To measure lung function by impulse oscillometry (IOS) and spirometry in recurrent wheezer pre-schoolers according to their asthma predictive index (API) condition.
MethodsWe performed a case–control study enrolling all pre-schoolers with recurrent wheezing episodes (>3 episodes confirmed by physician) who presented at a paediatric pulmonology clinic. The population was divided according to stringent API criteria into positive or negative.
ResultsIn the nine-month period, 109 pre-schoolers were enrolled. After excluding one patient (due to lung function technique problems) 108 pre-schoolers (56 males, age range from 24 to 72 months) completed the study; 50 belong to positive API and 58 to negative API group. There were no differences in demographics between groups. More use of ICS was found in those with positive API than with negative API (62% vs. 12%, respectively, p=0.001). No differences in basal lung function and post-bronchodilator response to salbutamol (by IOS or spirometry) were found between positive and negative API pre-schoolers. However, those positive API pre-schoolers with ICS had significantly higher central basal airway resistance (RA at 20Hz) and higher post-BD response (% change in FEF25–75 and in FEV0.5) than those positive API without ICS.
ConclusionRecurrent wheezer pre-schoolers with positive API and ICS used may have airway dysfunction. More studies are needed to confirm this finding.
Wheezing is a common symptom in the first years of life, but a minority of children will continue to experience wheezing symptoms in school years and beyond.1 Based on the epidemiological data on the natural history and temporal patterns of wheezing, several childhood wheezing phenotypes have been described. However, the use of these “epidemiological” phenotypes of wheezing is limited, since they can only be identified retrospectively; indeed, they were defined using statistical inference on longitudinally collected data, and not useful in the present as they are defined by events that will occur in the future.2 Thus, it has been proposed that wheezing phenotypes be based on the trigger(s) and temporality of symptoms (such as episodic viral wheeze [EVW] and multiple-trigger wheeze [MTW]) which can be ascertained in a clinic and could be more practical for making treatment decisions.3 However, it was reported that EVW and MTW had considerable overlap4; showing conflicting results as these differ in clinical features and have limited value in predicting asthma at school age.5
In the past 15 years, the diagnosis of asthma has hinged on the ability to predict the persistence of asthma at six years.6 Several asthma predictive rules have emerged. The Asthma Predictive Index (API)7 used clinical parameters (paternal asthma, rhinitis, dermatitis, wheezing without cold and eosinophils in peripheral blood) and originally developed in the Tucson cohort study, is the most widely used. The API is simple and cheap, and its major strength is its good positive likelihood ratio ∼7.4 (the post-test probability of disease can improve from 2 to 7 times) and high specificity (∼97%).8
Children with persistent wheeze, frequent asthma exacerbations, and multiple early atopy have diminished lung function (measured by specific airway resistance [sRaw]) throughout childhood, and are at risk of a progressive loss of lung function from age 3 to 11 years; these effects are more marked in boys.9 One study from the Tucson cohort suggested that for persistent wheezers the deficit in lung function is not present soon after birth (lung function measure at age four weeks by chest compression technique [RCT]), but is acquired by age six years (measured by spirometry).1
However, few studies on lung function and bronchodilator response were performed in children with API characteristics. A study on young Spanish children with a positive API had significant lower lung function (measured by RCT) than those with negative API.10 A trial in recurrent wheeze pre-schoolers with positive API or a positive screening test for atopy treated with inhaled corticosteroids (ICS) for 24 weeks modestly reduces wheeze exacerbation rates and improves lung function (measured by spirometry).11 A previous trial showed that ICS for 24 months improves lung function, but the effect disappears after the treatment is discontinued.12 However, no group of pre-schoolers with negative API was involved in those trials.
The objective of this case–control study is to compare the lung function (measured by impulse oscillometry [IOS] and spirometry) and post-bronchodilator (post-BD) response to salbutamol among pre-schoolers with positive and negative API.
MethodsWe prospectively enrolled all the recurrent wheezing pre-schoolers attended at the paediatric pulmonology clinic at Hospital Almirante Nef, Viña del Mar, Chile, during a nine-month period (May 2012 to February 2013). In accordance with international guidelines, their paediatricians treated each child with ICS (fluticasone propionate 125mcg bid or budesonide 200mcg bid by metered dose inhaler and spacer with face mask) or montelukast (4mg/d).13 The study was approved by the local ethics committee and parents gave written informed consent. Inclusion criteria: aged two to six years of age with recurrent wheezing, defined as having three or more wheezing episodes (confirmed by paediatrician) in the previous year; received ICS or montelukast for at least six months; and correctly performed IOS and spirometry according to ATS/ERS recommendation.14 Exclusion criteria: cardiac or other chronic respiratory diseases (e.g. cystic fibrosis, bronchopulmonary dysplasia, post-infectious bronchiolitis obliterans, ciliary dyskinesia), airway malformations, endocrinological and neurological diseases, prematurity (<37 weeks), low weight for gestational age, undernourishment, parental or guardian failure to sign the informed consent form.
A predefined questionnaire with demographic data and peripheral blood sample (during asymptomatic period) were performed on the children. According to the presence of stringent API6 we divided the population into two groups: positive API if they had one major or two minor criteria, or negative API if they did not fulfil those criteria.
Pulmonary lung function was performed only if the child was free of respiratory symptoms in the previous four weeks and following this protocol: first basal IOS, then basal spirometry, and finally post-BD measurements by IOS and spirometry after 15min of using two puffs (100mcg/puff) of salbutamol (Fesema®, GSK, Aranda de Duero, Spain) administered with three minutes of separation in between puffs and using a spacer with face mask (AeroChamber Plus® Flow-Vu®, Monaghan Medical Corp., USA). ICS treatment was not discontinued at the moment of lung function measurement. Weight and height were measured before lung function.
The IOS was done by one paediatric lung function technician (SMO). For all subjects, three replicate measurements of impedance respiratory (Zrs) were obtained using the system software (MasterScreen-IOS, Jaeger® Co, Germany). Impedance measurements were retained for analysis if reproducible, that is, if the coefficient of variation between replicates measurements was <10%. Briefly, the sitting child was asked to breathe for 15–20s using a rigid oval mouthpiece with a tongue guard, with the head in a neutral position, nose clip in place, and while supporting both cheeks. For the respiratory system, resistance represents the effective resistance of lungs and chest wall, whereas reactance is the net effect of the two opposite (one compliant and one inertial) components. Each recording on the MasterScreen IOS assessment yielded both the expiratory resistance (R5 and R20) and reactance (X5) [kPa/l/s] at different oscillatory frequencies between 5Hz and 35Hz within the flow range of normal tidal breathing. R5 measure the total resistance (central and peripheral), R20 the central resistance and X5 the peripheral reactance. In addition, the resonant frequency (Fres) [i.e. the frequency at which the reactance was zero] was also computed.
The spirometry (Jaeger Master-Screen®) was also performed by SMO. None of the participants had performed a spirometry before; each child did all possible expiratory force curves over a 15-minute period, with a nasal clip (unless it was not tolerated) and standing. Sometimes, according to operator criteria, animation programmes included in the software (e.g. candles, balloons) were used. We considered as acceptable spirometric curves those with evident peak expiratory forced (PEF), without abrupt end of flow >10% of PEF, with expiratory time >0.5s and without evidence of cough or glottis closure.14 For each test, we recorded time-volume and flow-volume curves and tabulated the real expiratory time of the curve with the best forced vital capacity (FVC). Moreover, to calculate the reproducibility, variation coefficient, and coefficient of repeatability, we registered the three best basal forced expiratory volume (FEV)0.5, FEV1, flow expiratory forced (FEF)25–75, FEF25, FEF50 and FEF75 values for each patient. Since no local spirometry reference values exist for pre-schoolers, we used values from the Spanish population.15
Statistical analysisWe compared children in the positive and negative API groups. For categorical variables, contingency tables were used (chi2 with Fisher exact test). For continuous variables with normal distribution, an average comparison and analysis of variance (ANOVA) were used; and Wilcoxon test was used in other cases. For lung function variables between groups, a Mann–Whitney was performed; and Pearson correlation was used for comparing the post-BD response to salbutamol. Statistically significant differences were considered for a p value <0.05. All statistical analyses were done with STATA® software v.11.0. To ensure that the bronchodilator response was a clinically significant change and was not due to technique variability, we considered it above the coefficient of repeatability.14
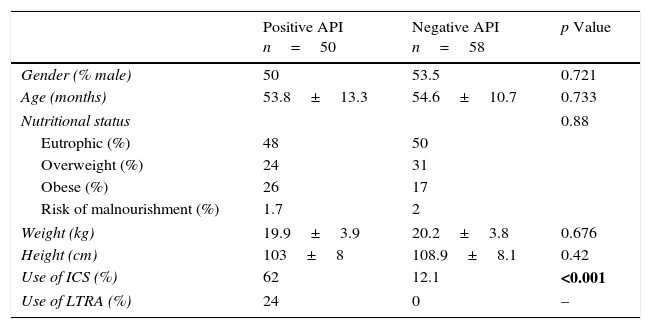
ResultsIn the nine-month period, we enrolled 109 pre-schoolers. One patient (belonging to the positive API) was excluded because of being unable to correctly perform lung function (post-BD IOS), therefore 108 pre-schoolers (56 males, age range from 24 to 72 months) completed the study; 50 belong to positive API and 58 to negative API group. There were no differences in demographic parameters (e.g. age, gender, nutritional status, weight and height) between groups (Table 1). There was more prevalence of ICS used in the pre-schoolers with positive API than negative API (62% vs. 12%, respectively, p=0.001). No difference in ICS type and daily doses was found between groups (data not shown).
Demographic characteristics between pre-schoolers with positive and negative API.
| Positive API n=50 | Negative API n=58 | p Value | |
|---|---|---|---|
| Gender (% male) | 50 | 53.5 | 0.721 |
| Age (months) | 53.8±13.3 | 54.6±10.7 | 0.733 |
| Nutritional status | 0.88 | ||
| Eutrophic (%) | 48 | 50 | |
| Overweight (%) | 24 | 31 | |
| Obese (%) | 26 | 17 | |
| Risk of malnourishment (%) | 1.7 | 2 | |
| Weight (kg) | 19.9±3.9 | 20.2±3.8 | 0.676 |
| Height (cm) | 103±8 | 108.9±8.1 | 0.42 |
| Use of ICS (%) | 62 | 12.1 | <0.001 |
| Use of LTRA (%) | 24 | 0 | – |
Numbers are expressed in % or median±SD.
API, asthma predictive index; ICS, inhaled corticosteroids; LTRA, leukotriene antagonist.
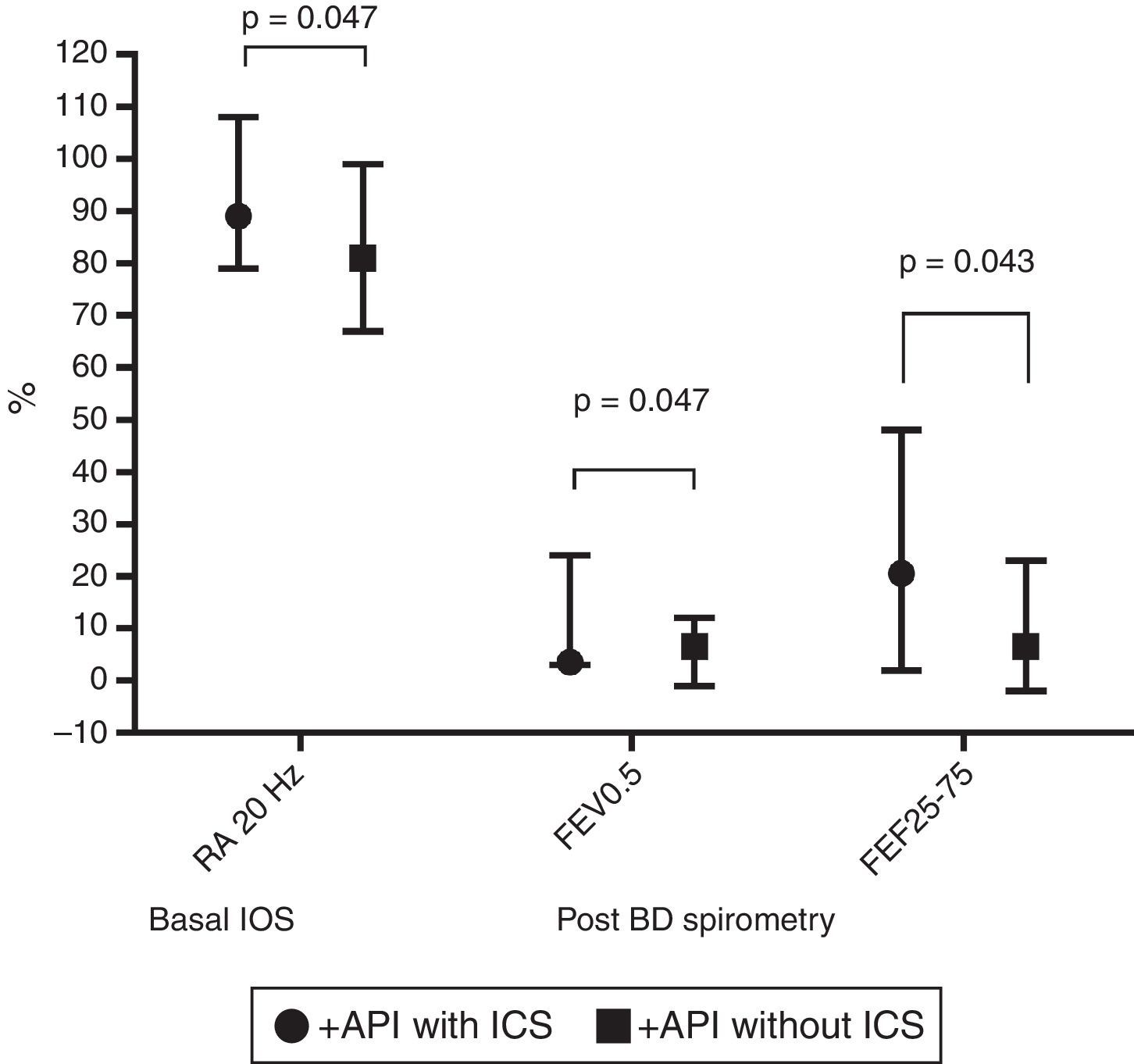
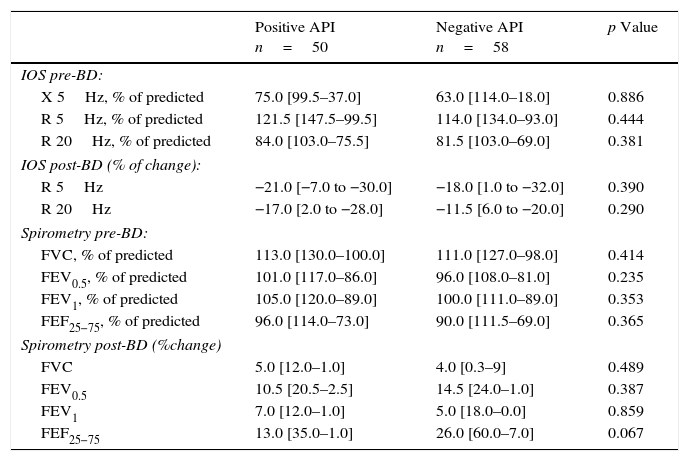
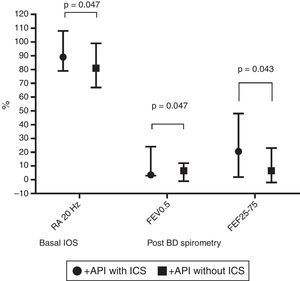
In terms of lung function (% of predicted), no significant difference between positive and negative API pre-schoolers was found in the basal or post-BD response in all the parameters in neither the IOS nor the spirometry (Table 2). In order to see if the effect of ICS therapy can modify the lung function, we did a comparison in pre-schoolers with positive API between those who used ICS (n=31) vs. did not use ICS (n=19). The only significant parameters between positive API with ICS vs. positive API without ICS were: basal resistance at 20Hz (median [interquartile range]) 89% [79–108] vs. 81% [67–99], respectively, p=0.047), post-BD change FEF25–75 (20.5% [2–48] vs. 6.5% [−2 to 23]), respectively, p=0.043), and post-BD change FEV0.5 (3.5% ([3–24] vs. 6.5% [−1 to 12], respectively, p=0.047), Figure 1. No significant differences were observed in IOS or spirometry parameters between pre-schoolers with negative API who used ICS (n=7) vs. did not use ICS (n=51) (data not shown).
Lung function (by IOS and spirometry) between pre-schoolers with positive and negative API.
| Positive API n=50 | Negative API n=58 | p Value | |
|---|---|---|---|
| IOS pre-BD: | |||
| X 5Hz, % of predicted | 75.0 [99.5–37.0] | 63.0 [114.0–18.0] | 0.886 |
| R 5Hz, % of predicted | 121.5 [147.5–99.5] | 114.0 [134.0–93.0] | 0.444 |
| R 20Hz, % of predicted | 84.0 [103.0–75.5] | 81.5 [103.0–69.0] | 0.381 |
| IOS post-BD (% of change): | |||
| R 5Hz | −21.0 [−7.0 to −30.0] | −18.0 [1.0 to −32.0] | 0.390 |
| R 20Hz | −17.0 [2.0 to −28.0] | −11.5 [6.0 to −20.0] | 0.290 |
| Spirometry pre-BD: | |||
| FVC, % of predicted | 113.0 [130.0–100.0] | 111.0 [127.0–98.0] | 0.414 |
| FEV0.5, % of predicted | 101.0 [117.0–86.0] | 96.0 [108.0–81.0] | 0.235 |
| FEV1, % of predicted | 105.0 [120.0–89.0] | 100.0 [111.0–89.0] | 0.353 |
| FEF25−75, % of predicted | 96.0 [114.0–73.0] | 90.0 [111.5–69.0] | 0.365 |
| Spirometry post-BD (%change) | |||
| FVC | 5.0 [12.0–1.0] | 4.0 [0.3–9] | 0.489 |
| FEV0.5 | 10.5 [20.5–2.5] | 14.5 [24.0–1.0] | 0.387 |
| FEV1 | 7.0 [12.0–1.0] | 5.0 [18.0–0.0] | 0.859 |
| FEF25−75 | 13.0 [35.0–1.0] | 26.0 [60.0–7.0] | 0.067 |
Numbers are expressed in median [interquartile range].
BD, bronchodilator; FEF, mid forced expiratory force; FEV, forced expiratory volume in 0.5s or 1s; FVC, forced vital capacity; R 5Hz, resistance at 5Hz; R 20Hz, resistance at 20Hz; X 5Hz, reactance at 5Hz.
Comparison in basal central airway resistance (R20) by IOS, and post BD changes in FEV0.5 and FEF25–75 by spirometry, between positive API with and without ICS. Footnote: API, asthma predictive index; BD, bronchodilator; FEF, mid forced expiratory force; FEV0.5, forced expiratory volume in 0.5s; ICS, inhaled corticosteroids; IOS, impulse oscillometry.
This case–control study shows that recurrent wheeze pre-schoolers with positive API had similar lung function (measured by IOS and spirometry) to those with negative API. However, those positive API pre-schoolers with ICS had significantly higher basal central airway resistance at 20Hz, and higher post-BD response in flow (% change in FEF25–75 and in FEV0.5) than those positive API without ICS, suggesting that a kind of airway dysfunction occurred in the former.
To our knowledge, only two other studies have measured lung function in pre-schoolers according to API condition. Borrego et al.16 using tidal and raised volume RTC in 50 recurrent wheezer pre-schoolers (aged 8–20 months) prior to receiving ICS or montelukast found that compared with healthy controls, those pre-schoolers with positive API (n=17) or negative API (n=33) had significantly lower FEV0.5, FEF75 and FEF25–75. After controlling for confounders, FVC z-score was significantly reduced in the positive API vs. negative API by 45ml [−89 to −2ml]; whereas, despite a trend towards lower values among positive API, there were no significant differences in FEV0.5 or FEF25–75. Later, Keklikian et al.10 measured lung function by using RCT in recurrent wheezer infants (11.9±4.9 months of age). Then, they adjusted by covarirantes and found that infants with positive API (n=51) had significant lower VmaxFRC z-score than those with negative API (n=41) (2.01±0.79 vs. 1.64±0.77, p=0.026, respectively).
We can speculate that in the present study pre-schoolers positive API with ICS have higher central airway resistance (increased RA at 20Hz) and higher BD response (in FEV0.5 and FEF25–75) than positive API without ICS because the former have more severe disease that make their paediatricians more prone to prescribe ICSs. However, unfortunately we do not have information about severity of recurrent wheezing in our population. Nevertheless, in general those pre-schoolers with ICS had a higher proportion of peripheral eosinophils >4% than those without ICS (data not shown). It is well known that high peripheral eosinophils is a marker for asthma severity in children and adult population.17
In a post hoc study on pre-schoolers aged four,18 atopic pre-schoolers with asthma had significantly more BD response to albuterol in the IOS parameters (RA at 5Hz, RA at 10Hz, and reactance at 10Hz) than atopic children without asthma; but no difference was seen in the spirometry. Among non-atopic children, there were no significant differences between asthmatic and non-asthmatic children at baseline or post-BD response measured by IOS nor spirometry.18 However, a study on 325 children (four years of age) showed that those children with persistent wheeze phenotype had significant larger baseline resistance at 4Hz (measured by force oscillation technique) than children with early transient wheeze, and the decrease in post-BD resistance at 4Hz was significantly larger than the children who never had wheeze and those with early transient wheeze.19
Data from the Tucson birth cohort showed that children with transient early and persistent wheezers had significantly lower FEF25–75, FEV1 and FEV:FEV1 ratio through age 16 years compared with never wheezers. Levels of lung function are established by age six years and do not appear to change significantly by age 16 years.1,20 However, in the COPSAC birth cohort, children developing asthma by age seven had a lung function deficit (FEF50) and increased bronchial responsiveness to methacholine as neonates; regardless of their atopic condition.21 Recently, the MAAS birth cohort9 demonstrated that persistent wheezers (but not transient and late-onset phenotypes) and multiple early atopy (but not dust mite, non-dust mite, multiple late phenotypes) have established airway dysfunction as early as three years of age; possibly because it was at least partly present at birth.21,22 However, since measures of sRaw after BD are not reported in the MAAS birth cohort,9 it is not known whether the observed increase in sRaw is due to increased airway tone or to an effect of remodelling on airway growth.
Low lung function at birth could lead to increased risk for wheeze, as suggested by both the transient and persistent wheeze groups.1,21 In the absence of early environmental sensitisation, the wheezing stops during school age in the transient non-atopic wheeze group.23 In contrast, early allergic airway inflammation exacerbation during viral infections may lead to remodelling, which then impairs airway growth with long-term increase in airway resistance and reduced FEF.24 Recently, a study in severe recurrent wheezer pre-schoolers showed that markers of remodelling and inflammation are unrelated, but atopy is associated with airway smooth muscle.25 In contrast, Saglani et al.26 reported reticular basement membrane (RBM) thickness in children with confirmed wheezers (median age 29 months) and controls, without difference in atopy characteristic. However, Berankova et al.,27 reported a significant difference in the thickness of the RBM between pre-schoolers (median age 13.5 months) with major criteria of the API (parental asthma or atopic eczema) before any wheezing episode occurred vs. controls.
A non-invasive common way to determine eosinophil airway inflammation is by exhaled nitric oxide (FeNO). Cross-sectional worldwide studies demonstrate that recurrent wheezer infant28 or pre-schoolers29,30 with positive API have significant higher FeNO than those with negative API or healthy controls. A recent report from the ALSPAC birth cohort31 showed that compared to never/infrequent wheeze during the first seven years of life, FeNO (assessed at 14–15 years) was significantly higher in intermediate-onset, late-onset and persistent wheezer phenotypes, and lower FEV1:FVC ratio and FEF25–75 (measured by spirometry at 14–15 years) were seen in those with intermediate-onset and persistent wheezing. Recently, the CAPS birth cohort32 using a latent transition model that can allow to see changes or transition of wheezing phenotype over time (from 1.5 years to 11.5 years) found that transition between phenotypes was common in early childhood, but less common in later childhood. The atopic asthma phenotype was much more stable over time (over 80%) and non-atopic asthma phenotype was less stable.
This study has limitations. First, no lung function before recurrent wheezing episodes or controller therapy was done. Second, no information about other factors that could influence lung function (e.g. secondhand-tobacco exposure or hospitalisation due to wheezing episodes) was available. Third, no exact data on duration of controller therapy was recorded; yet all of the patients were treated with ICS for at least six months. However, all except one of the enrolment pre-schoolers finished the study and correctly performed the spirometry/IOS tests according to international guidelines.
In conclusion, recurrent wheezers pre-schoolers positive API with ICS had significantly higher basal central airway resistance (at 20Hz), and higher response post-BD (% change in FEF25–75 and in FEV0.5) than positive API without ICS, suggesting a kind of airway dysfunction. More studies are needed to confirm these findings.
Conflicts of interestThe authors declare that there are no conflicts of interest.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
We thank Mr. Anthony Carlson for his editorial assistance.