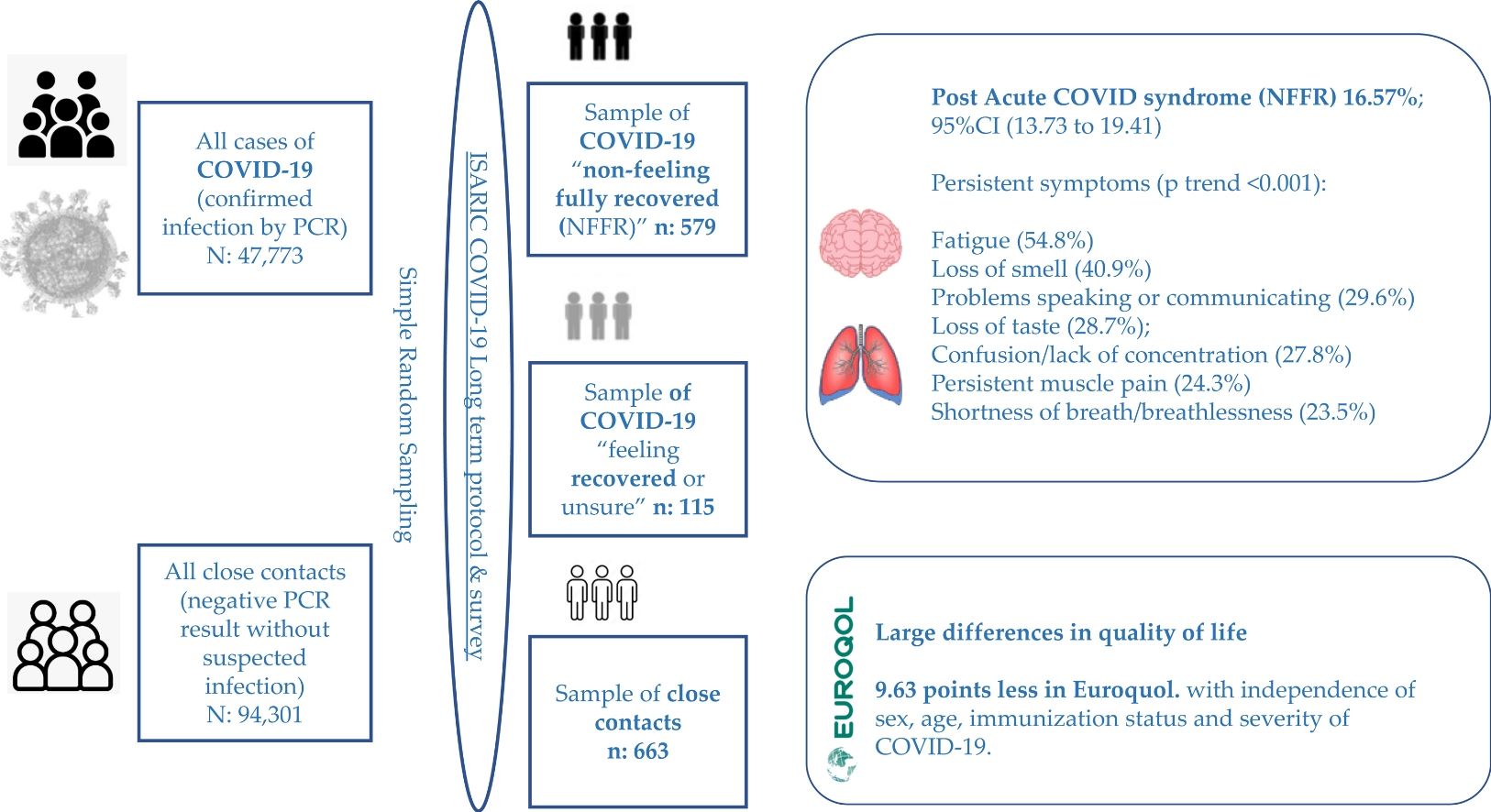
To characterize long-term patient-reported symptoms and quality of life, in adults after COVID-19.
Material and methodsCross-sectional study in Cantabria (Northern Spain) including adults with PCR-confirmed SARS-CoV-2 infection (n=694) with a time period between 4.7 and 24 month post-SARS-CoV-2 diagnosis, and their close contacts (n=663) (PCR negative and without suspected infection) obtained from simple random sampling of a total of 47,773 cases and 94,301 close contacts. The ISARIC survey was used as screening tool with self-reported “non-feeling fully recovery (NFFR)” defined as primary outcome.
Results16.57% (n=115/694) reported NFFR. Most prevalent symptoms were in order of frequency: Fatigue (54.8%); Loss of smell (40.9%); Problems speaking or communicating (29.6%); Loss of taste (28.7%); Confusion/lack of concentration (27.8%); Persistent muscle pain (24.3%) and Shortness of breath/breathlessness (23.5%). When comparing the three ordinal groups (Close contacts, COVID-19 feeling recovered, and COVID-19 NFFR) the prevalence of these symptoms was increasingly higher among each ordinal group (p<0.001). Female gender was significantly associated with NFFR: (adjusted odds ratio (aOR)=1.56); as well as older age: aOR per 10 year increment=1.15. Lastly, they scored on average 9.63 points less in Euroquol.
ConclusionsMore than 15% of patients in our real-life population-based study, reported NFFR, being female sex and older age independent predictors of this condition. Most symptoms in these patients were in accordance with WHO definition of post COVID-19 condition in adults, and were less prevalent in COVID-19 feeling recovered and close contact respectively, with a statistically significant dose-response pattern, and with a large decrease in quality of life according to Euroquol.
Caracterizar los síntomas y la calidad de vida informados a largo plazo después de un episodio agudo de COVID-19.
Material y métodosEstudio transversal en Cantabria (norte de España) que incluye adultos con infección por SARS-CoV-2 confirmada por PCR (n=694) tras un periodo entre 4,7 y 24meses desde el diagnóstico y sus contactos estrechos (n=663), obtenidos por muestreo aleatorio simple a partir de 47.773 casos y 94.301 contactos. Se utilizó la encuesta ISARIC, estableciéndose como variable resultado principal la respuesta «no-sentirse completamente recuperado (NSCR)».
ResultadosEl 16,57% (n=115/694) declararon NSCR. Los síntomas más prevalentes fueron, por orden de frecuencia: fatiga (54,8%), pérdida del olfato (40,9%), problemas para hablar o comunicarse (29,6%), pérdida del gusto (28,7%), confusión/falta de concentración (27,8%), dolor muscular persistente (24,3%) y dificultad para respirar/falta de aire (23,5%). Al comparar los tres grupos ordinales (contactos estrechos, COVID-19 recuperados y COVID-19 NSCR), la prevalencia de estos síntomas fue mayor en cada grupo (p<0,001). El sexo femenino se asoció significativamente con NSCR: Odds Ratio ajustada (aOR)=1,56), así como la edad avanzada: aOR por cada 10 años=1,15. Por último, obtuvieron en Euroquol una puntuación media de 9,63 puntos menos.
ConclusionesMás del 15% de los pacientes reportaron NSCR, siendo el sexo femenino y la edad factores predictores independientes. La mayoría de los síntomas en estos pacientes coincidieron con los de la definición de condición post-COVID-19 de la OMS y fueron menos prevalentes en contactos estrechos y COVID-19 que se sintieron recuperados, con un patrón dosis respuesta, y con una menor calidad de vida según Euroquol.
Epidemiological knowledge of long-term outcomes following a COVID-19 episode remains limited, although it is becoming increasingly clear that some patients who have had an acute COVID-19 experience, reports not being fully recovered several months after onset of COVID-19 symptoms accompanied by long-term persistent symptoms. It is known as long-COVID, post-COVID syndrome or Post-Acute Sequelae of SARS-CoV-2 infection (PASC).1–3 It appears to occur in both hospital and community settings. Among the long-term persistent symptoms, the following would stand out: fatigue, headache, chest pain, dyspnoea, neurological, psychological, and cardiovascular symptoms, with an impact on quality of life as assessed by the EuroQol tool.3–5
At this point of knowledge, the World Health Organisation (WHO) has developed a clinical case definition of post COVID-19 condition in adults by a Delphi consensus, stating that “Post COVID-19 condition occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19 with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis”. As common symptoms, WHO include fatigue, shortness of breath, cognitive dysfunction but also others, and these symptoms may be new onset following initial recovery from an acute COVID-19 episode or persist from the initial illness. Symptoms may also fluctuate or relapse over time.6
Due to the huge number of people affected by COVID-19 after the pandemic years and the growing evidence of long-term sequelae, a complete epidemiological understanding of long-term effects of COVID-19 seems to be necessary for policy makers and healthcare systems, and ultimately for the society that must understand the challenges faced by long-term COVID patients.7
The International Severe Acute Respiratory and emerging Infections Consortium (ISARIC) WHO Clinical Characterisation Protocol (CCP) was designed by international consensus in 2012 for any severe or potentially severe acute infection of public health interest.8 In the COVID-19 pandemic context, the ISARIC Global COVID-19 follow-up working group, has conducted an ongoing COVID-19 long term follow up study9 and an ISARIC survey (as a screening tool for persisting symptoms) has been developed, in collaboration with the WHO and a range of experts.10 The use of this “ISARIC COVID-19 follow-up survey foradults” allows a same standardized data collection among the different international studies in order to obtain valid comparisons of their results among the different countries and settings such as hospitalised and community settings. Its use therefore provides further insight into the burden of disease, and who is at greatest risk of developing long-term complications.
The objective of this study was to characterize long-term patient reported outcomes in adults after acute COVID-19 in Spain, by using the standardized data collection form mentioned above (the ISARIC survey as a screening tool for persisting symptoms).
Material and methodsStudy design and patientsCross-sectional study including adults (aged 18 years and over) with confirmed SARS-CoV-2 infection by Reverse-Transcriptase Polymerase Chain Reaction (RTPCR) (n=694) and their close contacts (with a negative PCR result and without suspected infection up to the time of survey) (n=663), obtained from a simple random sampling of a total of 47,773 cases and 94,301 close contacts, registered from June 2020 to December 2021 (18 months) in the whole community of Cantabria (Northern Spain). The ISARIC Global COVID-19 follow up protocol and associated standardized data collection form for adults were used.9,10 Persons institutionalized (residents of socio-health centres) or persons who live at home, but are disabled or dependent and cannot answer the survey for themselves were considered as exclusion criteria. The flow chart to obtain the final sample is shown in Fig. S1.
Data collection, variables, and outcomesParticipants were assessed via telephone by trained interviewers from the Epidemiological Surveillance and Intervention Unit [Unidad de Vigilancia Epidemiológica e Intervención (UVEI)] of Cantabria with a time period in a minimum of 4.7 months and a maximum of 24 month post-SARS-CoV-2 diagnosis, using the ISARIC COVID-19 follow-up survey for adults (ISARIC GLOBAL TIER 1 COVID-19 FOLLOW UP SURVEY. v1.2 21 Jan. 2021).10
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of Cantabria (code 2022.129). Informed consent was obtained from all participants in the moment of the telephone interview being recorded. All personal data were anonymized.
The primary outcome was self-reported “non-feeling fully recovery (NFFR)” at the time of follow-up. It regards to the question “Do you feel fully recovered from COVID-19?” of the ISARIC Initial Follow-up survey, with the following answers: Strongly disagree; Disagree; Neither disagree nor agree; Agree; Strongly Agree. The answers “Strongly disagree” or “Disagree” were computed as NFFR. The answer “Neither disagree nor agree” was computed as “unsure”, and the answers “Agree” or “Strongly Agree” to the question were computed as “Recovered”.
Secondary outcomes were the presence of new or persistent symptoms, in relation to the question: “Within the last seven days have you had any of these symptoms? (that you did not experience before onset of your Covid-19 illness)”. These symptoms were grouped into the following categories: Sensory; Neurological; Other neurological; Respiratory; Cardiovascular; Digestive; Urogenital; Dermatological; Joints and ligaments (see S Material).
Patient reported quality of life was assessed using the EuroQol overall health status tool with a range between 0 and 100, where zero denotes the worst and 100 the best possible health.11,12
The following variables were also recorded: sex, age, and previous COVID-19 immunization status in relation COVID-19 primary vaccination at the time of infection (or the time of contact tracing for close contact) in three ordinal categories: complete immunization (full primary vaccination), incomplete immunization, and non-immunization (no doses administered), or dichotomous categorized (complete versus “incomplete or non-immunization”) according to the Spanish criteria established by the National vaccination strategy at the time (see Table S1).
Statistical analysisFor categorical variables, proportions were estimated with their corresponding 95% confidence intervals (95%CI) and the comparison between independent groups were performed by using the Pearson's Chi-square test, or Fisher's exact test as appropriate. For continuous variables, means with their standard deviation (SD) or medians and interquartile ranges (IQR) were estimated and comparisons between two independent groups were performed by using the Student's T Test and Mann–Whitney's U Test respectively.
For mean and median comparisons between the three ordinal groups (close contact without COVID-19, COVID-19 “recovered or unsure”, COVID-19 NFFR) independent-samples Oneway ANOVA and Kruskal–Wallis test were used respectively. Linear-by-Linear Association (Mantel–Haenszel p-trend) was used to determine the dose-response pattern, when comparing the prevalence of symptoms between these three ordinal groups.
To study predictors of NFFR, crude and adjusted odds ratios (OR) with their 95%CI were obtained by using logistic regression models. In addition, crude and adjusted Mean Differences (MD) between patients “NFFR” and “recovered or unsure” In EuroQol results were obtained by using linear regression models.
A statistical significance level of 0.05 was considered for all hypothesis tests, and all tests were two-tailed. Statistical analysis of the data was performed using SPSS 25.0 (IBM SPSS, Inc., Armonk, NY) and Epidat 3.1 software (Consellería de Sanidade, Xunta de Galicia, Spain; Panamerican Health Organization (PAHO-WHO); CES University, Colombia).
ResultsDescription of the sampleOverall, 43.2% participants were male. Median age was 48 years (25th centile=35 to 75th centile=62 years old). Prevalence of obesity was 17%. Regarding time from COVID-19 diagnosis to complete survey, median time was 385 days (12.7 months) (25th centile=301 to 75th centile=505 days), with a minimum and maximum range between 143 days (4.7 months) and 670 days (22.1 months). 21% had a complete COVID-19 vaccination with a median time from vaccination to completing survey of 150 days (IQR 121–187). 9.4% (65/694) of patients required hospital or ICU admission. Table 1 shows the characteristics of participants who responded as a function of their COVID-19 status.
Characteristics of participants who responded.
| Without COVID-19 | With COVID-19 | ||||
|---|---|---|---|---|---|
| Close contacts (n=663) | “Recovered or unsure” (n=579) | NFFR (n=115) | All (n=694) | p-Value | |
| Age (years). Median (IQR) | 50 (37–63) | 46.25 (31–59) | 51 (20–84) | 47 (33–60) | 0.267 |
| Under 50. n (%) | 326 (49.2%) | 329 (56.8%) | 55 (47.8%) | 384 (55.3%) | 0.031 |
| 50–69. n (%) | 262 (39.5%) | 190 (32.8%) | 51 (44.3%) | 241 (34.7%) | |
| Over 70. n (%) | 75 (11.3%) | 60 (10.4%) | 9 (7.8%) | 69 (9.9%) | |
| Sex at birth | |||||
| Male. n (%) | 292 (44%) | 255 (44%) | 39 (33.9%) | 294 (42.4%) | 0.111 |
| Female. n (%) | 371 (56%) | 324 (56%) | 76 (66.1%) | 400 (57.6%) | |
| BMI. Median (IQR) | 25 (23–28) | 25 (23–29) | 26 (22–29) | 25 (23–29) | 0.427 |
| <18.5 (under weight) | 13 (2.0%) | 13 (2.3%) | 3 (2.7%) | 16 (2.3%) | 0.943 |
| 18.5–24.9 (normal weight) | 303 (46.6%) | 249 (43.7%) | 49 (43.4%) | 298 (43.6%) | |
| 25.0–29.9 (over weight). n (%) | 228 (35.1%) | 211 (37.0%) | 39 (34.5%) | 250 (36.6%) | |
| 30.0+ (obesity). n (%) | 106 (16.3%) | 97 (17.0%) | 22 (19.5%) | 119 (17.4%) | |
| Time from diagnosis to completing survey (days). Median (IQR) | n.a. | 378 | 414 | 385 | |
| COVID-19 vaccination | |||||
| Non-immunization (no doses administered). n (%) | 517 (81.4%) | 296 (54.0%) | 60 (56.6%) | 356 (54.4%) | <0.001 |
| Incomplete immunization. n (%) | 43 (6.8%) | 86 (15.7%) | 16 (15.1%) | 102 (15.6%) | |
| Complete immunization. n (%) | 75 (11.8%) | 166 (30.3%) | 30 (28.3%) | 196 (30.0%) | |
| Non-immunization or incomplete. n (%) | 560 (88.2%) | 382 (69.7%) | 76 (71.7%) | 458 (70.0%) | <0.001 |
| Complete. n (%) | 75 (11.8%) | 166 (30.3%) | 30 (28.3%) | 196 (30.0%) | |
| Time from complete vaccination to completing survey (days). Median (IQR) | 148 (124–177) | 153 (118–206) | 149 (121–188) | 153 (119–201) | |
| Severity of COVID-19 | |||||
| Without hospital admission. n (%) | n.a. | 531 (91.7%) | 98 (85.2%) | 629 (90.6%) | 0.029 |
| Hospital admission without ICU. n (%) | n.a. | 36 (6.2%) | 11 (9.6%) | 47 (6.8%) | |
| Length of hospital stay (days). Median (IQR) | n.a. | 7 (4–9) | 7 (6–14) | 7 (4–9) | 0.125 |
| Time from hospital discharge to completing survey (days). Median (IQR) | n.a. | 456 (298–513) | 490 (390–539) | 462 (302–530) | 0.273 |
| Hospital admission in ICU. n (%) | n.a. | 12 (2.1%) | 6 (5.2%) | 18 (2.6%) | |
| Without hospital admission. n (%) | n.a. | 531 (91.7%) | 98 (85.2%) | 629 (90.6%) | 0.029 |
| With hospital or ICU admission. n (%) | n.a. | 48 (8.3%) | 17 (14.8%) | 65 (9.4%) | |
Abbreviations: NFFR, non-feeling fully recovered; n.a., non applicable; BMI, body mass index; ICU, intensive care unit; IQR, interquartile range.
Of 694 participants with confirmed infection, 115 did “not feel they had fully recovered” at the time of their post-COVID survey: 16.57%; 95%CI (13.73–19.41) whereas the rest (n=579) did feel “recovered or unsure”: 83.43; 95%CI (80.59–86.27). When we stratified according to severity of COVID-19, this percentage was of 15.6% in patients who no required admission, and 23.4% and 33.3% in patients who required hospital and ICU admission respectively.
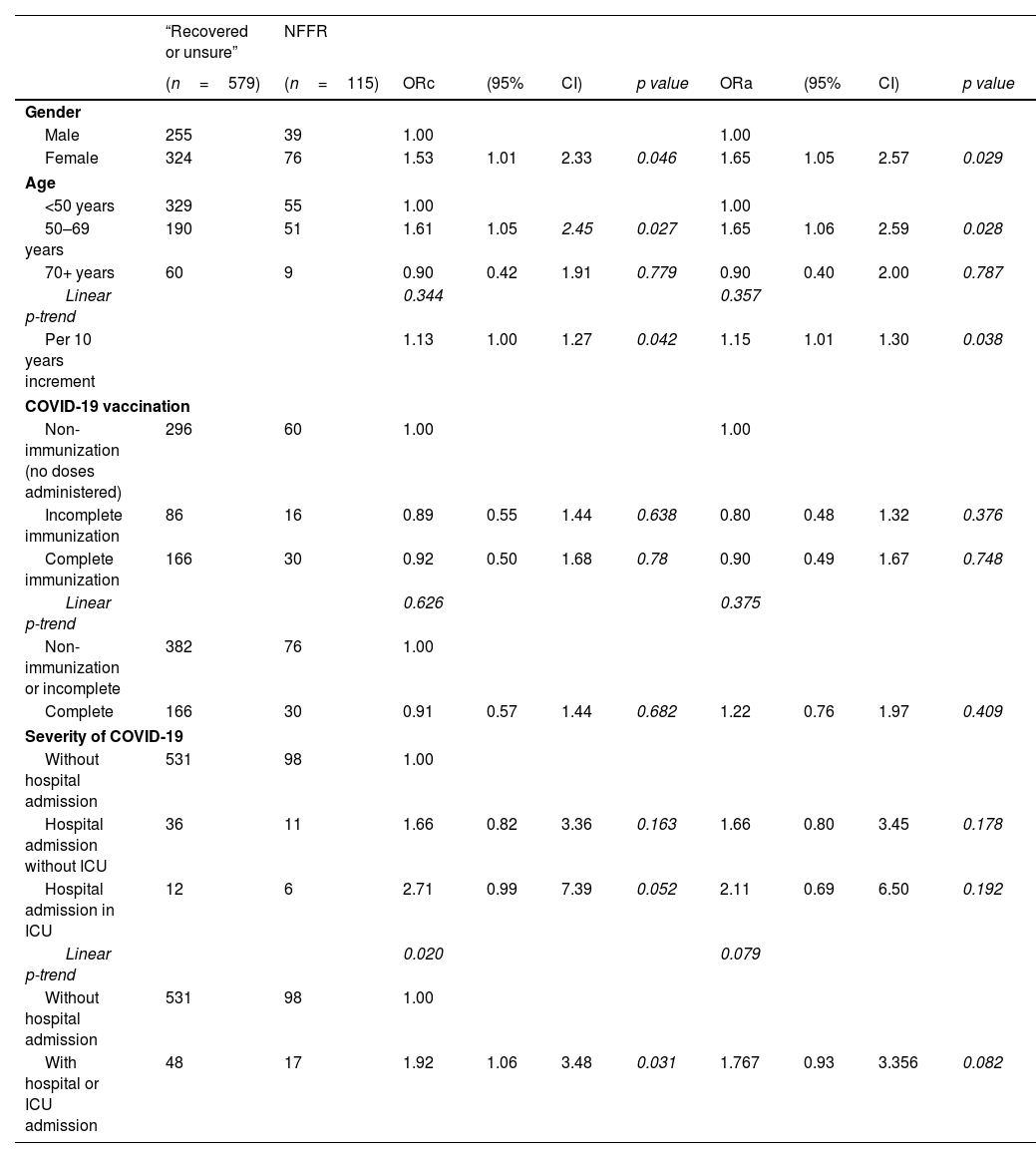
Female gender was significantly associated with NFFR, and this association remained after adjusting for age, immunization status and severity of COVID-19: adjusted OR 1.65; 95%CI (1.05–2.57), as well as older age. Each ×10 years increment of age, was associated to 1.15 times more likely to report NFFR with independence of gender, immunization status or severity of COVID-19. In relation to immunization status, a protective lower risk of small magnitude and non-statistical significant was obtained: adjusted OR=0.90; 95%CI (0.49–1.67), p=0.748. In contrast, evidence of association was found for severity of COVID-19. Patients who required hospital or ICU admission were two times more likely to report NFFR (crude OR=1.92, p=0.031) although this association diminished losing statistical significance after adjusting for sex, age and immunization status: adjusted OR=1.77; 95%CI (0.93–3.36), p=0.082. When severity of COVID-19 was ordinal categorized into “no admission, hospital, ICU admission”, a non-statistically significant dose response pattern was also obtained (see Table 2).
Predictors of “non-feeling fully recovery (NFFR)” from COVID-19 illness.
| “Recovered or unsure” | NFFR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n=579) | (n=115) | ORc | (95% | CI) | p value | ORa | (95% | CI) | p value | |
| Gender | ||||||||||
| Male | 255 | 39 | 1.00 | 1.00 | ||||||
| Female | 324 | 76 | 1.53 | 1.01 | 2.33 | 0.046 | 1.65 | 1.05 | 2.57 | 0.029 |
| Age | ||||||||||
| <50 years | 329 | 55 | 1.00 | 1.00 | ||||||
| 50–69 years | 190 | 51 | 1.61 | 1.05 | 2.45 | 0.027 | 1.65 | 1.06 | 2.59 | 0.028 |
| 70+ years | 60 | 9 | 0.90 | 0.42 | 1.91 | 0.779 | 0.90 | 0.40 | 2.00 | 0.787 |
| Linear p-trend | 0.344 | 0.357 | ||||||||
| Per 10 years increment | 1.13 | 1.00 | 1.27 | 0.042 | 1.15 | 1.01 | 1.30 | 0.038 | ||
| COVID-19 vaccination | ||||||||||
| Non-immunization (no doses administered) | 296 | 60 | 1.00 | 1.00 | ||||||
| Incomplete immunization | 86 | 16 | 0.89 | 0.55 | 1.44 | 0.638 | 0.80 | 0.48 | 1.32 | 0.376 |
| Complete immunization | 166 | 30 | 0.92 | 0.50 | 1.68 | 0.78 | 0.90 | 0.49 | 1.67 | 0.748 |
| Linear p-trend | 0.626 | 0.375 | ||||||||
| Non-immunization or incomplete | 382 | 76 | 1.00 | |||||||
| Complete | 166 | 30 | 0.91 | 0.57 | 1.44 | 0.682 | 1.22 | 0.76 | 1.97 | 0.409 |
| Severity of COVID-19 | ||||||||||
| Without hospital admission | 531 | 98 | 1.00 | |||||||
| Hospital admission without ICU | 36 | 11 | 1.66 | 0.82 | 3.36 | 0.163 | 1.66 | 0.80 | 3.45 | 0.178 |
| Hospital admission in ICU | 12 | 6 | 2.71 | 0.99 | 7.39 | 0.052 | 2.11 | 0.69 | 6.50 | 0.192 |
| Linear p-trend | 0.020 | 0.079 | ||||||||
| Without hospital admission | 531 | 98 | 1.00 | |||||||
| With hospital or ICU admission | 48 | 17 | 1.92 | 1.06 | 3.48 | 0.031 | 1.767 | 0.93 | 3.356 | 0.082 |
Abbreviations: NFFR, non-feeling fully recovered; ORc, crude odds ratio; ORa, odds ratio adjusted for gender, age, immunization status and severity of COVID-19 respectively; ICU, intensive care unit.
Most prevalent symptoms among patients NFFR, were in order of frequency: Fatigue, reported by 54.8% of these patients (63/115); Loss of smell (47/115) (40.9%); Problems speaking or communicating (34/115) (29.6%); Loss of taste 33/115 (28.7%); Confusion/lack of concentration (32/115) (27.8%); Persistent muscle pain (28/115) (24.3%) and Shortness of breath/breathlessness (27/115) (23.5%). When comparing prevalence of these symptoms between the three ordinal groups “close contact without COVID-19, COVID-19 recovered or unsure, COVID-19 NFFR)”, prevalence was increasingly higher among each ordinal group, with a statistically significant dose-response pattern (p<0.001) (see Table 3). In addition, positive statistically significant adjusted OR were obtained for these symptoms (see Table S2).
Prevalence of new symptoms (non-experienced before onset of their illness in COVID-19 patients).
| Without COVID-19 | With COVID-19 | ||||
|---|---|---|---|---|---|
| Close contacts (n=663) | “Recovered or unsure” (n=579) | Did not feel fully recovered (n=115) | All (n=694) | ||
| n (%) | n (%) | n (%) | n (%) | Linear p-trend | |
| Sensory | 23 (3.5%) | 63 (10.9%) | 62 (53.9%) | 125 (18.0%) | <0.001 |
| Loss of smell | 5 (0.8%) | 23 (4.0%) | 47 (40.9%) | 70 (10.1%) | <0.001 |
| Loss of taste | 3 (0.5) | 19 (3.3%) | 33 (28.7%) | 52 (7.5%) | <0.001 |
| Problems seeing | 5 (0.8%) | 14 (2.4%) | 11 (9.6%) | 25 (3.6%) | <0.001 |
| Ringing in ear | 11 (1.7%) | 20 (3.5%) | 15 (13.0%) | 35 (5.0%) | <0.001 |
| Neurological | 55 (8.3%) | 73 (12.6%) | 53 (46.1%) | 126 (18.2%) | <0.001 |
| Problems speaking or communicating | 3 (0.5%) | 8 (1.4%) | 12 (10.4%) | 20 (2.9%) | <0.001 |
| Problems sleeping | 45 (6.8%) | 45 (7.8%) | 34 (29.6%) | 79 (11.4%) | <0.001 |
| Confusion/lack of concentration | 16 (2.4%) | 27 (4.7%) | 32 (27.8%) | 59 (8.5%) | <0.001 |
| Other neurological | 93 (14.0%) | 120 (20.7%) | 80 (69.6%) | 200 (28.8%) | <0.001 |
| Headache | 34 (5.1%) | 38 (6.6%) | 28 (24.3%) | 66 (9.5%) | <0.001 |
| Fatigue | 30 (4.5%) | 55 (9.5%) | 63 (54.8%) | 118 (17.0%) | <0.001 |
| Cant feel one side of the body or face | 2 (0.3%) | 4 (0.7%) | 6 (5.2%) | 10 (1.4%) | <0.001 |
| Tingling feeling/“pins and needles” | 21 (3.2%) | 22 (3.8%) | 25 (21.7%) | 47 (6.8%) | <0.001 |
| Dizziness/light headedness | 27 (4.1%) | 27 (4.7%) | 19 (16.5%) | 46 (6.6%) | <0.001 |
| Fainting/blackouts | 9 (1.4%) | 3 (0.5%) | 4 (3.5%) | 7 (1.0%) | 0.566 |
| Seizures/fits | 1 (0.2%) | 1 (0.2%) | – | 1 (0.1%) | 0.832 |
| Tremor/shakiness | 4 (0.6%) | 2 (0.3%) | 2 (1.7%) | 4 (0.6%) | 0.496 |
| Respiratory | 60 (9.0%) | 36 (6.2%) | 41 (35.7%) | 77 (11.1%) | <0.001 |
| Persistent dry cough | 19 (2.9%) | 13 (2.2%) | 17 (14.8%) | 30 (4.3%) | <0.001 |
| Persistent cough with phlegm | 20 (3.0%) | 7 (1.2%) | 8 (7.0%) | 15 (2.2%) | 0.568 |
| Shortness of breath/breathlessness | 14 (2.1%) | 17 (2.9%) | 27 (23.5%) | 44 (6.3%) | <0.001 |
| Pain on breathing | 2 (0.3%) | 0 (0.0%) | 3 (2.6%) | 3 (0.4%) | 0.035 |
| Cardiovascular | 12 (1.8%) | 14 (2.4%) | 15 (13.0%) | 29 (4.2%) | <0.001 |
| Chest pains | 3 (0.5%) | 5 (0.9%) | 2 (1.7%) | 7 (1.0%) | 0.132 |
| Palpitations (heart racing) | 10 (1.5%) | 9 (1.6%) | 13 (11.3%) | 22 (3.2%) | <0.001 |
| Digestive | 49 (7.4%) | 44 (7.6%) | 26 (22.6%) | 70 (10.1%) | <0.001 |
| Weight loss | 21 (3.2%) | 12 (2.1%) | 9 (7.8%) | 21 (3.0%) | 0.225 |
| Loss of appetite | 5 (0.8%) | 10 (1.7%) | 8 (7.0%) | 18 (2.6%) | <0.001 |
| Stomach/abdominal pain | 13 (2.0%) | 13 (2.2%) | 11 (9.6%) | 24 (3.5%) | 0.001 |
| Feeling sick/vomiting | 10 (1.5%) | 11 (1.9%) | 4 (3.5%) | 15 (2.2%) | 0.197 |
| Constipation | 7 (1.1%) | 11 (1.9%) | 1 (0.9%) | 12 (1.7%) | 0.546 |
| Diarrhoea | 9 (1.4%) | 9 (1.6%) | 6 (5.2%) | 15 (2.2%) | 0.031 |
| Problems swallowing or chewing | 3 (0.5%) | 0 (0%) | 1 (0.9%) | 1 (0.1%) | 0.764 |
| Urogenital | 42 (6.3%) | 55 (9.5%) | 19 (16.5%) | 74 (10.7%) | <0.001 |
| Problems passing urine | 9 (1.4%) | 14 (2.4%) | 6 (5.2%) | 20 (2.9%) | 0.011 |
| Changes in menstruation (restricted to women n=771) | 29 (7.8%) | 42 (13.0%) | 14 (18.4%) | 56 (14.0%) | 0.002 |
| Erectile dysfunction (restricted to men n=586) | 4 (1.4%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.064 |
| Dermatological | 26 (3.9%) | 24 (4.1%) | 15 (13.0%) | 39 (5.6%) | 0.002 |
| Lumps or rashes (purple/pink) on toes | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0.0%) | – |
| Rash on face | 5 (0.8%) | 8 (1.4%) | 6 (5.2%) | 14 (2.0%) | 0.002 |
| Rash on trunk (stomach or back) | 5 (0.8%) | 6 (1.0%) | 3 (2.6%) | 9 (1.3%) | 0.126 |
| Rash on arms | 6 (0.9%) | 5 (0.9%) | 4 (3.5%) | 9 (1.3%) | 0.100 |
| Rash on legs | 6 (0.9%) | 7 (0.8%) | 3 (2.6%) | 10 (1.4%) | 0.174 |
| Rash on buttocks | 0 (0%) | 1 (0.2%) | 2 (1.7%) | 3 (0.4%) | 0.004 |
| Rash on the toes | 2 (0.3%) | 1 (0.2%) | 2 (1.7%) | 3 (0.4%) | 0.158 |
| Rash on fingers | 4 (0.6%) | 1 (0.2%) | 4 (3.5%) | 5 (0.7%) | 0.058 |
| Bleeding | 2 (0.3%) | 1 (0.2%) | 1 (0.9%) | 2 (0.3%) | 0.631 |
| Joints and ligaments | 65 (9.8%) | 68 (11.7%) | 52 (45.2%) | 120 (17.3%) | <0.001 |
| Swollen ankle(s) | 9 (1.4%) | 12 (2.1%) | 7 (6.1%) | 19 (2.7%) | 0.006 |
| Problems with balance | 6 (0.9%) | 7 (1.2%) | 3 (2.6%) | 10 (1.4%) | 0.174 |
| Weakness in arms or legs muscle weakness | 12 (1.8%) | 13 (2.2%) | 20 (17.4%) | 33 (4.8%) | <0.001 |
| Persistent muscle pain | 25 (3.8%) | 38 (6.6%) | 28 (24.3%) | 66 (9.5%) | <0.001 |
| Joint pain or swelling | 30 (4.5%) | 22 (3.8%) | 16 (13.9%) | 38 (5.5%) | 0.009 |
| Can’t fully move or control movement | 1 (0.2%) | 0 (0%) | 1 (0.9%) | 1 (0.1%) | 0.372 |
Mean of reported quality of life on the day of survey according to “EuroQol overall health status tool (range 0–100)” was 78.27 (SD=17.29) in close contact without COVID-19 and 79.4 (SD=16.42) in COVID-19 patients (p=0.182). In terms of medians, similar medians were obtained: medians=80 (25th centiles=70 to 75th centiles=90), p=0.165. However, when COVID-19 patients were split into two groups (“recovered or unsure” versus “NFFR”) higher statistically significant punctuations were obtained in those patients feeling “recovered or unsure” (p<0.001) (see Table S3 and Fig. S2).
When comparisons were restricted to COVID-19 patients, in average COVID-19 patients NFFR scored ten points less than those COVID-19 patients feeling “recovered or unsure”: crude MD=−10.52 points; 95%CI (−13.72 to −7.32), p<0.001. This MD remained after adjusting for sex, age, immunization status and severity of COVID-19: Adjusted MD=−9.63 points; 95%CI (−12.82 to −6.44), p<0.001 (see Table 4). When Euroquol results were dichotomous categorized according to median into low (≤the median of 80 points) and high values, patients NFFR were 2.6 times more likely to have low scores: adjusted OR=2.58; 95%CI (1.60–4.16), p<0.001 (see Table 5).
Mean differences in EuroQol scores between patients “non-feeling fully recovered (NFFR)” and “recovered or unsure” from their COVID-19 illness.
| MDc | (95% | CI) | p value | MDa | (95% | CI) | p value | |
|---|---|---|---|---|---|---|---|---|
| EuroQol overall health status tool (0–100) | −10.52 | −13.72 | −7.32 | <0.001 | −9.63 | −12.82 | −6.44 | <0.001 |
Abbreviations: MDc, crude mean difference; MDa, mean difference adjusted for gender, age, immunization status and severity of COVID-19 respectively.
Association between a low EuroQol punctuation (≤80) and “non-feeling fully recovered” from COVID-19 illness.
| “Recovered or unsure” | Non-feeling fully recovered | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n=576) (missing=3) | (n=115) | ORc | (95% | CI) | p value | ORa | (95% | CI) | p value | |
| EuroQol overall health status tool (0–100) | ||||||||||
| 80+ | 291 | 31 | 1.00 | 1.00 | ||||||
| ≤80 | 285 | 84 | 2.77 | 1.78 | 4.31 | <0.001 | 2.58 | 1.60 | 4.16 | <0.001 |
Abbreviations: ORc, crude odds ratio; ORa, odds ratio adjusted for gender, age, immunization status and severity of COVID-19 respectively.
More than 15% of patients, reported NFFR of their COVID-19 illness. This % is lower than that reported by other studies, but the mixed characteristics of patients (with and without hospital admission for COVID-19) has to be taken into account when comparing with other studies focusing only on patients with more severe disease requiring admission. In this regard, the meta-analysis performed by O’Mahoney et al. (2022)13 includes data from hospitalised (n=122 studies), non-hospitalized (n=11 studies), and mixed as our study (n=36 studies). Among studies with non-hospitalised patients, the pooled prevalence of COVID-19 survivors experiencing at least one symptom during follow-up was 34.5%. Among mixed studies including both groups, the pooled prevalence was 37.8% and lastly, in studies with patients requiring admission, the pooled prevalence was 52.6%.13 The meta-analysis published by Chen et al. (2022)7 would also support this dose–response pattern, with prevalences of PASC of 34% and 33% for non-hospitalized or mixed studies, and 54% for studies with hospitalised patients.
These meta-analyses also show that the prevalence of post-acute symptoms decreases as more time has elapsed since the COVID-19 illness. In this sense, our study would be very long-term compared to those of the studies included in the aforementioned meta-analyses, with means of follow-up of 126 days13 or being <120 days in most of published studies reported by Chen et al. (2022).7
The high vaccination rate in Cantabria and Spain must also be considered in the context of a COVID-19 complete immunization as a protective factor for long- COVID-19 symptoms. In the case of the meta-analysis with the highest prevalence of long-term symptoms (prevalence of at least one symptom of 80%), it is based on studies prior to January 2021, which implies that vaccination rates would be very low or non-existent. It would be also based on studies that in no case exceed an average of 110 days of follow-up.14 Nevertheless, it should be noted that the strength of the current evidence on vaccination as a protective factor for long-COVID-19 symptoms is limited and our results do not support a clinically relevant protective effect either.15–17 Finally, it is plausible that the earlier more virulent variants would be associated with more permanent symptoms.18 According to this hypothesis, studies with patients infected earlier in the pandemic (and thus with more virulent variants) could also report a higher prevalence of long-term symptoms.
In terms of the strengths of our study, the methodology used in the form of simple random sampling of the total number of cases in a whole autonomous community denotes that we have real-life conditions, providing a population-based view of real-life conditions at the community level. If we restrict to our patients with hospital or ICU admission, our percentage increase to 23.4% and 33.3% respectively. Percentages that would be more similar to those reported in other studies such as Sigrid et al. (2021)4 although lower than the pooled prevalences of the mentioned meta-analyses.7,13
Among our predictors of NFFR, having been admitted to ICU showed the strongest magnitude of association (adjusted OR=2.11). However, due to our small sample of patients who required ICU admission (n=18), this association did not yield statistical significance. Female sex and older age were independent predictors of NFFR, yielding statistical significance. Our results for the female sex are supported by other reviews and meta-analyses such as that of Aiyegbusy et al. (2021)5 or Chen et al. (2022)7 reporting an OR of 1.57 (95%CI 1.09–2.26) very similar to ours, and also with primary studies such as the Sigfrid et al. (2021)4 based on the same ISARIC survey as ours. Interestingly, for age, our results contrast with those of Sigfrid et al. in their prospective multicentre cohort study in adults discharged from UK hospitals after COVID-19, because in this study, women under 50 years old and those with more severe acute disease in-hospital, had the worst long-term outcomes. In terms of meta-analyses, those published by Aiyegbusy et al. (2021)5 and Chen et al. (2022)7 support the association between older age and higher prevalence of long-term symptoms, whereas that of O’Mahoney et al. (2022)13 show inconclusive results. The 70+ year olds in our study were less likely to report NFFR than the reference category (<50 years) (OR=0.90). It is possible that this result may be explained by a survival bias due to the fact that the most severe cases have died. Thus, if those >70 years of age susceptible to long-term sequelae have not survived, they have not had a chance to have them.
Main symptoms in our group NFFR (fatigue. headache. chest pain. shortness of breath & dyspnoea. cognitive dysfunction. neurological. psychological) coincide with those reported in primary studies, also listed in Annex 3 and Table 2 of the WHO report6 and as it is logical, coincide with those reported in most of the published systematic reviews and meta-analyses, with the percentages of each symptom being in very similar ranges.5,7,13,14,19–21 Regarding cardiovascular symptoms, in our study the prevalence was low, but it must be taken into account that we only included Chest Pain and Palpitations. If we compare these two specific cardiovascular symptoms, we find similar prevalences reported in meta-analyses: i.e. 9.3% and 11%.14,19
In terms of quality of life, no dose-response pattern was observed because higher punctuations were observed in COVID-19 patients feeling “Recovered or unsure” than in close contact without COVID-19. However, large differences in quality of life were found between COVID-19 patients. Restricting to COVID-19 patients, those NFFR scored on average 9.63 points less in Euroquol than those patients feeling “recovered or unsure”, with independence of sex, age, immunization status and severity of COVID-19. They were 2.6 times more likely to have low Euroquol scores (≤the median of 80 points). In this sense, Aiyegbusy et al. (2021) meta-analysis, includes five studies that specifically use Euroquol to analyze quality of life.1,22–25 One of these studies reports a difference of 10 points, very similar to ours.1
Our study includes the group of close contacts without evidence of infection as a comparative group, allowing a dose–response pattern between the three groups. The dose–response pattern is one of Bradford Hill's classic causality criteria and would therefore support the association between a higher prevalence of these symptoms and NFFR. Using the same tool based on the ISARIC WHO clinical characterization protocol, would allow valid comparisons with other studies. The similarity of symptoms found through this standardized survey, coinciding with other primary studies in other countries, and the mixed nature of our setting (with and without hospital admission for COVID-19) would support both the internal and external validity of our results, supporting the existence of a pattern after COVID-19 compatible with a complex clinical picture known as long-COVID, post-COVID syndrome or PASC. In terms of limitations, it should be noted that we only have a single cut-off point (the questionnaire was administered only once after infection). In this sense, prospective studies that take more than one visit, following the ISARIC protocol more completely, would support the persistence of these symptoms over time. Nevertheless, minimum time from diagnosis to completing our survey was 143 days (4.7 months) with a median time of 385 days (12.7 months), supporting the long-term post COVID-19 context of our results.
ConclusionsMore than 15% of patients in our real-life population-based study, reported NFFR of their COVID-19 illness after a minimum time of 4.7 month from diagnosis. Female sex and older age were in our study statistically significant independent predictors of NFFR. Most prevalence symptoms in our patients were in accordance with WHO definition of post COVID-19 condition in adults and were in order of frequency: Fatigue; Loss of smell; Problems speaking or communicating; Loss of taste; Confusion/lack of concentration; Persistent muscle pain and Shortness of breath/breathlessness. When comparing the three ordinal groups (Close contacts without infection, COVID-19 feeling recovered, and COVID-19 NFFR) the prevalence of these symptoms was increasingly higher among each ordinal group with a statistically significant dose-response pattern (p<0.001). Large differences in quality of life were found between patients NFFR and those patients feeling “recovered or unsure” reporting 9.63 points less in Euroquol with independence of sex, age, immunization status and severity of COVID-19.
FundingThere was no funding associated with this study.
Authors’ contributionsO.P. conceptualization, methodology, writing-review & editing. M.S. methodology, analysis and interpretation of data, drafted the work. L.R. analysis and interpretation of data, writing-review & editing. J.M.C. methodology, analysis and interpretation of data, writing-review & editing. A.H.L. conceptualization, methodology, supervision, writing-review & editing.
Conflicts of interestThere are no conflicts of interest associated with this study.
We thank the participant people and contact tracers for participating in this study.