A few experimental studies related to asthma have unveiled the beneficial effects of TNF alpha blocking agents on the airway histology, cytokine levels in bronchoalveolar lavage and bronchial hyper-responsiveness. In the current study, we aimed to assess the effect of adalimumab on the inflammation and histology of asthma in a murine model.
MethodTwelve-week-old BALB/c (H-2d/d) female rats (n=18) were allocated into three groups, including (group I) control (phosphate-buffered saline was implemented), (group II) asthma induced with OVA (n=6), and (group III) asthma induced with OVA+treated with adalimumab (n=6). Rats were executed on the 28th day of the study. The lung samples were fixed in 10% neutral buffered formalin. Lung parenchyma, alveolus, peribronchial and perivascular inflammation were assessed. Lung pathological scoring was performed.
ResultSeverity of lung damage was found to be reduced significantly in the asthma induced with OVA+treated with adalimumab group. When compared with the untreated group, adalimumab significantly reduced the inflammatory cells around the bronchi and bronchioles, and reduced inflammation of the alveolar wall and alveolar wall thickness as well (median score=1, p=0.52). Peribronchial smooth muscle hypertrophy and oedema were significantly reduced after adalimumab administration.
ConclusionAdalimumab (a human monoclonal anti-TNF alpha antibody) therapy significantly reduced the severity of lung damage by decreasing cellular infiltration and improvement on the lung histology in a murine model of acute asthma.
Asthma is a chronic inflammatory disorder of the airways in which many cells and cellular elements play a role.1 Asthma is a T helper 2 (Th2)-mediated disease characterised by increased airway eosinophilia, goblet cell hyperplasia with associated mucus production, neutrophilia and increased numbers of lung macrophages and activated mast cells.2 Additionally, cytokines play an important role in the aetiopathogenesis of asthma by promoting the development, differentiation, recruitment, activation and survival of inflammatory cells. TNF-alpha, which is a Th1 associated and pro-inflammatory cytokine with immunoregulatory activities, takes an important role in the aetiopathogenesis of asthma. TNF-alpha serves as a chemoattractant for the neutrophils and monocytes,3 increases vascular permeability, and activates4 T cells,5 eosinophils3,6 and mast cells. Few experimental studies in asthma have determined that different TNF-alpha blocking agents (anti-TNF antibody, recombinant human TNF receptor p80 Fc fusion protein) reduced the inflammation in the airways.7,8 Additionally, data about the effects of human monoclonal anti-TNF alpha antibody seem to be limited. In the present study, the aim was to ascertain the effects of adalimumab (a human monoclonal anti-TNF alpha antibody) on lung histology and lung inflammation in a murine model of asthma.
Materials and methodsAnimals and experimental designBALB/c (H-2d/d) female rats (n=18) were used for the study. Experimental protocols were approved by the Fatih University Animal Subject Committees. All animals were specified pathogen-free and were maintained under standard animal holding with water and food ad libitum at the Ankara D¿¿kap¿ Hospital, Research Center, Animals Research Laboratory in accordance with local and Turkish Home Office regulations. Twelve-week-old rats were randomly divided into three groups, including (group I) control (n=6) (Phosphate-buffered saline was implemented), (group II) asthma induced with OVA (n=6), and (group III) asthma induced with OVA+treated with adalimumab (n=6).
Administration of OVA and adalimumab250¿l of “Phosphate-buffered saline” (PBS) was given intraperitoneally to the rats in the control group on the first day and 14th day of the study. Additionally, rats were administered 250¿l of PBS by intranasal route under anaesthesia (0.3ml ketamine (6.5mg/ml)/xylazine (0.44mg/ml, intraperitoneally) on the 14th, 25th, 26th and 27th days of the study. Rats in groups I and II were sensitised with intraperitoneal 1mg ovalbumin (OVA) (Sigma Co., St. Louis, MO) emulsified in 250¿l of PBS on the first and the 14th day of the study; and 500¿g OVA in 250¿l of PBS by intranasal route (OVA challenge) was applied to the rats under anaesthesia (0.3ml ketamine (6.5mg/ml)/xylazine (0.44mg/ml, intraperitoneally) on the 14th, 25th, 26th and 27th days of the study. Also, 100¿g OVA in 100¿l of PBS was given intraperitoneally to all rats one hour before each intranasal administration. Adalimumab with a dose of 5mg/kg/day was applied intraperitoneally to the rats in the third group for five days, starting from the day before the first challenge. All rats were sacrificed by cervical dislocation on the 28th day of the study. Lung tissues were removed for the histopathological examination.
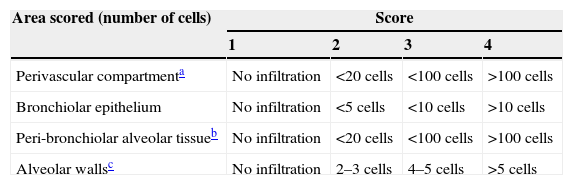
Lung histology and scoringThe lung samples were fixed in 10% neutral buffered formalin, after the routine tissue monitorisation, the sections were obtained and stained with haematoxylin and eosin (H&E) and evaluated under light microscope. The lung sections from all groups were examined in a blinded fashion and lung inflammation was scored on the sections stained with H&E. All slides were evaluated over 10 consecutive fields at ×200 magnification. In order to be scored, each field had to contain a complete transection of at least one bronchiole less than half a field width in diameter, a blood vessel and an alveolar airway. Inflammatory cell infiltrate, i.e. the number and type of inflammatory cells present, was evaluated for the perivascular area, the bronchiolar epithelium and the peri-bronchiolar alveolar tissue. Inflammation was also scored on the basis of increased alveolar wall thickness. The scoring system used to assess inflammation is shown in Table 1.9
Scoring system for assessing inflammation in a murine asthma model9
| Area scored (number of cells) | Score | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Perivascular compartmenta | No infiltration | <20 cells | <100 cells | >100 cells |
| Bronchiolar epithelium | No infiltration | <5 cells | <10 cells | >10 cells |
| Peri-bronchiolar alveolar tissueb | No infiltration | <20 cells | <100 cells | >100 cells |
| Alveolar wallsc | No infiltration | 2–3 cells | 4–5 cells | >5 cells |
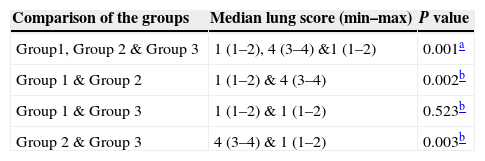
Statistical analysis was performed by the Statistical Package for Social Sciences (SPSS) 15.0 software (SPSS Inc., Chicago, IL). Quantitative variables (lung score) were expressed in medians. The median score of groups were compared by using Kruskal–Wallis test. Mann–Whitney U test with Benferoni correction was used to evaluate the differences between groups. A two-sided p<0.05 was considered statistically significant.
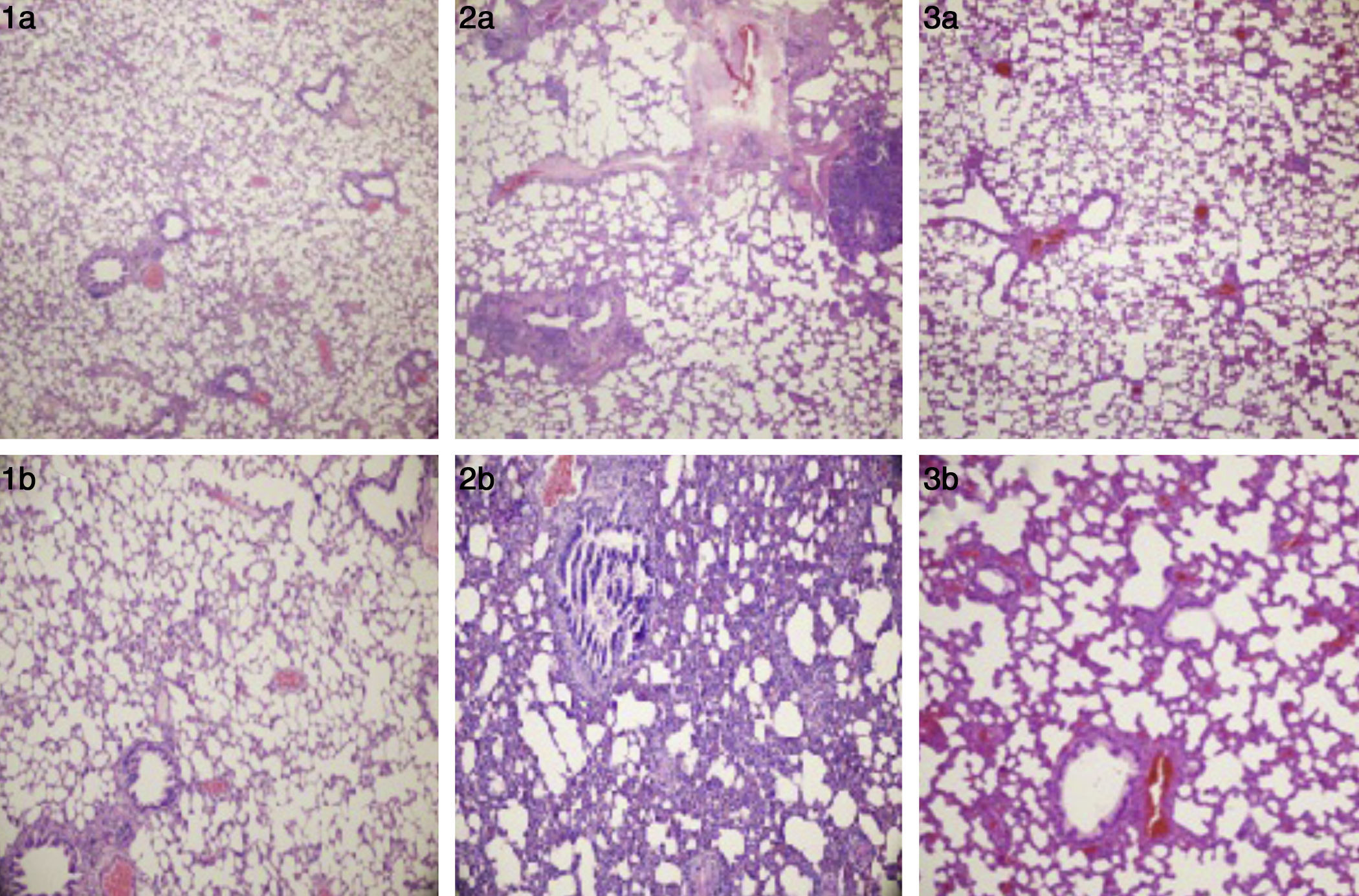
ResultsCell infiltration, inflammation and histological examinationWhen compared with the normal controls, dense inflammation was seen in the perivascular and peribronchiolar areas, including lymphocytes and eosinophils infiltration; and interstitial space (alveoli walls) was severely thickened due to intense inflammation in the lung tissues of asthma induced rats (median score: 4, p=0.02). Bronchial smooth muscle hypertrophy and oedema were observed in the lung tissues of asthma induced rats. In the group treated with adalimumab, the number of inflammatory cells surrounding the bronchi and bronchioles and alveolar wall thickness had a similar histomorphological appearance with the control group (median score: 1, p=0.52). Furthermore, when compared, peribronchial smooth muscle hypertrophy and oedema were similar between the control group and the adalimumab-treated group (Fig. 1). Statistically significant levels of the lung scores between the groups are shown in Table 2.
(1a and 1b) Normal alveoli and bronchi (control group), H&E ×40 and ×100. (2a) Peri-bronchiolar and perivascular mix inflammation (asthma group), H&E ×40. (2b) Thickened alveolar walls with inflammation (asthma group), H&E ×100. (3a) The lung parenchyma without inflammation (adalimumab-treated group), H&E ×40. (3b) Mild thickened alveolar septa (adalimumab-treated group), H&E ×100.
Comparison of the groups according to lung score.
In the current study, we evaluated the effects of adalimumab (a human monoclonal anti-TNF alpha antibody) on the lung histology and lung inflammation in a murine model of asthma. We found that adalimumab treatment decreased the cell inflammation, and showed similar histological findings to the control group. These findings support the opinion that anti-TNF alpha antibody could be a promising agent in the treatment of asthma.
Asthma is a disease mediated by Th2 cells, and specific cytokines such as IL4, IL-5, and IL-13 have important roles in the aetiopathogenesis of asthma.10 TNF alpha, which is a Th1 lymphocyte-associated cytokine, has been shown to play an important role in the pathogenesis of asthma in the studies of both animals and humans.11,12 TNF-alpha, a strong pro-inflammatory cytokine, is mainly synthesised and stored by mast cells and alveolar macrophages in the lung, and it also has immune-regulatory properties.13 Furthermore, it contributes to the development of remodelling in asthma by affecting on fibroblasts and triggering the release of matrix metalloproteinase-9.14 Some studies on rats exposed to house dust and in patients with severe asthma have determined increased TNF-alpha expression in the airways.8,15,16 Therefore, some other studies have been conducted to ascertain the beneficial effects of TNF alpha inhibiting agents on both the animal models and patients with severe asthma. Additionally, some experimental studies have reported that agents inhibiting synthesis and activity of TNF-alpha decrease the infiltration of inflammatory cells in the airways.17–19 However, these agents have been reported to be ineffective through some other studies.20,21 Furthermore, results of these studies vary according to blocking of TNF alpha activities by different steps. Although Nam et al. have reported that using soluble TNF-alpha receptor in a rat model of asthma reduces eosinophil infiltration; they have declared that no beneficial effects have been found on the airway inflammation, when compared to the control group. In contrast, Kim et al.22 have determined in an experimental model of asthma that TNF alpha antibodies have provided significant improvement on the pulmonary inflammation, bronchial hyper-responsiveness and pulmonary histology.
Recently, TNF alpha blocking agents have already been successfully used in the treatment of chronic diseases such as Crohn and Romatoid arthritis.23 Moreover, asthma is a chronic disease and TNF alpha is believed to have a major role in the pathogenesis of asthma. Therefore, TNF alpha blocking agents would provide favourable effects on the treatment of asthma. Some experimental studies related to asthma have unveiled the beneficial effects of TNF alpha blocking agents on the airway histology, cytokine levels in bronchoalveolar lavage (BAL), and bronchial hyper-responsiveness. However, few studies conducted on patients with severe asthma have indicated increased TNF alpha activity in BAL fluid and on the surface of peripheral blood monocytes.15,24 These patients were administered Etanercept (a soluble fusion protein combining two identical chains of the human p75 TNF receptors with an Fc portion of human IgG1) for 12 weeks. At the end of the treatment session, improvement on the asthma symptoms such as bronchial hyper-responsiveness, and pulmonary function tests has been determined.
All in all, asthma is a chronic disease of the airway, and it may sometimes have a severe nature and be treatment-resistant. TNF-alpha, a cytokine associated with Th1, is known to play an important role in the pathogenesis of asthma. It is also known that TNF-alpha blocking agents have beneficial effects on the airway inflammation, lung histology, and asthma symptoms. Adalimumab (a human monoclonal anti-TNF alpha antibody) is a different TNF alpha blocking agent. In the present study, we have evaluated the effects of adalimumab (a human monoclonal anti-TNF alpha antibody) on the lung histology and lung inflammation in a murine model of asthma. The work presented in the current study shows that adalimumab treatment suppresses the airway inflammation, and provides improvement in histological findings in asthma. We may suggest that adalimumab is a promising alternative for the treatment of patients with severe asthma which is unresponsive to treatment.
Conflicts of interestNo competing financial interests exist and there is no conflicts of interest for each author.
Ethical disclosuresPatients’ data protectionConfidentiality of Data. The authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
The authors thank Tuncay Delibas¿, M.D., and the Ankara D¿¿kap¿ Hospital, Research Center, Animals Research Laboratory's workers for providing help in nursing, immunisation with OVA and sacrificing of animals.