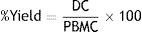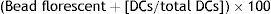Although the mechanism of asthma is not precisely understood in humans, clinical and epidemiological studies have offered a potential relationship between exposure to environmental fungi, such as Alternaria alternata (A. alternata) and the development and exacerbation of asthma. The aim of this project is to investigate the mechanisms of Th2 responses by A. alternata as a clinically relevant model for the environmental exposure.
Materials and methodsPlastic adherent monocytes were cultured with granulocyte macrophage colony stimulating factor (GM-CSF) and interleukin-4 (IL-4) to convert these cells into Monocyte-derived Dendritic cells (MoDc) and then matured in the presence of Monocyte-Conditioned Medium (MCM) as the control group and MCM+ A. alternata extract as the inductive groups.
ResultsThe results indicated that the expression of CD14 decreased and CD83 and anti-human leukocyte antigen-DR (HLA-DR) increased in the inductive groups in comparison with the control group. More importantly, A. alternata inhibited IL-12 production by activated dendritic cells (DCs), and the DCs exposed to A. alternata enhanced the Th2 polarisation of CD4+ T cells. The production amount of IL-10 overcame IL-12 as well as Il-23 increased significantly, and hand in T cells the production of cytokines Interferon-γ (IFN-γ) decreased. However, both IL-17 and IL-4 increased (p<0.05). Phagocytic activity in the inductive groups decreased significantly compared with the control group.
ConclusionThe asthma-related environmental fungus A. alternata, with an effect on dendritic cells profile mediates TH2/TH17. Such immunodysregulation properties of causative environmental fungi may explain their strong relationship with human asthma and allergic diseases.
Asthma exacerbation is a major agent of disease for the patients involved with moderate asthma. Sensitisation and exposure to the fungal allergen Alternaria alternata (A. alternata) are a risk factor for the start of severe asthma symptoms, including threatening and mortal responses.1–3 The exclusive relation between A. alternata and exacerbation of asthma is clear; but the aetiology of the exclusive pathogenesis of A. alternata has not been precisely understood. These responses are mediated by CD4-derived T-cells that are polarised to Th2 or Th17cells phenotypes.4 Dendritic cells (DCs) are one of the main cellular members regulating immune tolerance and response.5–7 DCs by producing various secretory substance or membrane ligand determine the eventuality of T cell responses, such as T-helper1 (Th1), T-helper2 (Th2), or T-regulatory (Treg) responses.8 Currently, limited data exist concerning how asthma patients develop such disturbance Th2 immune responses to environmental allergens. Overall, dendritic cell pulse for an innocuous antigen or medium is considered a tolerogenic occurrence.5,9 On the other hand, the mechanism of Th2 immune responses may reflect Th2 immune responses to parasite infection. In specific terms, asthma may offer overexpression of immune responses to chitin-containing organisms, specially mites, and fungi.10,11 However, the association of immunological mechanisms with impulsive due to chitin-containing organisms and development of Th2 immune responses are not fully understood. The aetiology of human asthma is complicated and multifactorial; probably it involves the interference between genetic factors and environmental motives. As a major environmental motive, the relationship between fungal exposure and asthma has been recognised pathologically and epidemiologically.1 In particular, there is a great deal of evidence suggesting a relationship between a ubiquitous environmental fungus A. alternata and asthma.2,12A. alternata is ubiquitous and unique both in outdoor and indoor,13 and for the high rates of spore germination and antigen propagation.14 Exposure to A. alternata is a major risk factor for asthma and allergic.15 Severe asthma and acute exacerbations of asthma have also been associated with increased airborne exposure to A. alternata spores and consequently its pollen pollution.16 In mice, the relationship between asthma and stimulation by fungal antigen, such as aspergillus and A. alternata, has been clinically and pathologically explained.1,2 The mouse models of asthma offer a wide range of experimental possibilities, but due to their limitations such as their different physiology, we decided to use other models.17 To survey the immunological mechanisms involved in Th2 responses, we used A. alternata to model environmental exposure correlated to asthma.
Materials and methodsThe objective of this study was to perceive the mechanisms engaged in asthma. The study was conducted in the cleaning room of the research centre of biotechnology at Urmia University and zoonosis researches central of Jahrom Universty of medical sciences from September 2013 to February 2014. Various tests have been used to evaluate Autologous T cell responses by co-culturing with DCs. In the meantime, the phagocytic activity of pulsed DCs by A. alternata was examined. These experiments were replicated five times.
Preparation of fungus extractA. alternata spore purchased from Iranian industrial and scientific research organisation (PTCC 5248) was cultured on Sabaroud dextrose agar at 25°C for five days. Mature fungi subculture on Czapek's agar was used to produce a large number of spores. In addition, the amount of 1×107 spores was collected by Hanks solution and passed through sterile Tampon, subsequently transported on liquid culture of Yeast nitrogen base (37°C, 5% CO2, 5% humidity) for 48h in order to improve the growth of mycelium. Then grown-up centrifuged myceliums (2000×g) were added to PBS buffer containing 2×10−3M protease inhibitor (Sigma-USA), 50×10−3M EDTA (Sigma-USA), 50×10−3 Tris–HCl and sonicated on a sonicator (20,000 AMP), 10s interval (apparatus 10s was off and 10s was on) and totally 5min duration. After sonication, the homogenised fungi were centrifuged (7000×g at 4°C), and the supernatant were dialysed by dialysing tube (cut off: 14,000) full off. The extract was dehydrated by freeze dryer (CHRIST ALPHA 1.4, UK) to reduce the volume of water.18 The resulted extract was filtered through fine pores (22μm in diameter) and protein of solution was measured by BRADFORD method.19 The final extract was considered 1mg/ml and it was stored at −70°C until use.
Preparation of Peripheral Blood Mononuclear Cell (PBMC)Heparinised blood was obtained from volunteer donors (200U/ml) in sterile conditions and mixed with the equal volume of culture medium RPMI-1640 (Gibco, UK). Diluents blood was transported gently on Ficoll-Hypaque (Sigma, USA) and centrifuged for 15min in 800×g. PBMC located between Ficoll-Hypaque and diluents blood were collected, and washed by RPMI-1640 then it was centrifuged for 10min in 480×g in order to be deleted from platelets. Cellular pellet was washed again by RPMI-1640 for 10min in 200×g. The number and viability of cells were assigned by Trypan blue.20
Production of control DCs in the presence of Monocyte-conditioned Medium (MCM) and its Inductive with A. alternata extractsMCM was prepared as described elsewhere. Briefly,20 PBMC was plated onto the human Ig-coated Petri dish for one hour, non-adherent cells were washed away and Ig-adherent cells were incubated in fresh complete medium with 1% autologous plasma at 37°C for 24h. The medium was collected and saved at −20°C until time of use. The isolation of T cells was performed using anti-CD3 magnetic bead cell sorting technique (Miltenyi Biotec, Germany). 4×106 PBMC in RPMI-1640 (6ml) were transferred to T-25 flask and after 2h the adhered cells to the T-25 flask were washed three times with RPMI-1640 and finally the adhered cells cultured with RPMI-1640 (5ml) supplemented 2% serum AB+. The preparation of control DC has been occurred at five stages; at the first stage and day 0, the adherent cells were converted to immature dendritic cells (ImDC) by using granulocyte macrophage colony stimulating factor (GM-CSF) and IL-4 (500U/ml). At the second stage (day 3), the equal amount of GM-CSF and IL-4 (Sigma, USA) was added to the culture for the maturation of DC. At the third stage (4 day) MCM was added to the DC culture. Additionally, 100μg/ml of A. alternata extracts and MCM (20%, v/v) were added to the induced group (Induced-group), but in the control group, only MCM (MCM-DC) was used. All of these stages occurred in sterile conditions and incubation performed at 37°C; 5% CO2. On day 7, DCs were harvested, and morphological changes of DC; stimulation of T cell phagocytic capability were assigned.20
Estimation of DC yield and viability from plated PBMCSeparated DCs from cell culture flasks in day 7 were submitted to the count and assessment of their viability by trypan blue exclusion test. The percentage of yield was estimated by the following formula21:
Microscopic analysisThe bottoms of the culturing flasks were observed daily by inverted microscope. The shape and size of cells as well as their composition were compared in both groups. Extended cells without projections were considered as macrophages20 and the round and unchanged cells were accounted as lymphocytes. Platelets were considered as the smallest cells with irregular surfaces.
Phenotypic analysisFor phenotypic analysis, direct immunofluorescence was used for the cell surface staining of DCs, which were stained in FACS buffer (0.2 BSA, 0.02 sodium azaid in PBS) contain 1×105 cells per staining. Staining was performed by incubation DC with FITC-conjugated mouse antibodies against CD14, CD83, anti-human leukocyte antigen-DR (HLA-DR) and the appropriate isotype-matched controls at 4°C for twenty minutes (DAKO, Denmark). Samples were analysed on FACS DAKO (Partec, Germany) using FlowMax Software.20
Cytokine production assayAfter DCs were stimulated with or without A. alternata extract, they were analysed for cytokine profile then were incubated with T cells and cytokine production of T cell was analysed the same as DCs by ELISA method. The non-adherent DCs were stimulated with or without 100μg/ml A. alternata extract for 24h. After that DCs were washed three times with Foetal Bovine Serum (FBS). 100μl of the suspended 2×105 cell/ml in RPMI 1640 supplemented 2% serum AB+ were seeded in round-bottom 96-well microplates. T cells, which were isolated from autologous human, were added to the wells at a 1:5 of DC:T cell ratio and co-cultured for 24. The concentrations of IL-12, IL-10 and IL-23 were measured from DC supernatant capture as well as IL-17, Il-4 and Interferon-γ (IFN-γ) were measured as DC-T cell co-culture profile (R&D Systems); sensitivities for IL-4, IFN-γ and IL-17 were 2, 3 and 0.5pg/ml, respectively.
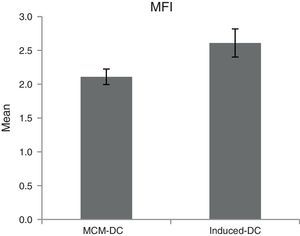
Evaluation of phagocytic activityThe phagocytic activity of DCs was computed by evaluating the uptake of FITC-conjugated latex beads (Sigma, Munich, Germany). 20μl latex bead florescent (FITC-conjugated) at concentration 2.5×105 bead per ml was incubated on human serum AB+ for seven minutes. Then, the amount of 25×105 mature dendritic cell (mDC) along with 60μl phagocytic buffer reached to the total volume 100μl in 96-well plate. DCs without beads were used as a negative control. After 48h of incubation, the cells were harvested and the extracellular fluorescence was quenched in quenching buffer. Finally, the number of engulfed latex beads in both MCM-DC and Induced-DC were assigned. Phagocytic activity was analysed in terms of percentage and mean fluorescence intensity (MFI) of phagocytic cell on DAKO flow cytometry system (Partec, Germany) and FlowMax Software by flow cytometry (DAKO, Germany). The percentage of phagocytosis was identified according to the following equation21:
All of the statistical analyses were calculated by SPSS-17 Software, and Tukey test was used for data analysis. p<0.05 was considered significant. Microsoft Excel was employed to draw diagrams. The data were presented as Mean±SEM.
ResultsYield and viabilityOur results indicated that by using MCM or A. alternata extract, either 5.56±1.45 or 6.69±1.85 percent of plated PBMCs was differentiated into DCs, respectively. The viability of MCM-DC and A. alternata extract DC (Induced-DC) were 70.66±9.8 and 91.65±7.08 percent, respectively. The differences in both yield and viability of the resultant DCs by the two methods were significant (p<0.05).
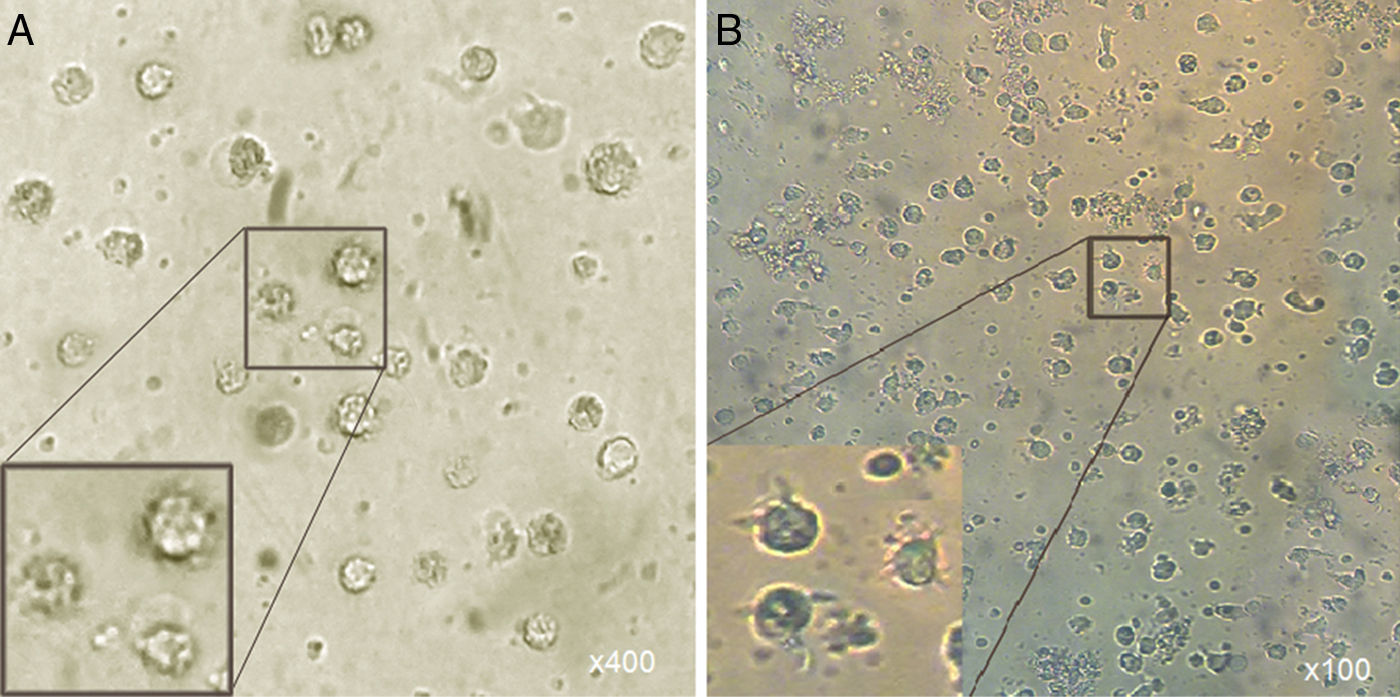
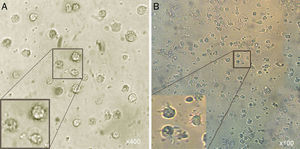
Microscopic analysisMorphological studies were conducted by inverted microscope at different magnifications and the results indicated that after five days, the adherent cells (at the present of GM-CSF, IL-4) lost their adhesion and caused the induced group of floating cells after adding A. alternata extract. These cells looked larger than monocytes containing large intracellular granules (Fig. 1).
Morphological appearance of MCM-DCs (A) and Induced-DCs (B). Analysis by inverted microscope revealed that in comparison, Induced-DC were longer and had more frills than MCM-DC. These cells (both groups) looked larger than monocytes containing large intracellular granules also were more non-adherent.
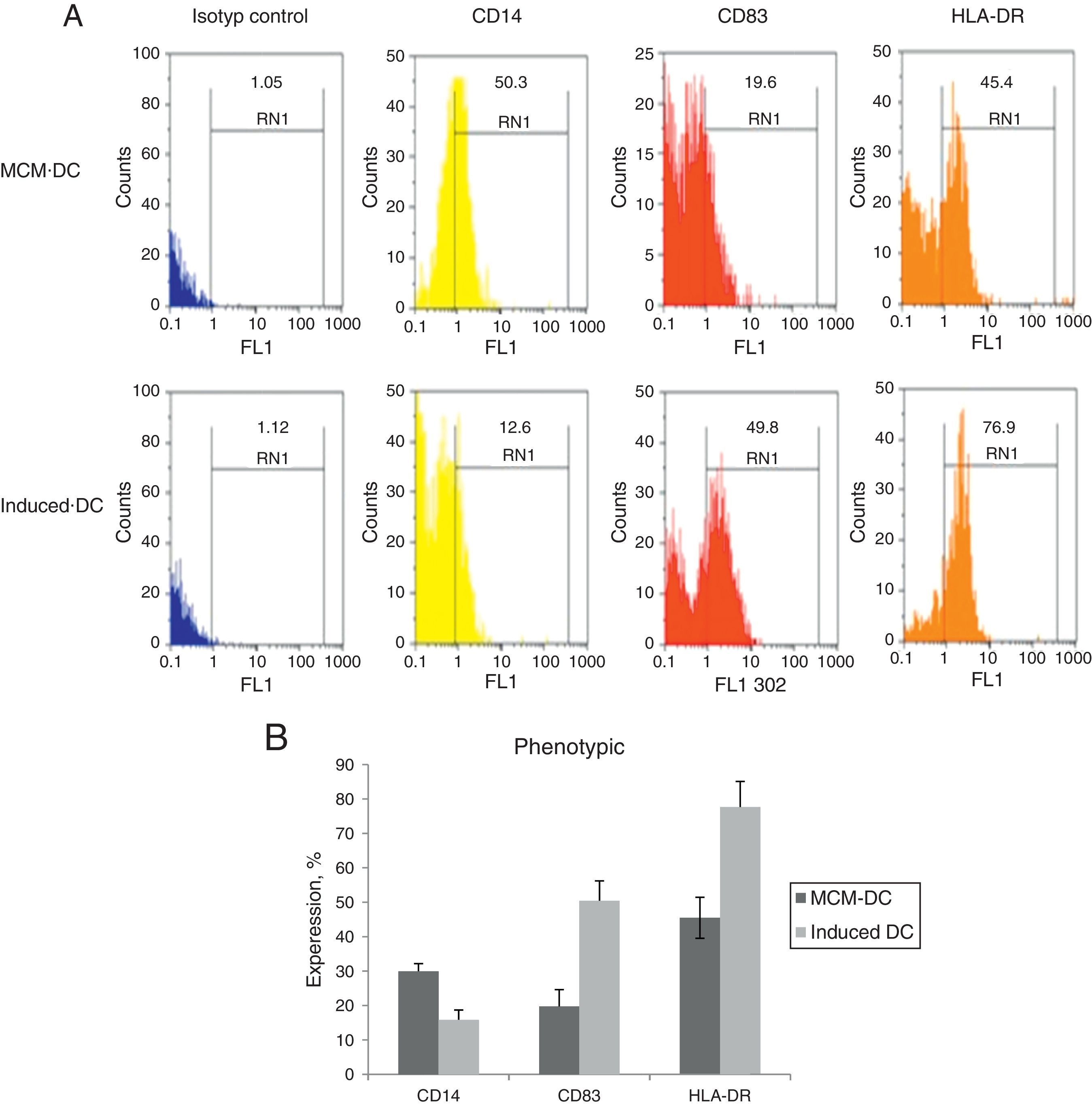
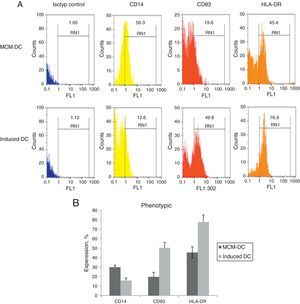
When DCs were incubated for 24h with A. alternata extract, their expression of MHC-II and costimulatory molecule including CD83, increased dramatically to the DCs which were cultured with only MCM. After 48h, A. alternata extract down-regulated the expression of CD14 that was implicating in DC maturation development. The flow cytometric analysis of DCs showed the significant increase of CD83 expressing DCs through A. alternata extract-DCs compared with MCM-DCs (19.7±3.9 vs. 50.4±4.5) (p<0.01). In addition, a higher percentage of HLA-DR were expressed by A. alternata extract-DCs (77.6±7 vs. 45.5±1.01) and lower percentage of CD14 (15.8±1.2 vs. 29.9.±4.1) were observed by MCM-DCs, with their differences being significant (p<0.05) (Fig. 2).
Immunophenotyping of DCs. (A) Representative flow cytometric histograms, obtained from MCM-DCs and Induced-DCs, stained with FITC conjugated mAb against CD14, CD83, and HLA-DR. As shown in histograms, the Induced-DCs (down) produced a higher fluorescent intensity relative to MCM-DCs (up) using the three stained markers. (B) Flow cytometric analysis showing increased CD83, HLA-DR and decreasing expression among Induced-DCs compared with MCM-DCs. Considering the data p<0.001 into CD83 and HLA-DR, as well as p<0.01 into CD14 significant differences existed between the two groups.
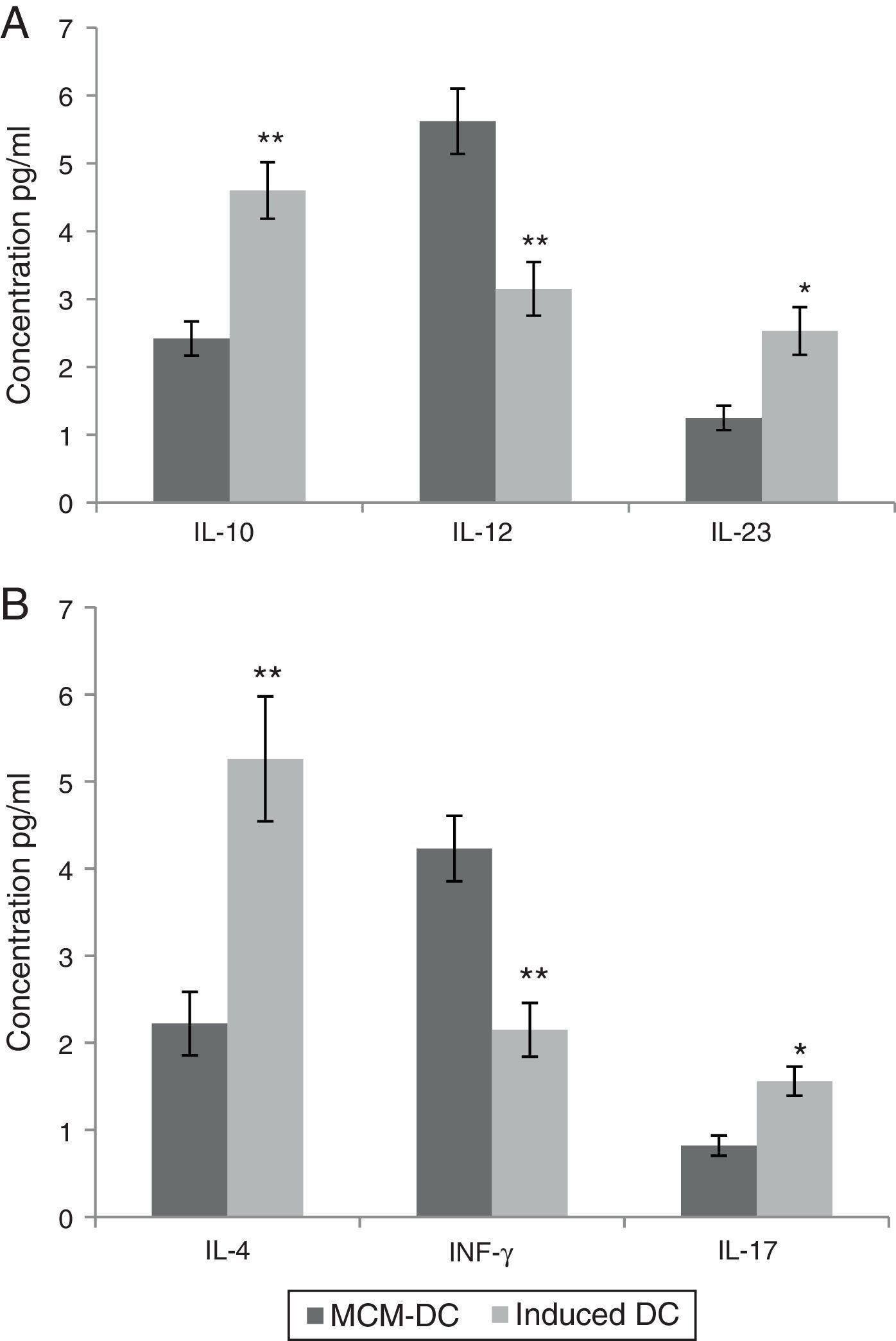
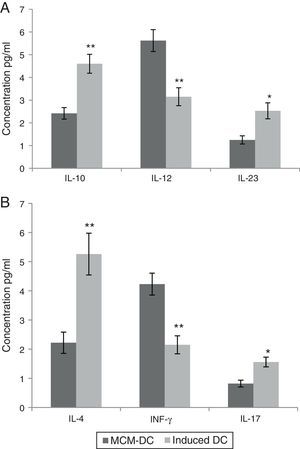
These in vitro observations suggested that DCs are activated by A. alternata extract and promoted CD4_T cell differentiation towards a Th2 type. Monocyte-derived DCs (MoDc) were stimulated with A. alternata extract or medium control, and washed, and then incubated with isogenic T cells. In this study, IL-10, IL-12 and IL-23 were evaluated as DC cytokine productions. Therefore, the DCs which had been stimulated by A. alternata were shown their IL-10 secretion dominated to IL-12 and IL-23 significantly (p<0.01). Isogenic T cells incubated with control DCs (stimulated with medium control) made IL-4, IL-17, and IFN-γ. Importantly, isogenic T cells incubated with A. alternata-stimulated DCs produced more IL-4 and IL-17 but they produced less IFN-γ. The results confirmed that the frequency of IL-4 and IL-17 producing T cells increased significantly (p<0.01), on the other hand IFN-γ producing T cells significantly decreased when those were incubated with A. alternata-stimulated DCs (p<0.05) (Fig. 3).
Both A and B show data using MCM-DCs and Induced-DCs, A show Concentrations of IL-10, IL-12 and IL-23 in the supernatant of DCs and B show IL-4, IFN-γ and IL-17 in the supernatant from the DC-T cell co-culture. The measurement was performed using commercially available ELISA kits. * and ** represent significant difference between these three tested groups p<0.05 and p<0.01 respectively
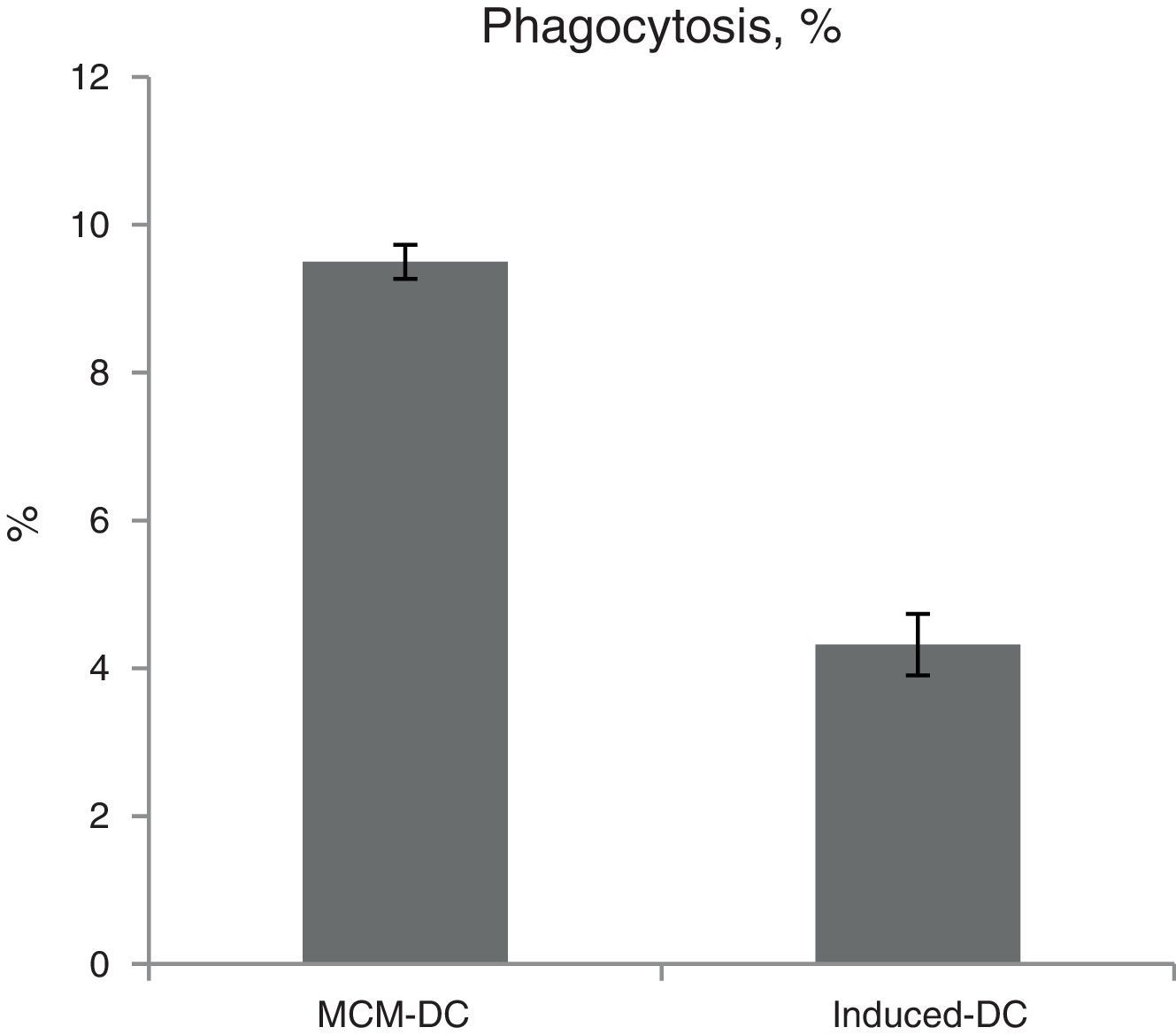
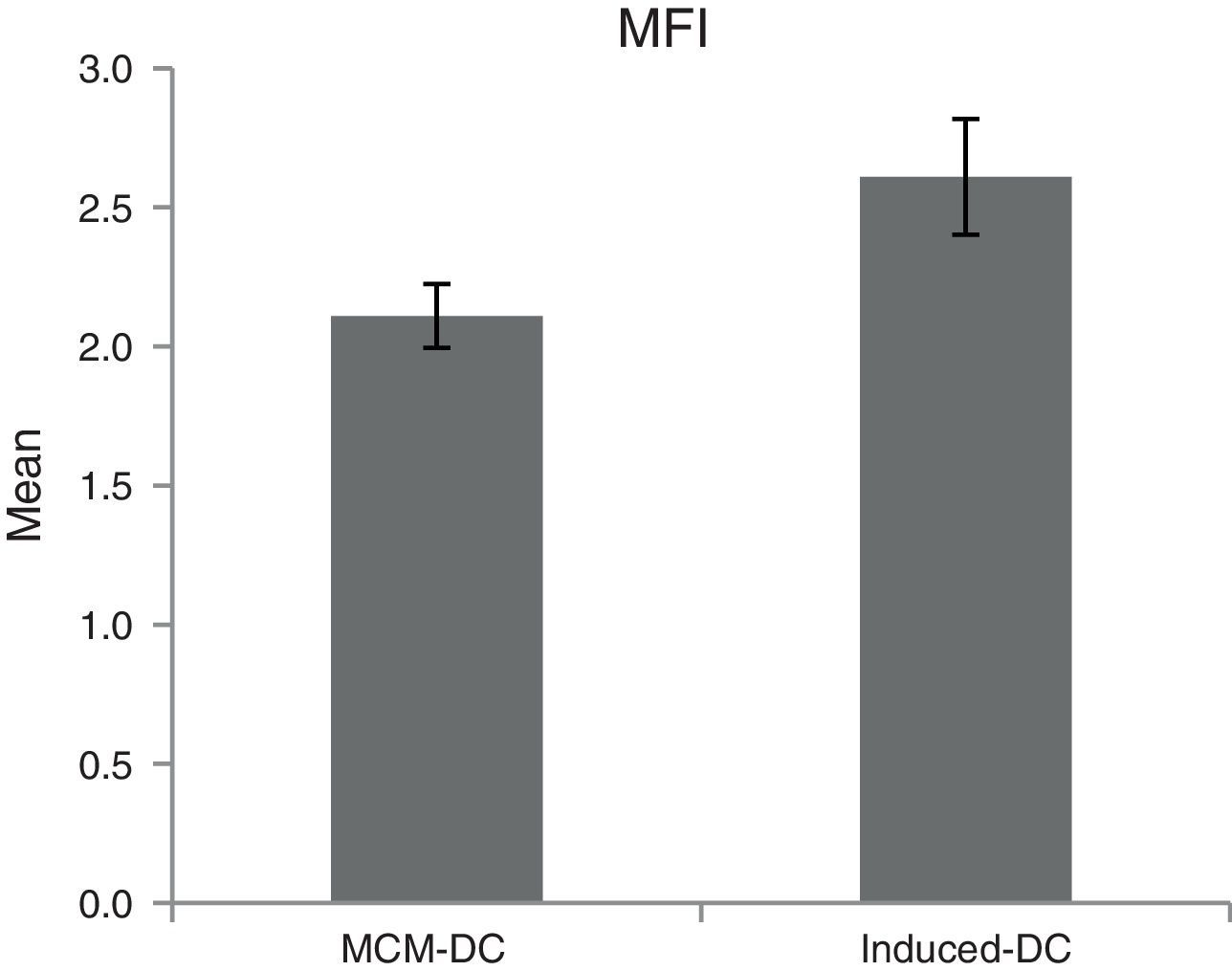
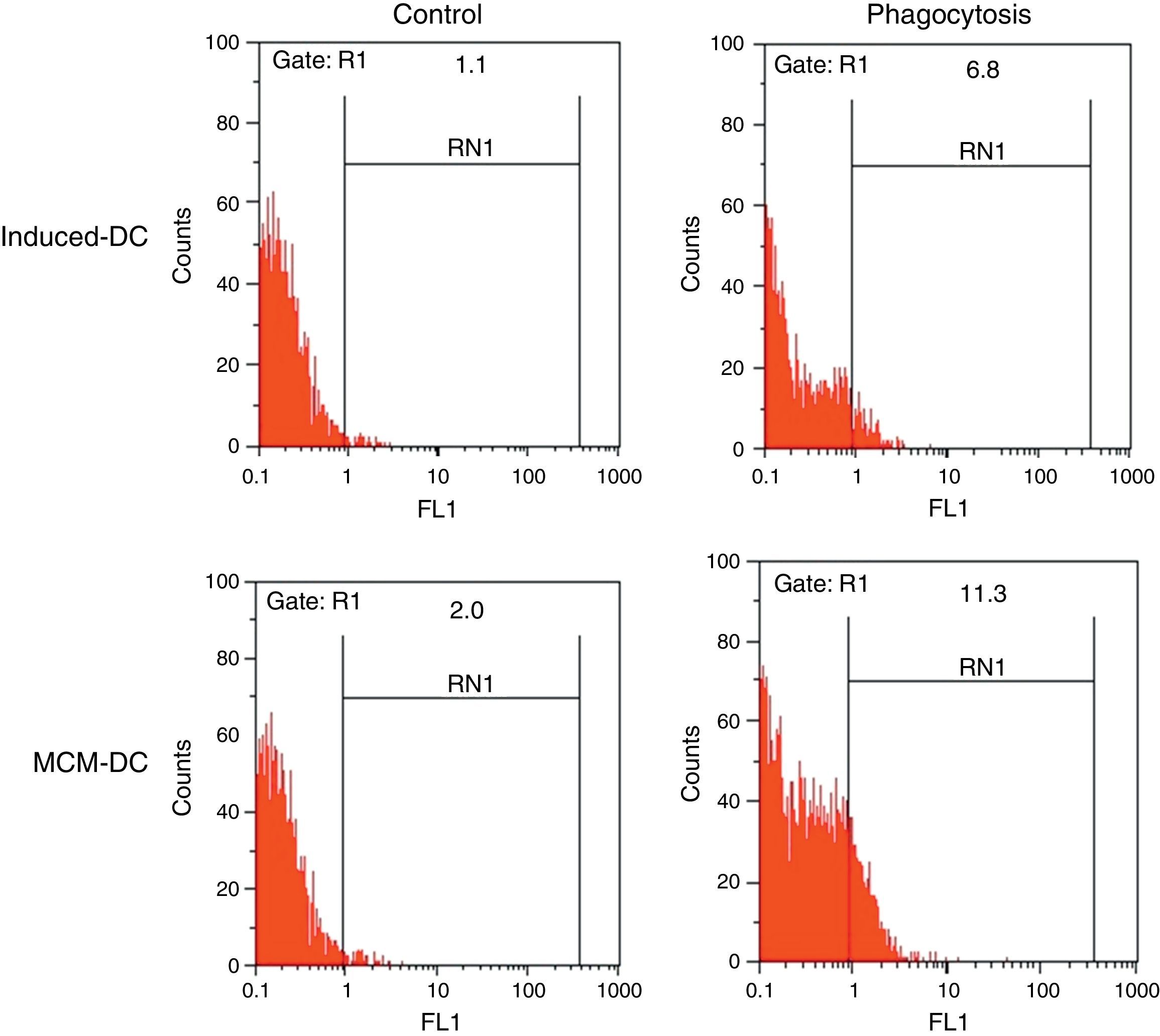
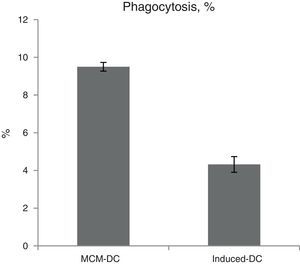
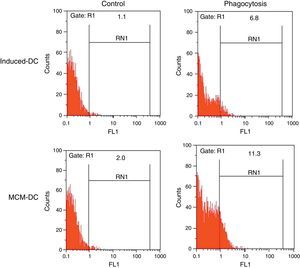
The results of phagocytic evolution have been shown in two forms: (A) the phagocytosis of thorough DCs and the percentage of phagocytosis in each DC (MFI), shown in Figs. 4 and 5, respectively. Furthermore, the data are shown graphically in Fig. 6. The results of florescence intensity and percentage of phagocytosis (shown in Fig. 4) indicated that the rate of phagocytosis in MoDc stimulated with A. alternata extract significantly decreased (p<0.01), but this percentage significantly increased in MFI (p<0.05).
The representative flow cytometric histograms obtained from phagocytic analysis of MCM-DC and Induced-DC. Both types of DCs were incubated with FITC-conjugated latex beads for 48h also DCs without beads were used as a negative control then washed with quenching buffer and subjected to flow cytometric analysis.
Although frequent studies have been conducted on the properties of dendritic activity in mice, their importance in the development of human allergic responses remains largely unknown. In this study, we have used an A. alternata-related asthma model to evaluate the importance of dendritic activities in the presence an A. alternata extract for triggering Th2 responses in vitro. MCM can perform DC maturation without specific antigen; such soluble non-pathogenic proteins, when delivered through DC, do not excite potent immune responses but instead induce a state of hypo responsive specific to medium.5 Consistent with previous reports,5 we found minimal sensitisation to MCM when DCs were exposed to only MCM. In contrast, when MCM was administered in the presence of A. alternata extracts, DC maturation and CD4-drived Th2 cytokine responses developed sturdily.22,23 Herein, when DCs were stimulated with A. alternata in vitro, we observed the following: they up-regulated their expression of MHC-II and costimulatory molecules, including CD83. Generally, when DCs show low levels of MHC-II and costimulatory molecules, they induce T cell tolerance or anergy.9 However, the A. alternata-mediated Th2 response was independent of TLR4 and TLR2.23 Now what products of A. alternata possess such strong Th2 adjuvant activity? Oliver, regarding this issue, mentions that protease from A. alternata is able to activate TLR4 and Myd88 signalling and the outcome development Th2 potent response.22,24 Whereas the IL-12 family cytokines are strong stimulator of the Th1 response thus DCs exposed to A. alternata can polarise T cell response to the Th2 type,24,25 one important condition for DCs to induce Th2 responses is likely the down-regulation of these cytokines. Several studies suggested that Th2 responses are likely resulted from the absence of IL-12 or over-existence of IL-10.21,23,26 This conclusion was conspicuous in our study. In contrast, other studies show that Th2 cell development requires ligands expression or soluble compound secretory with the mediation of DCs.23,26,27 For example, the interaction between OX40L on Antigen Presenting Cell and OX40 on T cells is a major key in the proliferation of CD4-T cell and participation of Th2 responses. One of our findings that was analogical with asthma subject showed the increase of IL-10 level in Induced-DC group, which is consistent with a previous report that there was an increased IL-10 mRNA expression in allergy and atopic asthma.28 On the other hand, it is possible that the discovery of IL-17 producing T cells has added an additional layer of complexity to the regulation of allergic inflammation. Although IL-10 was dominant in the presence of A. alternata extract, IL-17 promoted parallel one.1,29 Concerning this issue, Wang explained that a novel subset of Th2 memory/effector cells exists that co-expresses the transcription factors GATA3 and RORγt and co-produces Th17 and Th2 cytokines. This subset that is termed Classical Th2 memory/effector cells had the potential to produce IL-17 after stimulation with pro-inflammatory cytokines IL-1β, IL-6, and IL-2.30 It was also shown that chitin led to the production of IL-17A with involving TLR2 signalling through the MyD88- independent pathway.30 Recent studies have disclosed that IL-23 is an arch axis to maintain Th17 cells. Despite families and structural similarity between IL-12 and IL-23,31 it seems that IL-23, more than IL-12, plays pro-inflammatory roles in this study. On the contrary, in the previous report, we observed DCs co-pulsed with A. alternata and Myelin Basic Protein decreased IL-17.32 It is likely that DCs appear in the form of down regulatory when faced with multi-epitopes of IL-17 inducer. In our finding, observed T cells were significantly differentiated. Another notable characterisation of DC is phagocytic capabilities, so that it can be expected that turning ImDC into mDC can decrease the ability of DC to phagocytosis as well as all needs to engulf antigen such as surface receptor. In contrast, it increases the presentation of antigen, which leads to escalating T cell stimulation ability in DC.7 Furthermore, the IL-4 that abolished the release of cytokines in DC and macrophages had a minimal inhibitory effect on DC and macrophages from patients with asthma.29 According to the results, it was indicated that the rate of phagocytosis in Monocyte-derived DCs, which were stimulated with A. alternata extract was significantly decreased. DC exposure to A. alternata probably prevents tolerance to innocuous placebo as MCM and facilitates towards Th2 polarisation. Therefore, the potent biological activities of A. alternata facilitate both the sensitisation and effectors phases of immunologic Th2 responses. These activities and responses may offer the mechanism of triggering asthma and the well-perceived correlation between fungi, development and exacerbation of asthma and allergic airway diseases in humans.23,33,34 Our findings suggest that the DCs when accompanied with A. alternata antigen through interfering in cytokines or presentation and shift to the aberrant secretion of T cells pro-inflammatory cytokines such as IL-17 subsequent Th2/Th17 potent polarisation may be engaged in or exacerbate asthma. In the end, one question remained: If A. alternata is a strong Th2 response contrast with innocuous medium, why only some, but not all, individuals or animals promote allergic airway diseases? Further study of genetic analysis, individual specific immune and identification of antigens of A. alternata that interfere in the potent Th2 effects of A. alternata will show the mechanisms involved in the development of Th2 immune responses better.
Conflict of interestThe authors have no conflict of interest to declare.
FundingJahrom university of medical sciences and Urmia university.
Authors’ contributionsAll authors had equal role in design, work, statistical analysis and manuscript writing.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
This study was financed by Grant Numbers D-3-237 and K-2-422 equally from zoonosis researches central of Jahrom university of medical sciences and Urmia university respectively. The authors are grateful to Dr. M. Abtahi, Dr. Mohebalian for discussion and thank experts of immunology group Mr. Aliyari and Kzemnia for supplying material.