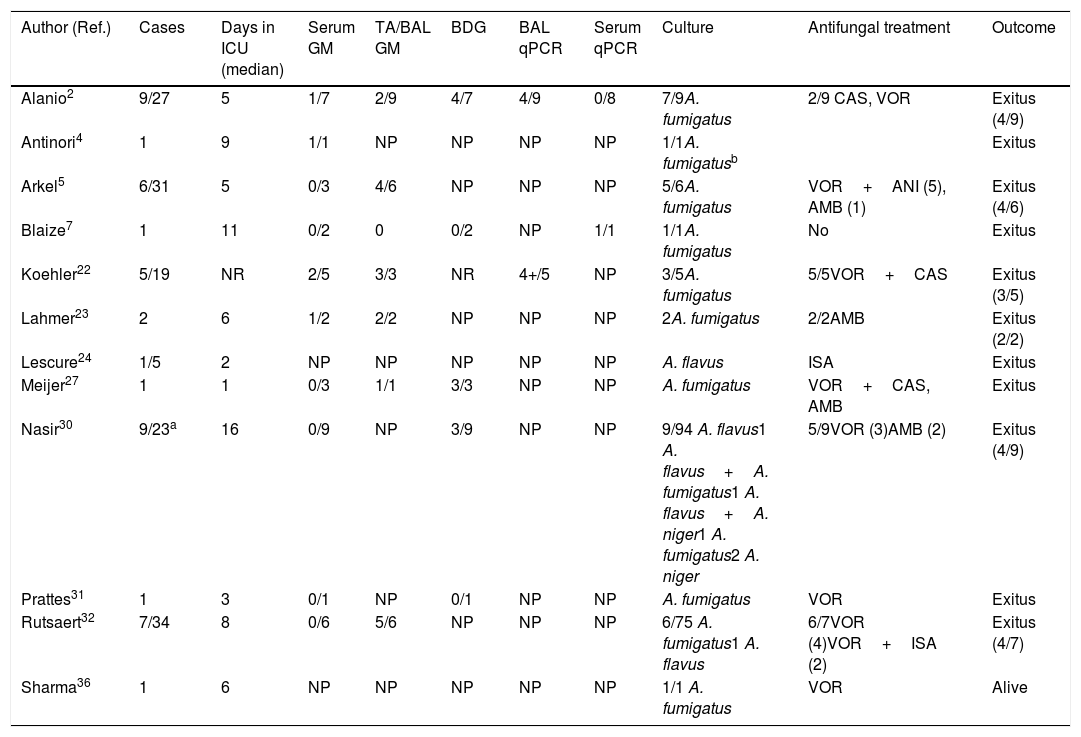
Of the thousands of studies published on COVID-19 since the beginning of the pandemic, very few deal with Aspergillus co-infections in these patients. Probably, the real burden of CAPA in patients requiring ICU admission is underestimated. Most CAPA episodes described are single case reports or small series, with Aspergillus fumigatus being the etiological agent in most infections, followed by Aspergillus flavus.2,4,5,7,22–24,27,31,32,36 The main characteristics of 44 published European, Australian and Pakistani CAPA episodes are summarized in Table 1. The largest European series are from France and Belgium and include 9 and 7 patients, respectively.2,32 Only in one patient the CAPA episode could be confirmed by necropsy, with the histopathological examination revealing bronchial wall ulceration, necrotizing pneumonia, and septate branching hyphae. Furthermore, the presence of Aspergillus DNA was verified by PCR-amplification on pulmonary tissue.4 Overall CAPA mortality was very high: 26 out of 44 patients (59.1%), listed in Table 1, died in the first ten days after the diagnosis of CAPA.
Clinical characteristics of patients suffering from COVID-19 and invasive pulmonary aspergillosis.
| Author (Ref.) | Cases | Days in ICU (median) | Serum GM | TA/BAL GM | BDG | BAL qPCR | Serum qPCR | Culture | Antifungal treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Alanio2 | 9/27 | 5 | 1/7 | 2/9 | 4/7 | 4/9 | 0/8 | 7/9A. fumigatus | 2/9 CAS, VOR | Exitus (4/9) |
| Antinori4 | 1 | 9 | 1/1 | NP | NP | NP | NP | 1/1A. fumigatusb | Exitus | |
| Arkel5 | 6/31 | 5 | 0/3 | 4/6 | NP | NP | NP | 5/6A. fumigatus | VOR+ANI (5), AMB (1) | Exitus (4/6) |
| Blaize7 | 1 | 11 | 0/2 | 0 | 0/2 | NP | 1/1 | 1/1A. fumigatus | No | Exitus |
| Koehler22 | 5/19 | NR | 2/5 | 3/3 | NR | 4+/5 | NP | 3/5A. fumigatus | 5/5VOR+CAS | Exitus (3/5) |
| Lahmer23 | 2 | 6 | 1/2 | 2/2 | NP | NP | NP | 2A. fumigatus | 2/2AMB | Exitus (2/2) |
| Lescure24 | 1/5 | 2 | NP | NP | NP | NP | NP | A. flavus | ISA | Exitus |
| Meijer27 | 1 | 1 | 0/3 | 1/1 | 3/3 | NP | NP | A. fumigatus | VOR+CAS, AMB | Exitus |
| Nasir30 | 9/23a | 16 | 0/9 | NP | 3/9 | NP | NP | 9/94 A. flavus1 A. flavus+A. fumigatus1 A. flavus+A. niger1 A. fumigatus2 A. niger | 5/9VOR (3)AMB (2) | Exitus (4/9) |
| Prattes31 | 1 | 3 | 0/1 | NP | 0/1 | NP | NP | A. fumigatus | VOR | Exitus |
| Rutsaert32 | 7/34 | 8 | 0/6 | 5/6 | NP | NP | NP | 6/75 A. fumigatus1 A. flavus | 6/7VOR (4)VOR+ISA (2) | Exitus (4/7) |
| Sharma36 | 1 | 6 | NP | NP | NP | NP | NP | 1/1 A. fumigatus | VOR | Alive |
Post-mortem lung examination confirmed invasive pulmonary aspergillosis and Aspergillus DNA was detected by PCR amplification on paraffin block tissue. AMB: Amphotericin B. ANI: Anidulafungin. BAL: Broncho-alveolar lavage. BDG: 1,3-β-d Glucan. CAS: Caspofungin. GM: Galactomannan. ISA: Isavuconazole. NP: Not performed. NR: Not reported. TA: tracheal aspirate.
Available information on CAPA in Asian patients is more imprecise, as published reports do not adequately differentiate between bacterial and fungal co-infection due to the lack of appropriate mycological studies. For instance, in China and other countries GM testing is rarely available.11,46 Thus, in a cohort of 221 ICU patients with COVID-19 in Wuhan, China, mycoses were diagnosed in seven individuals, but the causative pathogens were not identified.45 Moreover, in another cohort of 99 patients at the same hospital, only one episode of CAPA was diagnosed in a patient from whom A. flavus was isolated from a respiratory specimen.10 However, in a series of 257 COVID-19 patients in Jiangsu Province (China), Aspergillus DNA was detected in respiratory specimens from 59 patients (23%) using a specific real-time PCR.47 Recently, Nasir et al.30 have reported the isolation of Aspergillus from tracheal aspirates (TA) in 9 out of 23 COVID-19 patients requiring ICU admission (39.1%) at the same hospital in Karachi, Pakistan. Five of these nine patients (21.7%) suffered from CAPA, being the overall fatality 60% (3 out of 5 patients).
Diagnosing a CAPA episode is a real challenge for clinicians. The symptoms are non-specific and imaging techniques, including computed tomography scan, so useful in immunocompromised patients, are not helpful as the findings are similar to those seen in patients with COVID-19 and ARDS. In addition, detection of IFI biomarkers, such as GM and 1,3-β-d-glucan (BDG), and fungal DNA in serum or in respiratory secretions have been shown of limited value in the reported CAPA episodes (Table 1).
Despite the fact that Schauwvlieghe et al.33 reported that serum GM was positive in 65% of patients with IAPA, only 6 out of 30 patients with CAPA (20%) from the studies collected in Table 1 were serum GM positive. However, 17 out of 28 patients (61%) were BAL GM positive (Table 1). Nevertheless, restricted use of bronchoscopy has been recommended in COVID-19, because it is an aerosol-generating procedure that poses risks to patients and personnel. Bronchoscopy is recommended only when the intervention is considered lifesaving. As radiological and clinical signs of IPA in non-neutropenic patients are mostly unspecific, bronchoscopy should be indicated in critically ill patients with COVID-19 who are suspected of suffering a secondary infection. The reasons for the lower sensitivity of serum GM detection in COVID-19 patients versus those with influenza are unknown. Many of these patients have been treated with chloroquine or hydroxychloroquine since both drugs exhibit in vitro activity against A. fumigatus, but this treatment might have a negative effect on GM performance.17,41
Even if evidence for Aspergillus is recovered, uncertainty remains about whether patients truly develop invasive disease and require antifungal therapy. Only histopathology can prove IPA through necropsy in patients with suspected CAPA. If necropsy is contraindicated because of the risk of aerosol formation, post-mortem lung biopsy might be considered as an alternative to get tissue.41
So far, and considering the infectious risks associated to the collection of respiratory samples, the most valuable tool for the diagnosis of CAPA is the culture of respiratory secretions (tracheal aspirate, bronchoaspirate-BAS- or BAL) although it will never be easy to distinguish between colonization and infection after the isolation of Aspergillus in these clinical samples. Therefore, in our opinion, in the possibility of a fungal co-infection in a COVID-19 patient, the isolation of Aspergillus from respiratory secretions or some other microbiological or immunological marker from these patients should always be considered as an indicator of putative IPA. Consequently, an antifungal treatment (taking into account the isolated Aspergillus species) in accordance with guidelines should be recommended.15 First-line treatment options for IPA include voriconazole, posaconazole and isavuconazole. Echinocandins or nebulized amphotericin B in combination with anti-mold azoles are alternative therapeutic approaches. Intravenous liposomal amphotericin B should be considered in those regions with high rates of azole-resistant A. fumigatus. Achieving adequate drug exposure is challenging in ICU patients with multiple factors contributing to pharmacokinetic variability. Drug interactions are clinically relevant for the azoles and pharmacogenetic factors are important in inter-individual drug exposure variability.42
To control the alarming CAPA incidence in their hospital, Rutsaert et al.32 implemented a package of interesting measures including the sampling of room air and the oxygen and pressurized air supplies, the installation of high-efficiency particulate air filters (HEPA) in ICUs, systematic aspergillosis screening of all mechanically ventilated patients by performing serum GM assays twice weekly, requesting BAL GM indices and mold cultures, regardless of the indication for bronchoscopy, and starting prophylactic nebulization of liposomal amphotericin B in every mechanically ventilated patient without an established diagnosis of IPA. In our opinion, these measures would be difficult to carry out completely in many institutions but it would be worth proposing their adaptation as far as possible at most hospitals attending patients with moderate or severe COVID-19.
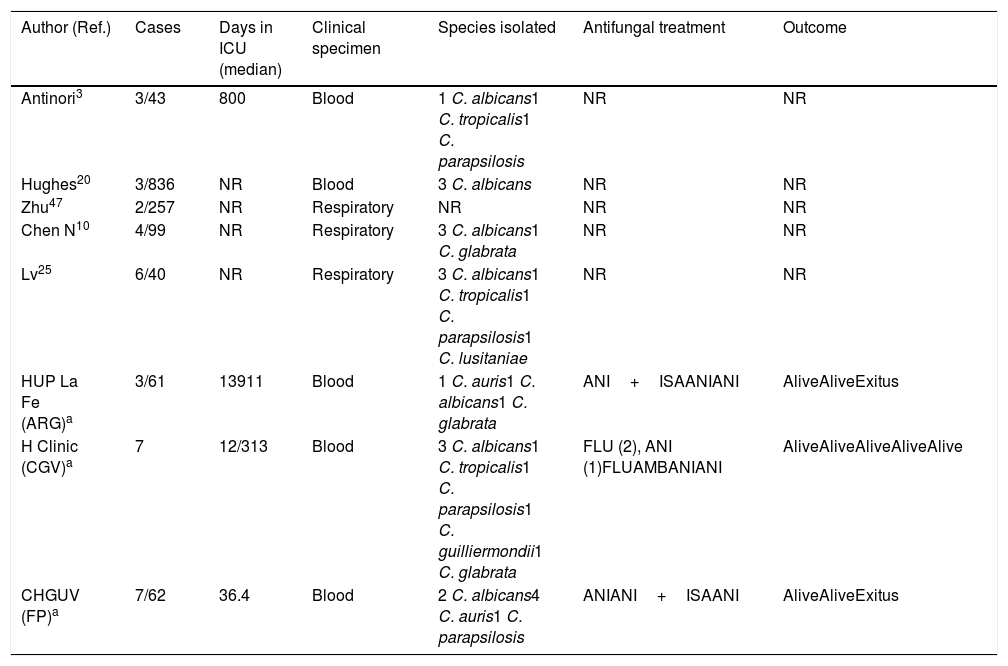
Invasive candidiasis in COVID-19 patientsIn addition to Aspergillus spp., severely ill COVID-19 patients are exposed to other fungal pathogens such as Candida species and P. jirovecii. All the classic risk factors for developing candidemia in a critically ill patient are present in COVID-19 patients admitted to the ICU: mechanical ventilation, parenteral nutrition, broad-spectrum anti-bacterial treatment, indwelling central venous or bladder catheters, older age, comorbidities, lymphopenia, corticosteroids, etc. In critically ill patients or in patients at COVID-19 advanced stages, to all these well-known risk factors must be added the use of tocilizumab, an IL-6 receptor monoclonal blocking agent administered to decrease the high levels of IL-6 associated with poor outcome in the setting of COVID-19 pneumonia. Recently, Antinori et al. have reported that in a cohort of 43 patients with COVID-19 treated with tocilizumab, three of them (16%) developed a candidemia episode: one patient by Candida albicans, with associated endophthalmitis and endocarditis, and the other two by Candida tropicalis and Candida parapsilosis each.3 In a recent published retrospective study, Huges et al. reported three candidemia episodes in a cohort of 836 COVID-19 patients admitted to a critical care wards, all of them caused by C.albicans and attributed to line-related infections.20 The authors found no difference in the incidence rate of candidemia when contrasting the COVID-19 cohort to a control group of 216 influenza positive patients admitted during 2019/2020 flu season. In our experience, candidemia crude mortality rate is lower (12%) than in other at risk groups of patients (Table 2).
Clinical characteristics of patients suffering from COVID-19 and potential invasive candidiasis.
| Author (Ref.) | Cases | Days in ICU (median) | Clinical specimen | Species isolated | Antifungal treatment | Outcome |
|---|---|---|---|---|---|---|
| Antinori3 | 3/43 | 800 | Blood | 1 C. albicans1 C. tropicalis1 C. parapsilosis | NR | NR |
| Hughes20 | 3/836 | NR | Blood | 3 C. albicans | NR | NR |
| Zhu47 | 2/257 | NR | Respiratory | NR | NR | NR |
| Chen N10 | 4/99 | NR | Respiratory | 3 C. albicans1 C. glabrata | NR | NR |
| Lv25 | 6/40 | NR | Respiratory | 3 C. albicans1 C. tropicalis1 C. parapsilosis1 C. lusitaniae | NR | NR |
| HUP La Fe (ARG)a | 3/61 | 13911 | Blood | 1 C. auris1 C. albicans1 C. glabrata | ANI+ISAANIANI | AliveAliveExitus |
| H Clinic (CGV)a | 7 | 12/313 | Blood | 3 C. albicans1 C. tropicalis1 C. parapsilosis1 C. guilliermondii1 C. glabrata | FLU (2), ANI (1)FLUAMBANIANI | AliveAliveAliveAliveAlive |
| CHGUV (FP)a | 7/62 | 36.4 | Blood | 2 C. albicans4 C. auris1 C. parapsilosis | ANIANI+ISAANI | AliveAliveExitus |
Different species of Candida have also been isolated from respiratory specimens (TA, BAS and BAL) from COVID-19 patients as unique pathogen and considered as cause of co-infections10,25 (Table 2). However, the etiological value of yeasts isolated from the respiratory tract as causal agents of infection of the lung parenchyma is controversial: it is in fact questioned whether Candida could be the cause of pneumonia. In contrast to the defined culture threshold for bacterial colony forming units in BAL, there is no such standard to distinguish Candida colonization from pulmonary candidiasis. Schnabel et al. were able to identify only five cases (0.7%) of Candida pneumonia in a series of BAL performed on 701 critically ill patients.34 The same way, Meersseman et al. did not find any single case of Candida pneumonia among ICU patients with evidence of pneumonia on autopsy in a two-year prospective study of 232 autopsies, despite frequent isolation of Candida from the airways.26 These studies indicate that Candida pneumonia is an extremely rare occurrence in ICU patients and only histopathology can establish the definite diagnosis. Unlike our advice to treat a potential CAPA that could save the life of patients, in the face of bronchopulmonary colonization by Candida, there is no clear indicator that antifungal treatment is advisable.
Pneumocystis pneumonia in COVID-19 patientsTo date, although we have observed cases of PJP (unpublished), only one episode of co-infection by P. jirovecii has been reported in a patient with COVID-19 and ARDS.28 In this patient, the high level of BDG detected in serum (305pg/ml) prompted to additional testing for P. jirovecii with a qualitative real-time PCR assay from a tracheal aspirate, which was positive. It is noteworthy that this patient had neither a known underlying immunodeficiency nor any other classical risk factor for PJP, such as malignancy, organ transplantation, or prolonged exposure to systemic corticosteroids. This case also highlights the potential utility of serum BDG for diagnosing PJP in COVID-19 patients, which is particularly relevant given concerns about healthcare transmission associated with performing bronchoscopy in these patients.
Other invasive mycoses in COVID-19 patientsBesides the fungal pathogens above mentioned, the high aggressive feature of the SARS-CoV-2 virus to the lung tissue and the large bilateral alveolo-interstitial lesions make the occurrence of other IFI very likely. There is a special concern for those IFI with a primary pulmonary entry and an airborne route transmission, such as mucormycosis and cryptococcosis. In endemic countries and in those with high mobility of travelers from or to these countries, the diagnosis of histoplasmosis should also be considered. To improve the diagnosis of these mycoses, in addition to mycological culture of respiratory samples from COVID-19 patients on suspicion of fungal respiratory co-infection, Cryptococcus antigenemia, blood and respiratory panfungal PCR, and a qPCR for mucorales and H. capsulatum could be performed to confirm or rule out the presence of these fungal pathogens.14 Additionally, two Saccharomyces cerevisiae bloodstream infections have been described in two COVID-19 patients in the ICU after receiving yeast supplementation.40
We can conclude that despite the serious pulmonary and systemic damage caused by SARS-CoV-2 in many patients, reported cases of IFI are rare, probably due to few bronchoscopies and necropsies performed in these patients because of the aerosolization risk. However, the presence of fungal markers in respiratory samples and usually sterile body fluids, with the exception of bronchopulmonary colonization by Candida, should make it advisable to early implement the most appropriate antifungal therapy for each patient.
Conflict of interestNone declared.
We thank Dr. Carolina Ferrer and Dr. Vicente Abril for their help in collecting the data.