Increased arginase activity in the airways induces reduced bioavailability of l-arginine and cause deficiency of bronchodilatating and anti-inflammatory nitric oxide (NO). Therefore, arginine and arginase inhibitors may have therapeutic potential in the treatment of asthma. Using a murine model of asthma, we aimed to investigate the effects of inhaled l-arginine and arginase inhibitor Nω-hydroxy-nor-l-arginine (nor-NOHA) and co-treatment on airway histology of asthmatic lung tissue.
MethodsForty-two BALB/c mice were divided into six groups: I (control), II (placebo), III, IV, V and VI. All mice except for control group were sensitised by an intraperitoneal injection of ovalbumin with alum adjuvant and then challenged with an aerosol of ovalbumin on three days of the week for eight weeks beginning from the 21st day of the study. Lung histology and bronchoalveolar lavage cell (BAL) counts were evaluated after treatment with inhaled l-arginine, nor-NOHA, l-arginine-nor-NOHA combination, budesonide and placebo. Interleukin(IL)-4 and IL-5 levels are determined in lung homogenates with ELISA.
Resultsl-Arginine group was similar to budesonide group in lowering all histological parameters. Results of groups treated with nor-NOHA were also similar to budesonide group except for epithelial thickness. The number of eosinophils in BAL decreased significantly in groups receiving study drugs. Decrease was only noted in IL-4 levels in group receiving nor-NOHA.
ConclusionWe demonstrated that inhaled l-arginine administration alleviated all histological parameters similar to budesonide and treatment with arginase inhibitor improved not all but some of the pathological changes in chronic asthma. Combination therapy had no additive effect on either treatment.
Bronchial asthma is characterised by airway hyperresponsiveness, remodelling and inflammation. Lung inflammation in allergic asthma is mediated by a cascade of reactions involving various mediators including nitric oxide (NO). Nitric oxide is thought to have an important role in smooth muscle relaxation, bronchodilation and neurotransmission besides participating in inflammation.1 Nitric oxide is formed by the metabolism of l-arginine by nitric oxide synthase (NOS). Three NOS mammalian isoenzymes have been identified: NOS-1, NOS-2 and NOS-3. Neuronal NOS or NOS-1 and endothelial NOS (NOS-3, eNOS) are expressed in airway epithelium and involved in neurotransmission and smooth muscle relaxation and bronchodilation, respectively. Their activity is regulated by calcium and they produce small amount of NO. Inducible NOS (NOS 2, iNOS) is also expressed in airway epithelium but regulated by pro-inflammatory stimuli with the ability of producing large amounts of NO over hours.2 Deficiency of eNOS derived NO is involved in inflammatory response during asthmatic reaction. Thus it has been shown that e-NOS derived NO inhibits airway inflammation by suppressing expression of inflammatory cytokines and iNOS.4 In inflamed asthmatic airways, expression of iNOS is increased in airway epithelium by cytokines like TNF-α and IFN-γ and levels of exhaled nitric oxide are increased in patients with persistent asthma.3 iNOS-derived NO is shown to be associated with epithelial damage, infiltration of inflammatory cells, stimulation of TH2-mediated response, mucus hypersecretion and vascular permeability but it is suggested that formation of peroxynitrite from iNOS-derived NO contributes to airway hyperresponsiveness rather than NO itself.1,2,5 It is thought that increased formation of peroxynitrite is the result of decreased bioavailability of l-arginine to NOS. Increasing the l-arginine concentration in macrophages is found to stimulate NO production and inhibit the formation of peroxynitrite.6,7
l-Arginine is metabolised by arginase which has also gained an important role in regulating endogenous NO production. Arginase is an urea cycle enzyme that hydrolyses l-arginine to urea and l-ornithine. Two isoforms of arginase (arginase I and II) are expressed in airways and arginase reduces the bioavailability of l-arginine for NOS and therefore affects NO production. l-Ornithine is also involved in synthesis of polyamines and l-proline which are involved in promotion of cell growth and repair and collagen synthesis, respectively. Experimental models of asthma have demonstrated that arginase activity is induced following challenge with allergens.8,9 Studies in human asthma also confirm the importance of arginase in the pathogenesis of experimental asthma.10,11 The lung function of severe asthmatics correlates directly with l-arginine bioavailability and inversely with serum arginase activity, indicating that serum arginase activity reduces circulating l-arginine levels which contribute to NO deficiency within airways.11
Increased understanding of the role of arginase in the pathogenesis of asthma represents a drug target that opens novel therapeutic perspectives for the treatment of asthma. In this study we determined the effects of l-arginine, arginase inhibitor Nω-hydroxy-nor-l-arginine (nor-NOHA) and co-treatment of these on airway inflammation in a murine model of asthma.
Materials and methodsAnimalsForty-two male, conventionally raised, 6–8-week-old, BALB/c mice weighing 18–20g were used in the experiment. The animals were fed a commercial diet ad libitum and housed in an air-conditioned facility on a 12-h light/12-h dark cycle. The studies adhered to the National Institutes of Health guidelines for the experimental use of animals. The Institutional Animal Care and Use Committee granted approval for the study.
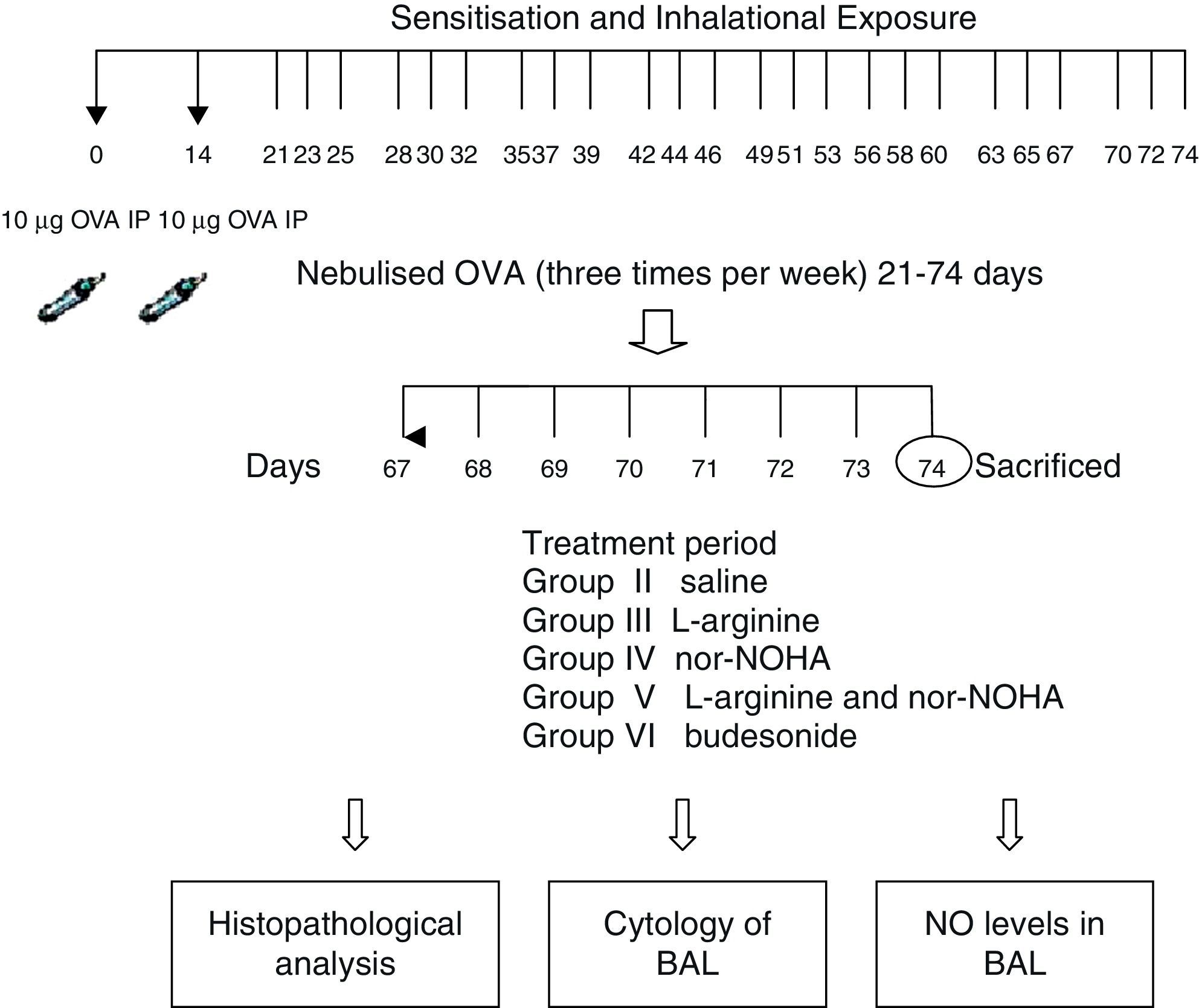
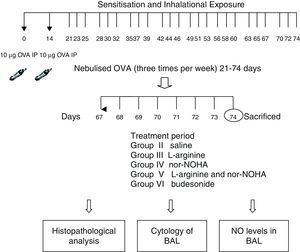
Experimental protocolMice were sensitised by an intraperitoneal (ip) injection of ovalbumin (10μg/0.1ml, 2 weeks apart, i.e. on Day 0 and Day 14) consisting of chicken egg albumin (ovalbumin, grade V, ≥98 pure, Sigma, St. Louis, MO, USA) with alum adjuvant as described by Temelkovski et al.12 Mice in study groups II, III, IV, V and VI were then challenged with an aerosol of 5ml 2.5% ovalbumin (OVA) in saline for 30min per day on 3 days of the week for 8 weeks beginning from the 21st day of the study. The mice in the control group (Group I) received normal saline with alum intraperitoneally on Days 0 and 14 of the experiment and aerosolised saline without alum for 30min per day on 3 days of the week for 8 weeks beginning from the 21st day of the study. Exposures were carried out in a whole body inhalation exposure system in a plexiglass chamber with 40×60×120 diameters designed for the placement of cages. Temperature and relative humidity were maintained at 20–25°C and 40–60%, respectively. A solution of 2.5% ovalbumin in normal saline was aerosolised by delivery of compressed air to a sidestream jet nebuliser with a flow rate of 6L/min (Medicair, UK) and injected into a chamber. The aerosol generated by this nebuliser comprised >80% particles with a diameter of <4μm. Particle concentration was maintained in the range of 10–20mg/mm3 in the chamber.13
Study drugsMice in Group III received nebulised l-arginine 1M (174mg/ml),14 Group IV received nebulised nor-NOHA 20μM (3.5μg/ml),15 Group V received both l-arginine and nor-NOHA, Group VI received nebulised budesonide 0.5mg/2ml,16, and Group II received nebulised saline once a day for the last 7 days of the challenge period. l-Arginine was obtained from Sigma–Aldrich (St. Louis, MO, USA) and nor-NOHA from Cayman Chemical (Ann Arbor, MI, USA). Experimental protocol and study drugs are presented in Fig. 1.
Preparations of lung homogenatesAnimals were sacrificed by an overdose of ketamine 24h after the last drug administration. Lungs were removed and washed with cold PBS three times in order to remove blood. Lung tissues were taken into 2ml microcentrifuge tubes and stored at −80°C until analysis. On the study day, frozen lungs were thawed, weighed (60–80mg), transferred into different tubes on ice containing 5ml of stainless beads, 0.1% SDS, protease inhibitor cocktail (Sigma–Aldrich, St. Louis, MO, USA) and 0.1mg/ml phenylmethanesulfonyl fluoride (PMSF) in PBS. Microcentrifuge tubes were transferred to pre-cooled Tissuelyser LT racks and placed into TissueLyser (Qiagen, Germany) homogenisator. Frequency and time were set to 50 and 5min, respectively. The homogenates were then centrifuged at 15,000×g for 1h at 4°C. The supernatants were stored at −80°C.
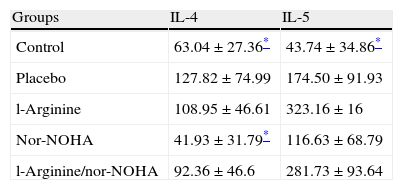
Measurement of cytokinesLevels of IL-4 and IL-5 were quantified in the supernatants of the lung tissue by standard ELISA protocols using commercial mouse IL-4 and IL-5 (Bender MedSystems, MedSystems Diagnostics GmbH, Vienna, Austria). Detection levels were 2.0pg/ml for IL-4 and 3.3pg/ml for IL-5.
Measurement of l-arginine in lung tissue supernatantsL-Arginine content of lung homogenates was assessed by the method described by Frank et al.17 In brief, airway homogenate aliquots of 100μl were added to 5% HClO4 and centrifuged at 15,000×g for 15min. 100μl of supernatant was mixed with the equal amount of OPA and after 30min, 10μl was analysed with HPLC.
Bronchoalveolar lavageBronchoalveolar lavage fluid (BAL) was immediately collected from euthanised mice by instillation and recovery of 1ml 0.99% NaCl through the tracheal cannula. BAL was centrifuged at 3000×g for 10min and supernatant was removed and stored at −80°C until assay of NO. Histological smear samples were prepared. A differential cell count was performed using May-Grünwald Giemsa staining and classified as eosinophils, neutrophils, and lymphocytes on the basis of morphological criteria and staining characteristics. Differential cell counts were performed in a blinded fashion by counting at least 200 cells per slide under light microscopy.
Determination of NONO levels in BAL samples were measured by a Griess reaction.18 The colorimetric kit (Sigma–Aldrich Co., USA) was used according to the manufacturer's manual. Results are determined spectrophotometrically on 540nm (BioTek Synergy HT, USA).
Histological evaluationExaminations were conducted in a blind fashion by two of the investigators. Tissue specimens were obtained from the mid-zone of the left lung of mice. Samples were fixed in 10% formalin for light microscopic evaluation. Some tissue samples of 1–2mm3 taken from adjacent regions were stocked in 2% gluteraldehyte for electron microscopic evaluation. After fixation, samples were embedded in paraffin for light microscopic evaluation. Paraffin blocks were placed in Leica RM2125 rotary microtome (Germany) and serial sections of 5μm thickness were obtained. After choosing the first section randomly, 10 sections in each mouse were selected by skipping over 10 sections and proceeding to staining process. For light microscopic evaluation, four different staining processes were used. The first 10 samples were stained with haematoxylin and eosin (H&E). In these samples, general tissue features were examined and thicknesses of epithelium and subepithelial smooth muscle layers of the medium and small airways were measured. In order to evaluate the thicknesses of epithelium and subepithelial smooth muscle layers, measurements were performed from four points of each airway at levels of 3, 6, 9, and 12 o’clock. Considering that each section contained two to three airways, nearly 20 or more airways were evaluated in each mouse.
Photomicrographs were taken by Olympus DP25 (Japan) camera, which was adapted on Olympus CX-41 model microscope (Olympus Optical, Tokyo, Japan). The histological analysis was carried out with a computer-assisted image analyser system (DP2-BSW).
The 10 consecutive sections were stained with Toluidine Blue and the other 10 sections with periodic acid–Schiff (PAS). Last sections were stained with Masson Trichrome stain for demonstration of collagen deposition. Photomicrographs were taken randomly from five fields of each section which were stained with Toluidine Blue. For mast cell enumeration, a standard transparent counting frame representing an area of 20,000μm2 was used manually and 10 fields in each photograph were examined for each mouse. Goblet cells stained with PAS were enumerated in 10 sections of each mouse. In each section, three to five randomly selected airways were photographed. Circumferences of all airways were measured and goblet cell numbers in these areas were recorded. For standardisation, goblet cell numbers in 100μm were analysed by division of total goblet cell number to the total length of airway circumferences and multiplying the result by 100.
For ultrastructural investigations, the tissues were postfixed with osmium tetroxide (OsO4), dehydrated in a graded series of alcohol, and then embedded in Araldite® CY212. The thin (60–90nm) sections were obtained with ultra-microtome (Leica) and stained with uranyl acetate and lead citrate, examined on a transmission electron microscope (Carl Zeiss Libra 120 EFTEM, Germany), and digitally photographed. In each mouse, five to seven ultrathin sections were taken from each two blocks and epithelium of the airway, the surrounding structures, and the intercellular connections were evaluated. Thicknesses of the basement membrane of the respiratory epithelium were measured from 20 points that were at equal distances to each other and the data were recorded.
Statistical analysisThe SPSS 15.0 package program was used in the statistical analysis. Data were presented as mean±standard deviation (SD) in each group. All data were analysed by Kruskal–Wallis and then Mann–Whitney test for dual comparisons. p<0.05 was considered statistically significant.
ResultsWe set out to determine if nebulised arginase inhibitor nor-NOHA was effective in vivo. In order to answer this question, we compared the concentration of l-arginine in lung homogenates in nor-NOHA treated and untreated Balb/c mice. In the mice treated with nor-NOHA, the concentration of l-arginine was significantly higher compared to the mice receiving OVA alone (mean: 4.39±1.38 and 1.68±1.38mol/L, respectively; p=0.024). So it was concluded that the dose and the route of nor-NOHA treatment were effective in raising the concentration of l-arginine in the lung of mice receiving this arginase inhibitor.
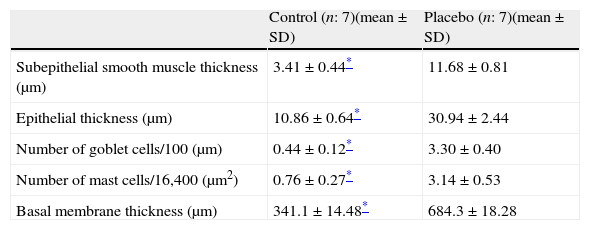
Every group consisted of seven mice at the beginning of the experiment, and one mouse died in Group VI during the study. We compared the histopathological features of the control group with the placebo in order to show that the model of asthma was established. In the chronic asthma group (placebo), thickness of basal membrane, epithelium and subepithelial smooth muscle layer as well as the number of mast cells and goblet cells were significantly higher than the control group (Table 1 and Fig. 2a). These results showed that the chronic asthma model was established.
Histological comparison of the placebo and the control group.
| Control (n: 7)(mean±SD) | Placebo (n: 7)(mean±SD) | |
| Subepithelial smooth muscle thickness (μm) | 3.41±0.44* | 11.68±0.81 |
| Epithelial thickness (μm) | 10.86±0.64* | 30.94±2.44 |
| Number of goblet cells/100 (μm) | 0.44±0.12* | 3.30±0.40 |
| Number of mast cells/16,400 (μm2) | 0.76±0.27* | 3.14±0.53 |
| Basal membrane thickness (μm) | 341.1±14.48* | 684.3±18.28 |
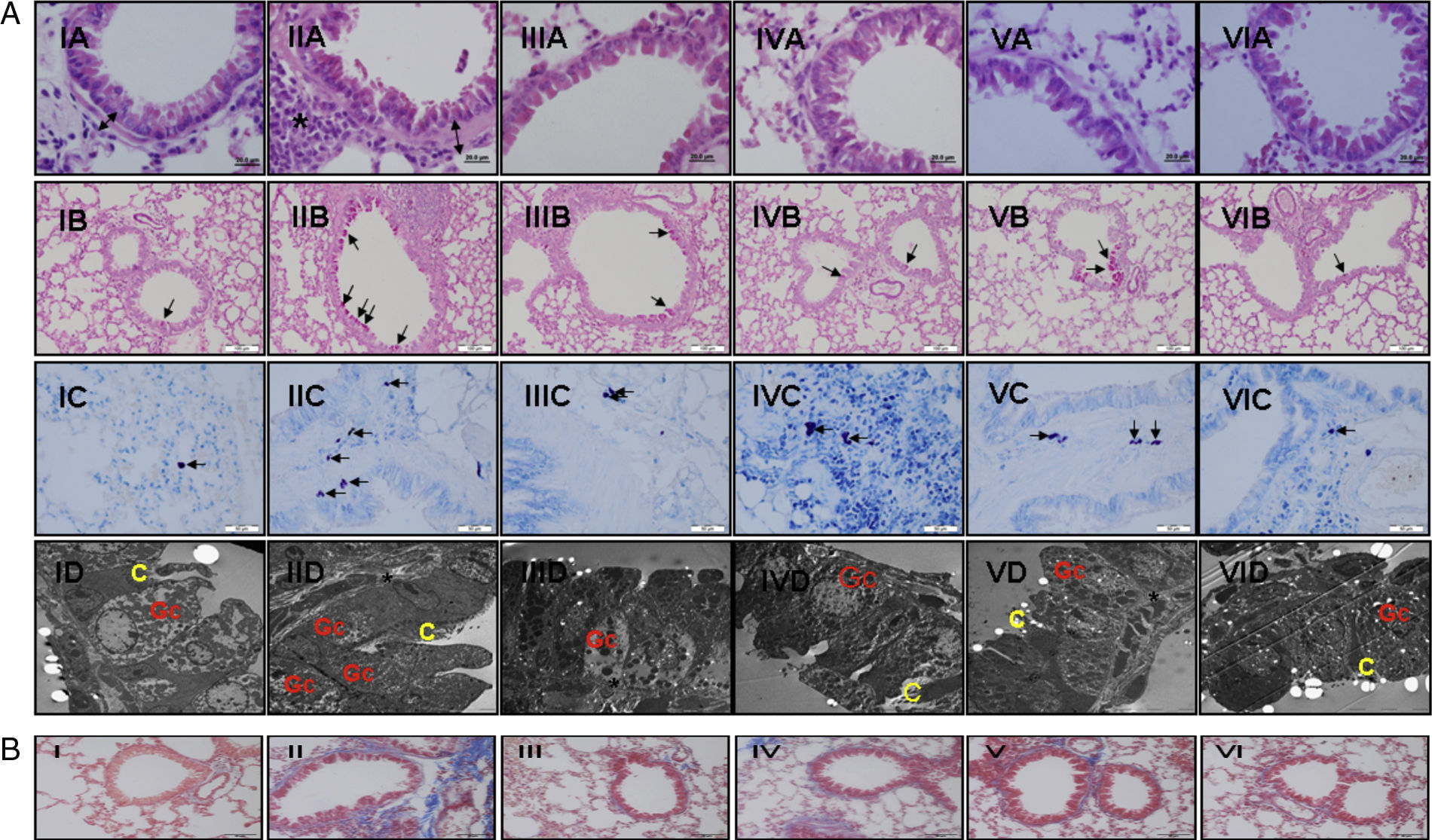
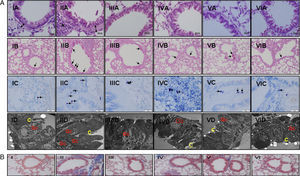
(a) Light and electron microscopic findings of study groups: (I) control (n: 7); (II) placebo (n: 7); (III) l-arginine (n: 7); (IV) nor-NOHA (n: 7); (V) l-arginine+nor-NOHA (n: 7); (VI) budesonide (n: 6). (A) H&E; (B) PAS; (C) Toluidine staining. (A) Two-headed arrow: subepithelial smooth muscle thickening; (*) peribronchial mononuclear cell infiltration. (B) Arrows: goblet cells; (C) arrows: mast cells. (D) Electron microscopic views. Increased goblet cells in placebo group (Gc), basal membrane thickening (*). C: cilia. (B) Masson Trichrome staining. Masson Trichrome staining (blue colour) showed minimal peribronchial and perivascular collagen accumulating in control (I) and budesonide (VI) groups. Increased collagen deposition was seen with Trichrome staining in the OVA-induced asthma mice (II), as compared to normal tissue. Collagen deposition was reduced in groups III, IV and V. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
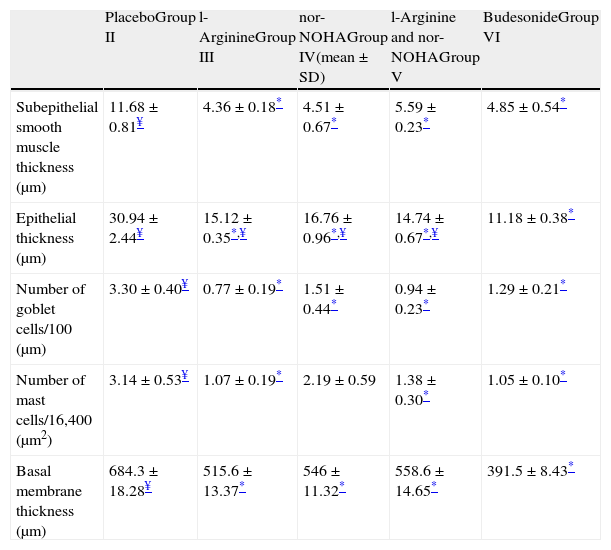
In Group III (L-arginine), subepithelial smooth muscle thickness, epithelial thickness, basal membrane thickness, number of mast and goblet cells were significantly lower compared to placebo group and no difference was noted in histological parameters with regard to gold standard budesonide treated group except epithelial thickness (Table 2 and Fig. 2a).
Histological comparison of the study groups.
| PlaceboGroup II | l-ArginineGroup III | nor-NOHAGroup IV(mean±SD) | l-Arginine and nor-NOHAGroup V | BudesonideGroup VI | |
| Subepithelial smooth muscle thickness (μm) | 11.68±0.81¥ | 4.36±0.18* | 4.51±0.67* | 5.59±0.23* | 4.85±0.54* |
| Epithelial thickness (μm) | 30.94±2.44¥ | 15.12±0.35*,¥ | 16.76±0.96*,¥ | 14.74±0.67*,¥ | 11.18±0.38* |
| Number of goblet cells/100 (μm) | 3.30±0.40¥ | 0.77±0.19* | 1.51±0.44* | 0.94±0.23* | 1.29±0.21* |
| Number of mast cells/16,400 (μm2) | 3.14±0.53¥ | 1.07±0.19* | 2.19±0.59 | 1.38±0.30* | 1.05±0.10* |
| Basal membrane thickness (μm) | 684.3±18.28¥ | 515.6±13.37* | 546±11.32* | 558.6±14.65* | 391.5±8.43* |
n: 7 in each group except Group VI which includes 6 mice.
In Group IV (nor-NOHA), all histological parameters except the number of mast cells were significantly better compared to placebo and improvement was similar to budesonide in all parameters except epithelial thickness (Table 2 and Fig. 2a).
In Group V in which the study drugs were l-arginine and nor-NOHA combination, all histological parameters were improved compared to the placebo group but this combination did not add any improvement on administration of l-arginine and nor-NOHA alone (p>0.05).
In Masson Trichrome staining, increased subepithelial and perivascular deposition of collagen was seen in OVA-induced asthma mice (Fig. 2b, II). Control and budesonide groups showed a thin collagen accumulating in peribronchiolar subepithelial and perivascular regions (Fig. 2b, I and VI). Collagen deposition in these regions was reduced in groups receiving l-arginine, nor-NOHA and Larginine and nor-NOHA combination.
Interleukin 4 and 5 levels were significantly higher in the placebo group compared to the control group as expected. But significant decreases of interleukins were not detected in study groups except nor-NOHA treated group in which the levels of IL-4 decreased (Table 3).
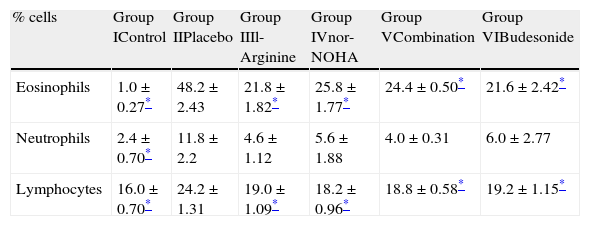
The number of eosinophils in BAL fluid was significantly reduced in groups receiving l-arginine and nor-NOHA when compared with placebo. The number of neutrophils was also reduced in study groups but this was not statistically significant (Table 4).
Differential cell counts of bronchoalveolar fluid in study groups.
| % cells | Group IControl | Group IIPlacebo | Group IIIl-Arginine | Group IVnor-NOHA | Group VCombination | Group VIBudesonide |
| Eosinophils | 1.0±0.27* | 48.2±2.43 | 21.8±1.82* | 25.8±1.77* | 24.4±0.50* | 21.6±2.42* |
| Neutrophils | 2.4±0.70* | 11.8±2.2 | 4.6±1.12 | 5.6±1.88 | 4.0±0.31 | 6.0±2.77 |
| Lymphocytes | 16.0±0.70* | 24.2±1.31 | 19.0±1.09* | 18.2±0.96* | 18.8±0.58* | 19.2±1.15* |
n: 5 in each group.
Mean bronchoalveolar NO levels were 0.064, 0.056, 0.054 and 0.060μM in groups III, IV, V and VI, respectively. There was no statistically significant difference in NO levels between groups receiving both l-arginine and nor-NOHA when compared to other study groups (p>0.05).
Discussionl-Arginine and arginase inhibitors have been studied in experimental models of asthma in various studies but the effect of cotreatment is firstly investigated in our study. Using a histologically well established murine model of asthma, we demonstrated that l-arginine and nor-NOHA were effective in reversing abnormal histological findings of asthma. We hypothesized that both arginase inhibition and l-arginine administration will increase l-arginine bioavailability and increase production of NO much more but cotreatment did not have any additive effect and NO levels did not increase as expected.
l-Arginine has been used as a potential therapy in various disease states including asthma.19 Since l-arginine bioavailability is shown to be reduced in asthma, it has been hypothesized that exogenous administration of l-arginine may increase the bronchodilation effect by constitutive forms of NO. Oral, inhaled and intraperitoneal routes were chosen for l-arginine administration in animal studies14,20,21 and oral and inhaled routes were used for humans.22,23 In an asthma model, delivery of dietary l-arginine increased eosinophilia and levels of IL-5 production in the study by Takano et al.20 and intraperitoneal l-arginine treatment also increased eosinophils and neutrophils in bronchoalveolar lavage and increased airway responsiveness to inhaled methacholine in another study.21 Inhaled l-arginine is used in two studies involving healthy and asthmatic subjects and effect of l-arginine on exhaled NO is studied.23,24 These studies showed different results: one suggesting that l-arginine increases the formation of endogenous NO, and the other ignoring the rise in exhaled NO. In a guinea pig model of asthma using perfused tracheal preparations ex vivo, it was demonstrated that inhaled l-arginine reversed airway hyperresponsiveness.14 In another study it was found that l-arginine administration restored mitochondrial dysfunction and reduced airway injury in murine allergic inflammation.25 These different results can be explained with the species of animals used, methodology of the studies and the dosage of l-arginine, as suggested by Mabalirajan et al.26 They state that low doses of l-arginine may not be sufficient to increase the substrate availability of eNOS and the high dose of l-arginine administered orally in their study enhanced eNOS expression, downregulated both iNOS and arginase and these resulted in reduction of airway inflammation and airway hyperresponsiveness. We chose to use inhaled l-arginine administration in order to reach higher concentrations in airways and bypass the systemic effects of other routes. Our study was mainly based on histological outcome of l-arginine treatment and we noted improvement in most of the parameters similar to gold standard budesonide.
It is thought that chronic administration of l-arginine would increase the activity of the arginase leading to enhanced formation of downstream products of l-ornithine that are involved in airway remodelling, and prolonged treatment of asthma with l-arginine may be contraindicated.14 Then the interest has been focused on arginase and arginase inhibitors. Arginase plays a role in airway remodelling and fibrosis by decreasing NO production through competition for l-arginine and reducing anti-fibrotic and anti-proliferative effects of NO. However, contradictory effects of arginase inhibition have been reported in animal studies.14,27,28 In a guinea pig model of asthma, specific arginase inhibitor 2(S)-amino-6-boronohexanoic acid (ABH) administration reduced airway sensitivity to inhaled allergen and protected against early and late asthmatic reactions including airway hyperresponsiveness and airway inflammation.14 Nor-NOHA, another arginase inhibitor reduced total inflammatory cell content of lung lavage fluid in C57BL/6 mice exposed to OVA. This study also showed that NOS2−/− strain mice treated with nor-NOHA did not show a reduction in eosinophilic influx, indicating that when l-arginine concentrations were increased by arginase inhibition eosinophilic inflammation is decreased directly or indirectly through NOS2.29 In mice sensitised to ovalbumin, another arginase inhibitor S-(2-boronoethyl)-1-cysteine (BEC) given orally, increased the peribronchial and perivascular inflammation in association with increases in protein S-nitrosylation and tyrosine nitration.28 The results of our study were consistent with the former two studies as we found improvement of histological parameters and decrease in Th2 cytokines in nor-NOHA treated group. Differences among studies can be seen because of the different species of study animals and type of arginase inhibitors and their route of administration.
We originally hypothesized that arginase inhibition with l-arginine administration will lead to a better histological outcome. We may speculate that hyperargininaemia occurring from these treatments limited the rate of NO production by NOS or led to systemic effects apart from local effects on lungs and had no benefit over l-arginine or nor-NOHA alone. These complex interactions should be highlighted in studies investigating arginase and NOS expressions in models treated with l-arginine and arginase inhibitor combination. In order to confirm the effects of study drugs on NO production we planned to measure NO in BAL supernatants, but the results were similar in study groups indicating that this hypothesis was not valid.
In conclusion, inhaled administration of l-arginine and arginase inhibitor nor-NOHA alleviated airway inflammation and remodelling in murine model of asthma but combination therapy did not have additive effects. Arginine and arginase represent drug targets that have potentials to open new therapeutic perspectives. However, specific and off-target effects and mechanisms should be elucidated in oncoming studies of inhaled l-arginine and arginase inhibitors.
Conflict of interestNone of the authors have conflict of interest to declare.
ContributorsZAA and NU contributed in the design of the study, OY and MK (M. Karaman) participated in animal study of the work, MK (M. Karaman) was involved in determination of NO and cytokine levels, MK (M. Kiray) carried out conventional histological work, AB carried out electron microscopy, TT performed statistical analysis, OK participated in design and coordination of the work. ZAA wrote the manuscript; all authors read and approved the manuscript.
Ethical disclosuresProtection of human subjects and animals in researchWe declare that the procedures followed were in accordance with the regulations of the responsible Animal Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Patients’ data protectionWe declare that no patient data appears in this article.
Right to privacy and informed consentWe declare that no patient data appears in this article.
This Project was supported by Dokuz Eylül University Scientific Research Foundation Grant BAP-Project no. 2010.KB.SAG.23.