Brain abscess is a severe focal infection of the central nervous system (CNS) with an annual incidence of up to 8% in developing countries. This article aims to present a comprehensive review of the literature on the pathophysiology, clinical presentation, diagnosis, and treatment of brain abscess.
MethodsWe conducted a narrative review of the literature employing the PubMed, Embase, and Google Scholar databases. The terms “brain abscess,” “physiopathology,” “clinical presentation,” and “treatment” were combined using the Boolean operator AND (eg, “brain abscess” AND “physiopathology” or “brain abscess” AND “clinical presentation”). We analysed the titles and abstracts of all articles in English and Spanish, applying no restrictions regarding their publication date, selecting 69 articles for full-text review.
ConclusionsBrain abscess is a frequent infectious disease of the CNS whose prevalence is higher in developing countries. The main pathophysiological mechanisms are related to bacterial spread from adjacent or distal foci. Laboratory findings can be nonspecific. Therefore, neuroimaging and microbiology tests play a fundamental role in establishing the most appropriate treatment for each patient.
El absceso cerebral es una infección focal grave del sistema nervioso central (SNC) con una incidencia anual de hasta el 8% en países en desarrollo. El objetivo de este artículo es presentar una revisión exhaustiva de la literatura científica sobre la fisiopatología, cuadro clínico, diagnóstico y tratamiento del absceso cerebral.
MétodosSe llevo a cabo una revisión narrativa de la literatura en las bases de datos Pubmed, Embase y Google Scholar, empleando los términos ‘Brain abscess’, ‘Physiopathology’, ‘Clinical presentation’ y ‘Treatment’, combinándolos mediante el operador booleano ‘AND’ teniendo como término principal ‘Brain abscess’ (ej: ‘Brain abscess’ AND ‘physiopathology’ o ‘Brain abscess’ AND ‘Clinical presentation’). Posteriormente, se analizaron por título y abstract todos los artículos en inglés y español sin restricción respecto a su fecha de publicación, seleccionando 69 de ellos para su revisión a texto completo.
ConclusionesEl absceso cerebral es una patología infecciosa frecuente del SNC cuya prevalencia es mayor en países en vías de desarrollo. Los principales mecanismos fisiopatológicos están relacionados con la diseminación bacteriana por contigüidad o desde focos infecciosos distales. El diagnóstico por laboratorio puede ser inespecífico, por lo cual, las neuroimágenes y las pruebas microbiológicas juegan un papel fundamental permitiendo individualizar el tratamiento de cada paciente.
A brain abscess is a focal infection of the central nervous system (CNS) frequently characterised by areas of localised cerebritis and central necrosis surrounded by a well vascularised capsule.1–5 Brain abscesses account for approximately 8% of all intracranial space-occupying lesions; they are most frequent in men aged 30 to 50 years, and are associated with a mortality rate of up to 53%.2,6–8
Before the 1980s and the outbreak of the human immunodeficiency virus (HIV) epidemic, the incidence of brain abscesses in the United States ranged from 0.3 to 1.3 cases per 100,000 person-years.2,5,8 Currently, the annual incidence of brain abscesses is 1%–2% in developed countries and 8% in developing countries.5,9,10 According to the United States Centers for Disease Control and Prevention, brain abscesses in that country account for 14% of all post-craniotomy infections.
Although a large percentage of brain abscesses are polymicrobial, the most frequently isolated pathogens are Staphylococcus aureus and Streptococcus viridans.2,8,9 Anaerobic microorganisms are detected in up to 40% of cases, and enteric gram-negative bacilli in up to 33%; the latter are more frequent in patients with otomastoiditis, septicaemia, immunosuppression, or history of neurosurgery.4,10–12
The proportion of brain abscesses of fungal aetiology has increased with the use of immunosuppressants and broad-spectrum antibiotics, amounting to approximately 1% of all brain abscesses.2,8 Brain abscesses associated with Nocardia spp. infection may occur as isolated CNS lesions or as part of a disseminated infection, associated with lung or skin diseases, and represent fewer than 1% of all cases.13
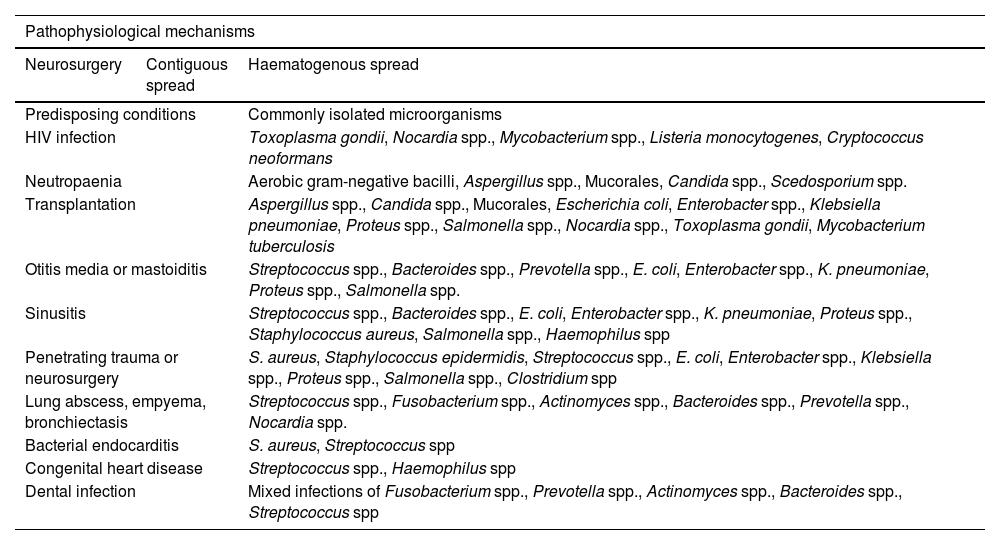
PathophysiologyA strong association has been described between the pathophysiological mechanisms of brain abscesses and the predisposing factors (Table 1).1 Three main pathophysiological mechanisms have been described: inoculation of pathogenic microorganisms normally found on the skin (eg, in the context of head trauma or neurosurgery), contiguous spread of bacteria (eg, mastoiditis, otitis media, sinusitis), and haematogenous spread from distal foci (eg, bacterial endocarditis, lung abscess, skin and dental infections).2–4,6,10,14–33
Pathophysiological mechanisms, predisposing conditions, and microorganisms frequently isolated in patients with brain abscesses. The anaerobic microorganisms most frequently isolated are Bacteroides spp., Fusobacterium spp., Clostridium spp., Streptococcus spp., and gram-negative bacilli.
| Pathophysiological mechanisms | ||
|---|---|---|
| Neurosurgery | Contiguous spread | Haematogenous spread |
| Predisposing conditions | Commonly isolated microorganisms | |
| HIV infection | Toxoplasma gondii, Nocardia spp., Mycobacterium spp., Listeria monocytogenes, Cryptococcus neoformans | |
| Neutropaenia | Aerobic gram-negative bacilli, Aspergillus spp., Mucorales, Candida spp., Scedosporium spp. | |
| Transplantation | Aspergillus spp., Candida spp., Mucorales, Escherichia coli, Enterobacter spp., Klebsiella pneumoniae, Proteus spp., Salmonella spp., Nocardia spp., Toxoplasma gondii, Mycobacterium tuberculosis | |
| Otitis media or mastoiditis | Streptococcus spp., Bacteroides spp., Prevotella spp., E. coli, Enterobacter spp., K. pneumoniae, Proteus spp., Salmonella spp. | |
| Sinusitis | Streptococcus spp., Bacteroides spp., E. coli, Enterobacter spp., K. pneumoniae, Proteus spp., Staphylococcus aureus, Salmonella spp., Haemophilus spp | |
| Penetrating trauma or neurosurgery | S. aureus, Staphylococcus epidermidis, Streptococcus spp., E. coli, Enterobacter spp., Klebsiella spp., Proteus spp., Salmonella spp., Clostridium spp | |
| Lung abscess, empyema, bronchiectasis | Streptococcus spp., Fusobacterium spp., Actinomyces spp., Bacteroides spp., Prevotella spp., Nocardia spp. | |
| Bacterial endocarditis | S. aureus, Streptococcus spp | |
| Congenital heart disease | Streptococcus spp., Haemophilus spp | |
| Dental infection | Mixed infections of Fusobacterium spp., Prevotella spp., Actinomyces spp., Bacteroides spp., Streptococcus spp | |
When the blood–brain barrier is damaged (eg, due to brain trauma or a neurosurgical procedure), the brain becomes extremely vulnerable to bacterial infection. According to studies with different animal models, inoculation of at least 102 colony-forming units of a single microorganism is sufficient for a brain abscess to form. These models also showed that the abscess capsule tends to be thinner and more fragile in the vicinity of the lateral ventricles, which may be linked to reduced blood irrigation in this area as compared to the cerebral cortex, for example.4,5,19,24
Brain abscess development has classically been divided into 4 stages: 1) early cerebritis (perivascular infiltration of neutrophils, plasma cells, and mononuclear cells); 2) late cerebritis (macrophage and fibroblast infiltration is also observed); 3) early capsule formation; and 4) late capsule formation. From a histological viewpoint, 5 main areas have been described: 1) a necrotic centre, 2) a peripheral area of inflammatory cells, 3) a capsule, 4) an area of neovascularisation, and 5) astrogliosis and perilesional oedema.3,4,7,15,16,29,34–36
Symptoms and diagnosisThe most frequent signs and symptoms of brain abscesses are headache (69%), fever (53%), focal neurological deficits (48%), impaired consciousness (48%), nausea and vomiting (47%), papilloedema (35%), meningeal signs (32%), and seizures (25%). Other findings may be linked to predisposing conditions, pathophysiological mechanisms, and the location, number, and size of the lesions.2–5,7,8,10,28–30,36
Diagnosis of brain abscess requires detailed clinical history taking and a thorough physical examination, which help in identifying the predisposing factors and potential pathophysiological mechanisms involved (Table 1). Due to the variable specificity of clinical findings, neuroimaging and laboratory studies are essential tools in the diagnostic process.30,37
Lumbar puncture and cerebrospinal fluid (CSF) analysis may be indicated in cases of abscess rupture into the ventricular system or suspected concomitant meningitis; however, there is no consensus on its use due to the high risk of herniation secondary to abrupt changes in intracranial pressure and the high likelihood of obtaining negative culture results.11,14,23,25,30,33,38 When indicated, lumbar puncture must be performed after neuroimaging studies have ruled out intracranial hypertension.2 Another alternative is stereotactic aspiration, which enables microbiological study and may also act as an adjuvant therapy.8,32,39
In a systematic review and meta-analysis published by Brouwer et al.,8 the main blood analysis findings were elevated erythrocyte sedimentation rate (72%), leukocytosis (60%), elevated C-reactive protein (CRP) levels (60%), and positive blood culture (28%). The main CSF analysis findings were pleocytosis (71%), elevated protein levels (51%), and positive CSF culture (24%).
Neuroimaging studies enable us to determine the localisation, extension, and characteristics of the lesions.27 Depending on the radiological pattern, which varies according to the developmental stage of the abscess, several differential diagnoses may be considered, ranging from infectious lesions to tumours.30
In up to 85% of cases, contrast-enhanced head CT reveals a lesion with a hypodense nucleus (necrosis) surrounded by a thin contrast-enhancing ring (capsule) and perilesional oedema of variable extension.2,4,5,10,12,18,26,28,33
Contrast-enhanced brain MRI is the diagnostic technique of choice due to its high sensitivity and specificity. This technique is able to detect early cerebritis, intraventricular and subarachnoid infectious dissemination, and satellite lesions.5,10,25,30,40,41 T1-weighted sequences reveal a hypointense lesion surrounded by a isointense or slightly hyperintense halo presenting contrast enhancement (ring-enhancing lesion).23,30 On T2-weighted FLAIR sequences, perilesional oedema displays signal hyperintensity whereas the capsule appears as a hypointense halo due to the presence of paramagnetic oxygen produced by macrophages.27,42–44
These techniques differentiate purulent material from a brain abscess from cell debris generated by tumoural tissue necrosis. On DWI sequences, purulent material is hyperintense, as it restricts the movement of water molecules, whereas the necrotic tissue of a brain tumour is typically hypointense, with a high apparent diffusion coefficient (ADC).22,44 Another alternative is magnetic resonance spectroscopy (MRS), which detects the presence of amino acids (leucine, valine, acetate, succinate, and alanine) resulting from extracellular proteolysis and bacterial metabolism.44
On DWI sequences, bacterial brain abscesses display signal hyperintensity, with low ADC values. Tumoural lesions appear hypointense and present high ADC values. Recognising this pattern decreases diagnostic errors, improving the sensitivity and specificity of MRI.45
In MRS images, healthy brain tissue exhibits high concentrations of creatine (3.0 ppm [ppm]), N-acetyl aspartate (2.0 ppm), choline (3.2 ppm), and myo-inositol (3.5 ppm).43 On MRS images, brain abscesses are characterised by the absence of phosphocreatine, creatine, N-acetyl aspartate, and the trimethyl group of choline-type compounds; images also identify acetate, lactate, and such amino acids as alanine, glycine, valine, leucine, isoleucine, and phenylalanine, as well as fatty acids.46,47
TreatmentIn most cases, management of brain abscesses combines surgery (drainage, excision, or stereotactic aspiration) and pharmacological treatment (oral, intravenous, or intrathecal antibiotics).6,11,14,21,23,30,35,48
Pharmacological treatmentEligibility criteria for non-surgical treatment are the presence of multiple cerebral abscesses measuring <1.5 cm diameter, presence of a single lesion measuring <1.5 cm diameter, lesion location in eloquent areas, and presence of concomitant infection (eg, meningitis, ependymitis).32,39,48,49
In order to select the most appropriate drug, we must consider the pharmacokinetic and pharmacodynamic characteristics of each compound, previous treatments, administration routes, the patient's predisposing conditions, the potential pathophysiological mechanism underlying the abscess, and the microorganism involved.25 Some authors recommend starting empirical antibiotic therapy as soon as samples are collected for microbiological study.4,5,26,32,39,48,50,51
Success rates for antibiotic therapy are higher when treatment is started during the stage of early cerebritis, if the lesion measures <1.5 cm diameter, in patients with progression times <2 weeks, and in patients displaying symptom improvement within a week of treatment.49 Empirical broad-spectrum antibiotic therapy (Table 2) should be maintained for 6–8 weeks, and modified according to microbiological study results to ensure the best possible therapeutic effectiveness (Table 3). Table 4 presents the recommended doses for the different antibiotics described.4,25–27,51–61
Empirical broad-spectrum antibiotic therapy in patients with brain abscesses.
| Indication | Treatment |
|---|---|
| Standard empirical treatment | Cefotaxime, cefepime, or ceftriaxone, plus metronidazole and vancomycin |
| Transplant patients, patients receiving immunosuppressants | Cefotaxime, cefepime, or ceftriaxone, plus metronidazole, vancomycin, and trimethoprim-sulfamethoxazole |
| Patients with HIV infection | Cefotaxime, cefepime, or ceftriaxone, plus metronidazole, vancomycin, pyrimethamine, and sulfadiazine. Isoniazid, pyrazinamide, and ethambutol may also be considered for potential Mycobacterium tuberculosis infection. |
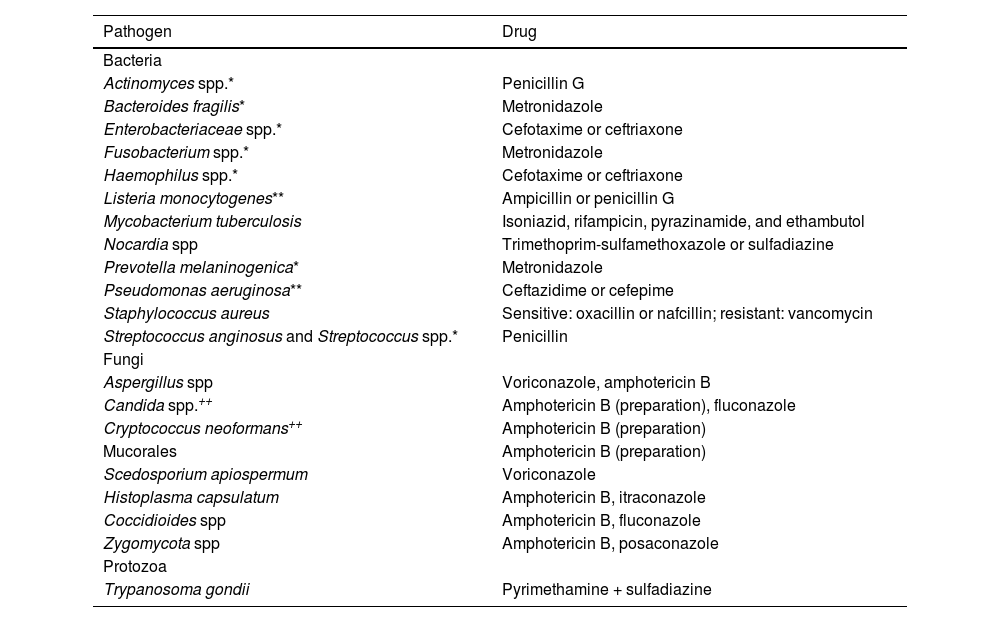
Antibiotic therapy guided by microbiological study results. Selection of specific antibiotics depends on microbiological study results and antibiotic susceptibility test results. * Microorganisms frequently isolated in mixed infections (combination antibiotic therapy may be needed). ** In patients presenting infection with these microorganisms, treatment should include an aminoglycoside antibiotic (gentamicin 1.7 mg/kg/8 h). ++ In patients presenting infection with these microorganisms, consider adding flucytosine 25 mg/kg/6 h.
| Pathogen | Drug |
|---|---|
| Bacteria | |
| Actinomyces spp.* | Penicillin G |
| Bacteroides fragilis* | Metronidazole |
| Enterobacteriaceae spp.* | Cefotaxime or ceftriaxone |
| Fusobacterium spp.* | Metronidazole |
| Haemophilus spp.* | Cefotaxime or ceftriaxone |
| Listeria monocytogenes** | Ampicillin or penicillin G |
| Mycobacterium tuberculosis | Isoniazid, rifampicin, pyrazinamide, and ethambutol |
| Nocardia spp | Trimethoprim-sulfamethoxazole or sulfadiazine |
| Prevotella melaninogenica* | Metronidazole |
| Pseudomonas aeruginosa** | Ceftazidime or cefepime |
| Staphylococcus aureus | Sensitive: oxacillin or nafcillin; resistant: vancomycin |
| Streptococcus anginosus and Streptococcus spp.* | Penicillin |
| Fungi | |
| Aspergillus spp | Voriconazole, amphotericin B |
| Candida spp.++ | Amphotericin B (preparation), fluconazole |
| Cryptococcus neoformans++ | Amphotericin B (preparation) |
| Mucorales | Amphotericin B (preparation) |
| Scedosporium apiospermum | Voriconazole |
| Histoplasma capsulatum | Amphotericin B, itraconazole |
| Coccidioides spp | Amphotericin B, fluconazole |
| Zygomycota spp | Amphotericin B, posaconazole |
| Protozoa | |
| Trypanosoma gondii | Pyrimethamine + sulfadiazine |
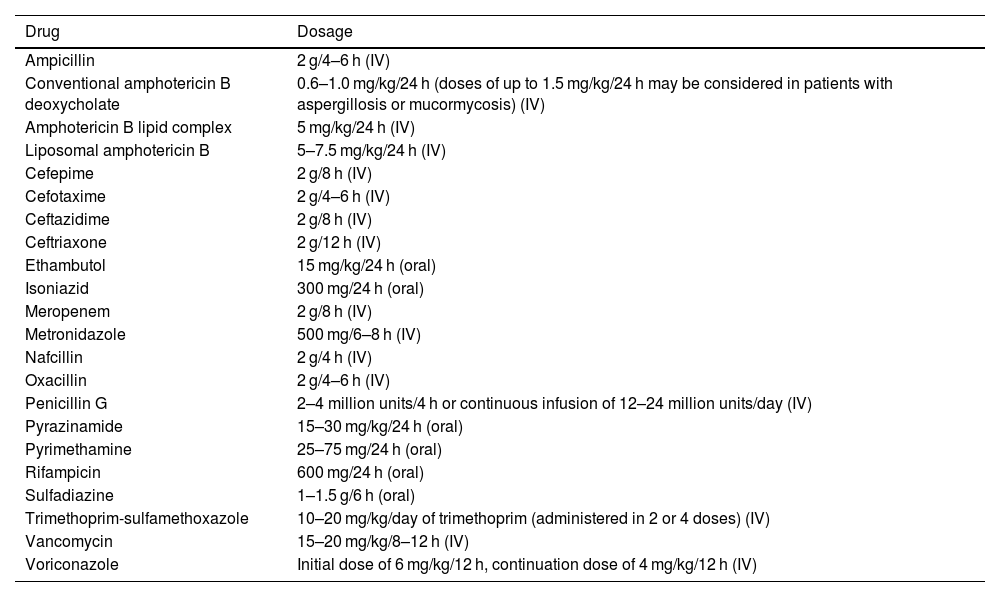
Dosing of the recommended antibiotics. The doses indicated are for adults with normal liver and kidney function.
| Drug | Dosage |
|---|---|
| Ampicillin | 2 g/4–6 h (IV) |
| Conventional amphotericin B deoxycholate | 0.6–1.0 mg/kg/24 h (doses of up to 1.5 mg/kg/24 h may be considered in patients with aspergillosis or mucormycosis) (IV) |
| Amphotericin B lipid complex | 5 mg/kg/24 h (IV) |
| Liposomal amphotericin B | 5–7.5 mg/kg/24 h (IV) |
| Cefepime | 2 g/8 h (IV) |
| Cefotaxime | 2 g/4–6 h (IV) |
| Ceftazidime | 2 g/8 h (IV) |
| Ceftriaxone | 2 g/12 h (IV) |
| Ethambutol | 15 mg/kg/24 h (oral) |
| Isoniazid | 300 mg/24 h (oral) |
| Meropenem | 2 g/8 h (IV) |
| Metronidazole | 500 mg/6–8 h (IV) |
| Nafcillin | 2 g/4 h (IV) |
| Oxacillin | 2 g/4–6 h (IV) |
| Penicillin G | 2–4 million units/4 h or continuous infusion of 12–24 million units/day (IV) |
| Pyrazinamide | 15–30 mg/kg/24 h (oral) |
| Pyrimethamine | 25–75 mg/24 h (oral) |
| Rifampicin | 600 mg/24 h (oral) |
| Sulfadiazine | 1–1.5 g/6 h (oral) |
| Trimethoprim-sulfamethoxazole | 10–20 mg/kg/day of trimethoprim (administered in 2 or 4 doses) (IV) |
| Vancomycin | 15–20 mg/kg/8–12 h (IV) |
| Voriconazole | Initial dose of 6 mg/kg/12 h, continuation dose of 4 mg/kg/12 h (IV) |
IV: intravenous route.
Patients receiving immunosuppressant or immunomodulatory drugs should receive empirical treatment with third-generation cephalosporins (ceftriaxone or cefotaxime); fourth-generation cephalosporins (cefepime) should be administered if Pseudomonas infection is suspected, and combined with metronidazole, vancomycin, or trimethoprim-sulfamethoxazole (the latter in cases of suspected Nocardia spp. infection). Voriconazole is suggested for fungal infection, particularly those caused by Aspergillus. In cases of Toxoplasma gondii infection, voriconazole should be supplemented with pyrimethamine and sulfadiazine.5,25
Antibiotic therapy should be administered for 6–8 weeks; treatment for less than 45 days is associated with increased risk of recurrence.38 Clinical progression allows us to evaluate the efficacy of pharmacological treatment.25,32 If no significant improvement is observed after a minimum of 2 weeks of treatment, neuroimaging studies should be repeated. Other criteria include normalisation of blood analysis results and acute-phase protein levels.25 Persistently elevated serum CRP levels after completing the recommended treatment schedule may indicate pharmacological treatment failure.48,53,62
SurgeryNeurosurgical management enables microbiological characterisation and reduction of abscess size.6,11,63–65 Drainage may be performed either with open surgery or with minimally-invasive techniques.27 Open surgery focuses on draining the purulent material and resecting the capsule to minimise the risk of recurrence. Stereotactic aspiration, on the other hand, is being used increasingly frequently for abscess drainage. However, the decision to choose one technique or the other must be made on an individual basis.62,66
Open surgery may be indicated in the following cases: 1) lesions measuring ≥2.5 cm, 2) midline shift ≥5 mm, 3) proximity to the ventricular system, and 4) brain herniation.25 Complete surgical resection of the abscess may be indicated in the following cases: 1) abscesses located in a superficial, non-eloquent area; 2) suspected fungal, Mycobacterium tuberculosis, Actinomyces spp., or Nocardia spp. infection; 3) abscesses resulting from a congenital or acquired fistulous path; 4) multiloculated abscesses; 5) abscesses caused by parameningeal septic foci; and 6) failure of previous treatments.27,62
With stereotactic surgery and neuronavigation, aspiration can be performed regardless of the phase of the abscess, enabling microbiological and histopathological studies.23,27,32,33,67 In patients with multiple small abscesses, drainage of the largest lesion should be considered. The remaining lesions may be drained if they are surrounded by marked oedema, if the patient's clinical status worsens, and/or when response to antibiotic therapy is insufficient.27
When stereotactic surgery and neuronavigation are not available, intraoperative ultrasound is an alternative technique that may facilitate drainage of the abscess; however, this technique is not recommended in patients with small, deep-seated lesions.27,68
Catheter placement in the central region of the abscess for continuous drainage of the purulent material and direct antibiotic administration aims to lower rates of surgical reintervention, although this approach is no longer recommended for routine use.66 Reintervention may be necessary due to inadequate aspiration, lack of a drainage catheter in large abscesses, history of immunosuppresion, or inadequate antibiotic therapy, among other factors.2,27,62
Regarding imaging follow-up, Yamamoto et al.69 recommend performing a CT study at least once per week, depending on the patient's clinical status.
ConclusionsBrain abscesses are focal infections of the CNS, and account for approximately 8% of all intracranial space-occupying lesions. The most frequently isolated pathogens are S. aureus and Streptococcus viridans, with anaerobic microorganisms being found in up to 40% of cases. The main pathophysiological mechanisms are: 1) inoculation of microorganisms normally found on the skin (eg, in the context of head trauma or neurosurgery), 2) contiguous spread of bacteria (eg, mastoiditis, otitis media, sinusitis), and 3) haematogenous spread (eg, lung abscess, bacterial endocarditis). The most common signs and symptoms are headache, fever, focal neurological deficits, and impaired consciousness. Laboratory findings are nonspecific, with leukocytosis, elevated CRP levels, pleocytosis, and elevated CSF protein levels being the most frequent findings. In up to 85% of patients, contrast-enhanced head CT may reveal one or several lesions surrounded by a contrast-enhancing ring; these lesions may be better characterised with contrast-enhanced MRI and such other MRI modalities as DWI and MRS. Treatment of brain abscesses is based on 2 main pillars, and should be established on an individual basis. To select the most appropriate pharmacological treatment, we must take into account multiple pharmacokinetic and pharmacodynamic factors, as well as microbiological findings. The indication of neurosurgery, whether open or minimally-invasive, depends on the size, number, and location of the lesions.
Conflicts of interestNone.
The authors wish to thank Yanneth Olaya Urrego for her advice regarding microbiology findings.