In the management of acute bronchiolitis there is a generalised use of treatments that have not been shown to be useful or efficacious in clinical studies. The objective of this study was to determine the appropriateness in the treatment of acute bronchiolitis of different severity within different clinical care settings.
MethodsThis is a cross-sectional, descriptive study of 5647 cases of acute bronchiolitis in 91 Spanish hospitals and primary care centres. We classified the appropriateness of the treatments according to the recommendations of a consensus conference.
ResultsThere was an inappropriate use of treatments in 58.3% of the cases during the acute phase and in 45.4% during the maintenance phase. There was a generalised use of inhaled beta 2 agonists, regardless of the severity of the patients (hospitalised patients 69.3%, emergency care 63.2% and ambulatory 64.1%). Adrenaline was used in 30.1% of hospitalised cases and in 80.2% of intensive care patients. Systemic corticosteroids were not only used in one-third of hospitalised patients but also in 25.8% of ambulatory cases.
ConclusionsIn acute bronchiolitis in Spain there is a wide use of treatments that are not recommended by the available clinical practice guidelines. Beta 2 agonist bronchodilators and corticosteroids are widely used and maintained, regardless of the severity of the patients.
Acute bronchiolitis is the main cause of hospital admissions related to acute lower respiratory airway infections in infants. It has significant repercussions at all health care levels. Literature on the management of bronchiolitis is very abundant in diagnostic as well as preventive-therapeutic aspects. The published information has been revised in depth, and different clinical practice guidelines (CPGs) are available.1–5 The evidence suggests that in the treatment of bronchiolitis, the use of symptomatic support measures is fundamental for the management of fever, respiratory secretions, hyporexia, respiratory distress and hypoxaemia. Other treatments, in spite of their wide use, have not shown enough efficacy in clinical trials and present unfavourable benefit-risk ratios. A therapy trial with inhaled beta 2 agonists or adrenaline (better with hypertonic saline solution) has been proposed by some CPGs, but only for moderate-to-severe cases. These treatments can only be maintained if there is a documented improvement that compensates their costs and adverse effects.
The objective of our study was to analyse the appropriateness in the treatment of bronchiolitis in a large and representative sample of different health care settings in Spain. This study complements a preliminary one, conducted within the aBREVIADo Project, which describes the global variability in the clinical management. Here, we present an analysis of the appropriateness of the treatments in relation to the severity of the patients.6
Materials and methodsDesignThis was a cross-sectional, descriptive study of acute bronchiolitis cases in a sample of hospitals, emergency services and primary care centres or offices in Spain. The participating centres belonged to 12 autonomous communities (25 provinces) and corresponded to 31 hospital centres (18 complete hospitals, 7 hospitalisation services, and 6 emergency services) and 60 primary care centres or offices (Annex 1). The information of this descriptive study is part of the aBREVIADo Project (Bronchiolitis-Study of Variability, Adequacy, and Adherence), in which the recommendations made by the consensus conference of bronchiolitis were used as reference standards.7
Study periodFrom October 2007 to March 2008.
Inclusion criteriaAll bronchiolitis cases were diagnosed during the study period according to the McConnochie criteria8: first acute episode of respiratory distress with wheezing preceded by a cold-like clinical picture of the upper respiratory airway (rhinitis, cough, with/without fever), which affects children younger than two years of age.
Exclusion criteriaPatients with previous wheezing episodes.
Data gatheringData gathering included collecting the consecutive records of cases diagnosed by collaborating doctors in the study as well as the periodical review of databases and lists or copies of reports for the records of cases diagnosed by other doctors.
We designed a questionnaire for the collection of the study's variables that included general data, signs-symptoms, risk factors, diagnostic tests, and treatments. A complete description of these items is available in a previous article.7 We designed a score of the severity of disease by gathering the variables that have been shown in previous studies to have an adequate interobserver concordance, including the following: respiratory rate (<45; 45–60; >60 per minute), pulmonary ventilation (normal; hypoventilation; silent chest), wheezing (mild expiratory; all expiration; expiratory and inspiratory), retractions (not or mild intercostal; moderate intercostal-suprasternal; severe or nasal flaring), and consciousness (normal; agitated; lethargic); these variables were measured after adequate aspiration of secretions (0–2 for each component; maximum score of 10). The treatments were differentiated according to their use in the acute or maintenance phases of the disease. We considered acute phase treatments in inpatients: those received during admission; in ambulatory patients: treatments administered at the place of diagnosis and those recommended during the following 24h.
The treatment was classified according to its appropriateness following the recommendations of the consensus conference as: first choice, alternative or inappropriate.4,7 Patients admitted to the intensive care unit (ICU) were excluded from this classification. The consensus conference was conducted and published after gathering the cases, so that it did not influence in the management of patients.
Ethical aspectsIt was specifically recommended not to modify, in any way, the routine management of patients with bronchiolitis. Data were gathered anonymously without registering the patients’ identifying data.
Statistical aspectsStatistical processing was performed with SPSS version 11.5.1 (serial number 9036057). We did not conduct an estimation of the sample size necessary for each setting because in almost all of the centres, all of the patients diagnosed with bronchiolitis were included. However, we had calculated that a subsample of 300 patients would allow the estimation of percentages with a precision of ±5% as well as the ability to discriminate differences of at least 12% (for theoretical most unfavourable percentages of 50%, α value of 5%, and β of 20%).
We estimated confidence intervals (CI) for the main measurements. We compared the variables by health care setting (offices, emergency, hospitalisation, and intensive care) using the χ2 test or exact tests for the qualitative variables and variance analysis for the quantitative variables.
ResultsBetween October 2007 and March 2008, we gathered 5647 cases of bronchiolitis from 31 hospitals and 60 primary care centres/offices. The cases were predominantly diagnosed in the emergency departments (2914; 51.6%) and in hospitalisation wards (1576; 27.9%); 1060 (18.8%) were made in primary care offices, and 86 (1.5%) were made in the ICU. The health care setting was not specified in 11 cases.
Of the cases, 1874 (34.7%) children required hospitalisation. Because of the system used for gathering cases, this percentage did not allow for the calculation of overall risk upon admission; however, this was estimated for two of the subsamples: 2655 cases gathered from the emergency wards of ten tertiary hospitals, with 23.5% of admissions (CI 95%: 21.8–25.1%), and 1142 cases from primary care centres (including 181 cases diagnosed at hospital level with 9.1% of admissions; CI 95%: 7.5–11.0%). The mean hospital stay was 5.73 days (CI 95%: 5.54–5.92).
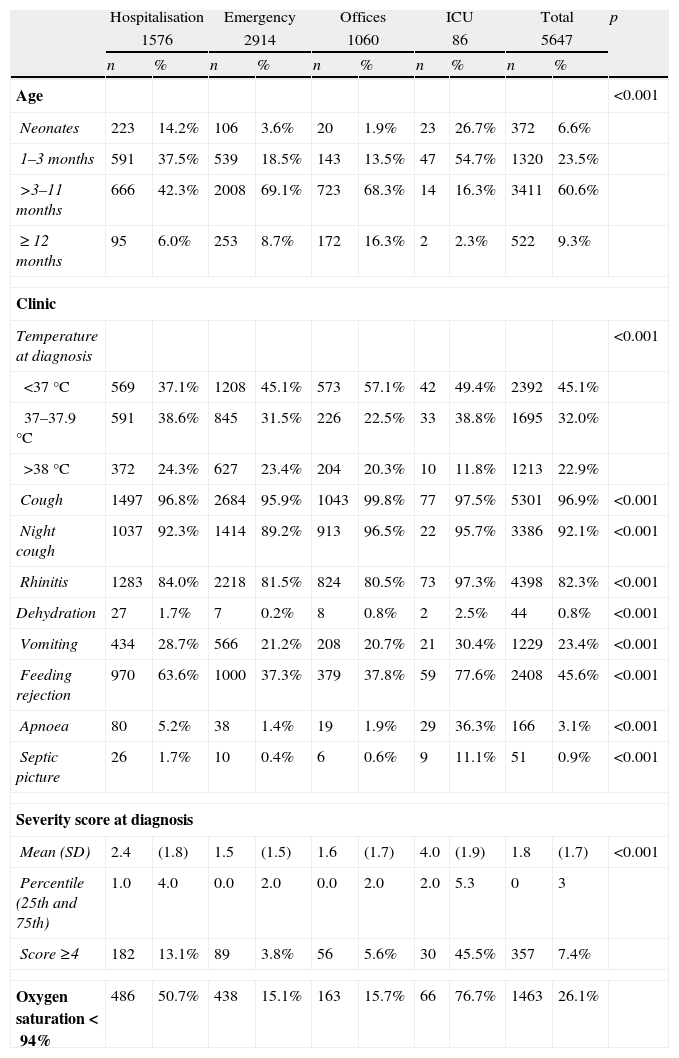
A total of 58.1% of the cases were male, and no differences were observed between different health care settings with regard to gender. The mean age was 0.34 years (CI 95%: 0.32–0.35) with a predominance of children between three months and one year of age (Table 1). Clinical presentation varied by health care setting, and the highest presence of all symptoms was seen among the hospitalised patients (Table 1). Hospitalised and ICU-admitted children had a higher level of respiratory failure, which was reflected in higher scores on the severity score (Table 1).
Frequency distributions for the main variables. Distribution according to the place of diagnosis and total.a
| Hospitalisation | Emergency | Offices | ICU | Total | p | ||||||
| 1576 | 2914 | 1060 | 86 | 5647 | |||||||
| n | % | n | % | n | % | n | % | n | % | ||
| Age | <0.001 | ||||||||||
| Neonates | 223 | 14.2% | 106 | 3.6% | 20 | 1.9% | 23 | 26.7% | 372 | 6.6% | |
| 1–3 months | 591 | 37.5% | 539 | 18.5% | 143 | 13.5% | 47 | 54.7% | 1320 | 23.5% | |
| >3–11 months | 666 | 42.3% | 2008 | 69.1% | 723 | 68.3% | 14 | 16.3% | 3411 | 60.6% | |
| ≥ 12 months | 95 | 6.0% | 253 | 8.7% | 172 | 16.3% | 2 | 2.3% | 522 | 9.3% | |
| Clinic | |||||||||||
| Temperature at diagnosis | <0.001 | ||||||||||
| <37°C | 569 | 37.1% | 1208 | 45.1% | 573 | 57.1% | 42 | 49.4% | 2392 | 45.1% | |
| 37–37.9°C | 591 | 38.6% | 845 | 31.5% | 226 | 22.5% | 33 | 38.8% | 1695 | 32.0% | |
| >38°C | 372 | 24.3% | 627 | 23.4% | 204 | 20.3% | 10 | 11.8% | 1213 | 22.9% | |
| Cough | 1497 | 96.8% | 2684 | 95.9% | 1043 | 99.8% | 77 | 97.5% | 5301 | 96.9% | <0.001 |
| Night cough | 1037 | 92.3% | 1414 | 89.2% | 913 | 96.5% | 22 | 95.7% | 3386 | 92.1% | <0.001 |
| Rhinitis | 1283 | 84.0% | 2218 | 81.5% | 824 | 80.5% | 73 | 97.3% | 4398 | 82.3% | <0.001 |
| Dehydration | 27 | 1.7% | 7 | 0.2% | 8 | 0.8% | 2 | 2.5% | 44 | 0.8% | <0.001 |
| Vomiting | 434 | 28.7% | 566 | 21.2% | 208 | 20.7% | 21 | 30.4% | 1229 | 23.4% | <0.001 |
| Feeding rejection | 970 | 63.6% | 1000 | 37.3% | 379 | 37.8% | 59 | 77.6% | 2408 | 45.6% | <0.001 |
| Apnoea | 80 | 5.2% | 38 | 1.4% | 19 | 1.9% | 29 | 36.3% | 166 | 3.1% | <0.001 |
| Septic picture | 26 | 1.7% | 10 | 0.4% | 6 | 0.6% | 9 | 11.1% | 51 | 0.9% | <0.001 |
| Severity score at diagnosis | |||||||||||
| Mean (SD) | 2.4 | (1.8) | 1.5 | (1.5) | 1.6 | (1.7) | 4.0 | (1.9) | 1.8 | (1.7) | <0.001 |
| Percentile (25th and 75th) | 1.0 | 4.0 | 0.0 | 2.0 | 0.0 | 2.0 | 2.0 | 5.3 | 0 | 3 | |
| Score ≥4 | 182 | 13.1% | 89 | 3.8% | 56 | 5.6% | 30 | 45.5% | 357 | 7.4% | |
| Oxygen saturation<94% | 486 | 50.7% | 438 | 15.1% | 163 | 15.7% | 66 | 76.7% | 1463 | 26.1% | |
SD, standard deviation; ICU, intensive care unit.
A total of 11.1% of cases were preterm born (2.6% with ≤32 weeks of gestation), and 2.3% of cases had congenital heart disease. Other risk factors were infrequent and included the following: bronchopulmonary dysplasia (0.9%), other chronic lung diseases (0.2%), immunodeficiency (0.1%), and neuromuscular disease (0.11%). Amongst the hospitalised patients, there was a slightly higher frequency of preterm born (hospitalised 13.9%, emergency services 9.9%, offices 9.4%, and ICU 22.4%; p<0.001), congenital heart disease (hospitalised 3.5%, emergency services 1.8%, offices 1.6%, and ICU 2.4%; p=0.002), and bronchopulmonary dysplasia (hospitalised 1.7%, emergency services 0.8%, offices, 0.1%, and ICU 1.2%; p<0.001).
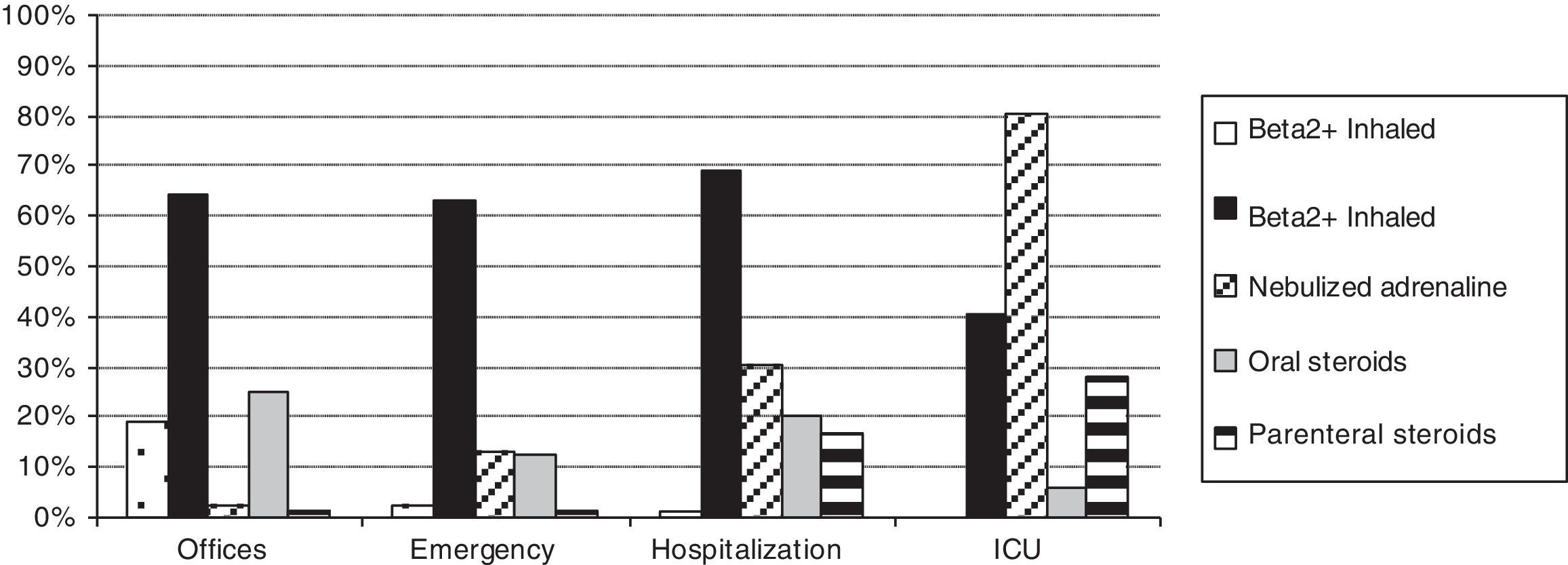
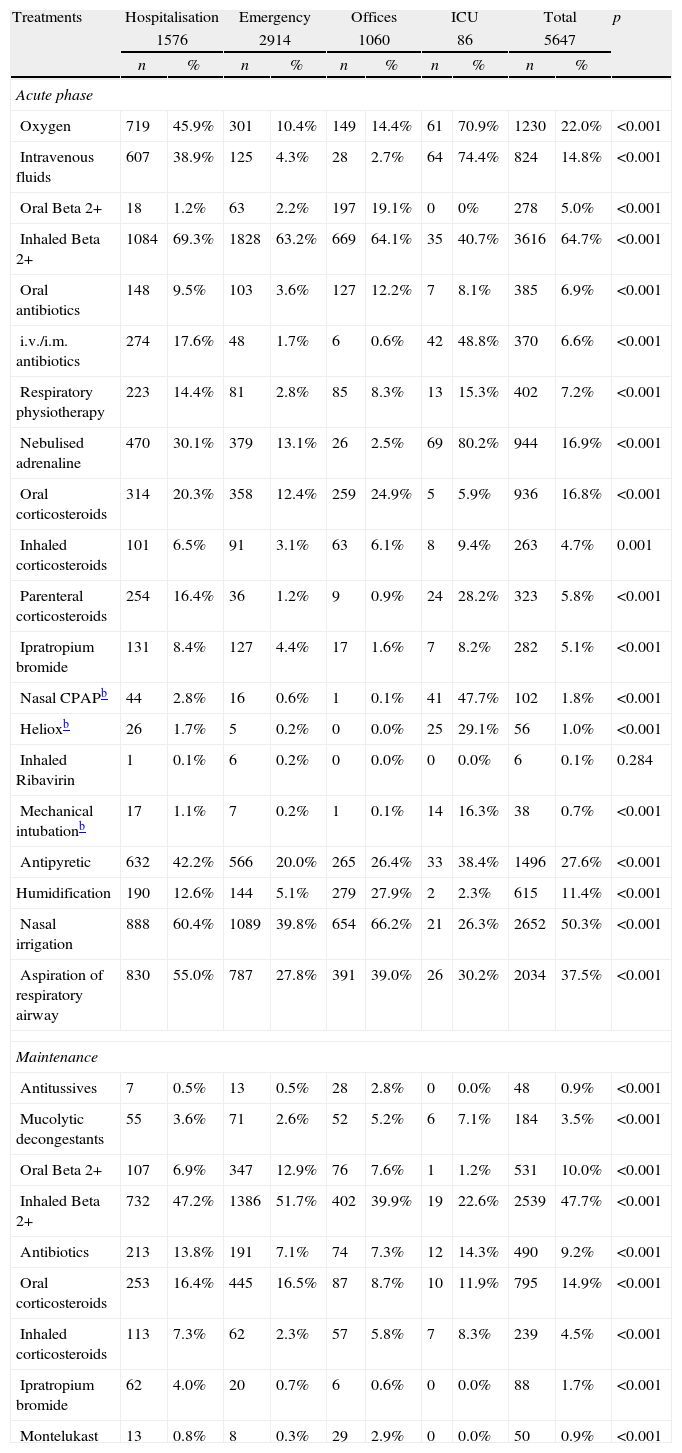
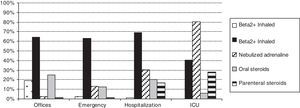
Table 2 shows the treatments used during the acute and maintenance phases of bronchiolitis. Fig. 1 shows a comparison of the percentages of use of the main treatments in each health care setting. Table 3 shows the classification of appropriateness of treatments by health care setting, excluding ICU patients.
Main treatments before diagnosis and during the acute and maintenance phases. Distribution according to the place of diagnosis and total.a
| Treatments | Hospitalisation | Emergency | Offices | ICU | Total | p | |||||
| 1576 | 2914 | 1060 | 86 | 5647 | |||||||
| n | % | n | % | n | % | n | % | n | % | ||
| Acute phase | |||||||||||
| Oxygen | 719 | 45.9% | 301 | 10.4% | 149 | 14.4% | 61 | 70.9% | 1230 | 22.0% | <0.001 |
| Intravenous fluids | 607 | 38.9% | 125 | 4.3% | 28 | 2.7% | 64 | 74.4% | 824 | 14.8% | <0.001 |
| Oral Beta 2+ | 18 | 1.2% | 63 | 2.2% | 197 | 19.1% | 0 | 0% | 278 | 5.0% | <0.001 |
| Inhaled Beta 2+ | 1084 | 69.3% | 1828 | 63.2% | 669 | 64.1% | 35 | 40.7% | 3616 | 64.7% | <0.001 |
| Oral antibiotics | 148 | 9.5% | 103 | 3.6% | 127 | 12.2% | 7 | 8.1% | 385 | 6.9% | <0.001 |
| i.v./i.m. antibiotics | 274 | 17.6% | 48 | 1.7% | 6 | 0.6% | 42 | 48.8% | 370 | 6.6% | <0.001 |
| Respiratory physiotherapy | 223 | 14.4% | 81 | 2.8% | 85 | 8.3% | 13 | 15.3% | 402 | 7.2% | <0.001 |
| Nebulised adrenaline | 470 | 30.1% | 379 | 13.1% | 26 | 2.5% | 69 | 80.2% | 944 | 16.9% | <0.001 |
| Oral corticosteroids | 314 | 20.3% | 358 | 12.4% | 259 | 24.9% | 5 | 5.9% | 936 | 16.8% | <0.001 |
| Inhaled corticosteroids | 101 | 6.5% | 91 | 3.1% | 63 | 6.1% | 8 | 9.4% | 263 | 4.7% | 0.001 |
| Parenteral corticosteroids | 254 | 16.4% | 36 | 1.2% | 9 | 0.9% | 24 | 28.2% | 323 | 5.8% | <0.001 |
| Ipratropium bromide | 131 | 8.4% | 127 | 4.4% | 17 | 1.6% | 7 | 8.2% | 282 | 5.1% | <0.001 |
| Nasal CPAPb | 44 | 2.8% | 16 | 0.6% | 1 | 0.1% | 41 | 47.7% | 102 | 1.8% | <0.001 |
| Helioxb | 26 | 1.7% | 5 | 0.2% | 0 | 0.0% | 25 | 29.1% | 56 | 1.0% | <0.001 |
| Inhaled Ribavirin | 1 | 0.1% | 6 | 0.2% | 0 | 0.0% | 0 | 0.0% | 6 | 0.1% | 0.284 |
| Mechanical intubationb | 17 | 1.1% | 7 | 0.2% | 1 | 0.1% | 14 | 16.3% | 38 | 0.7% | <0.001 |
| Antipyretic | 632 | 42.2% | 566 | 20.0% | 265 | 26.4% | 33 | 38.4% | 1496 | 27.6% | <0.001 |
| Humidification | 190 | 12.6% | 144 | 5.1% | 279 | 27.9% | 2 | 2.3% | 615 | 11.4% | <0.001 |
| Nasal irrigation | 888 | 60.4% | 1089 | 39.8% | 654 | 66.2% | 21 | 26.3% | 2652 | 50.3% | <0.001 |
| Aspiration of respiratory airway | 830 | 55.0% | 787 | 27.8% | 391 | 39.0% | 26 | 30.2% | 2034 | 37.5% | <0.001 |
| Maintenance | |||||||||||
| Antitussives | 7 | 0.5% | 13 | 0.5% | 28 | 2.8% | 0 | 0.0% | 48 | 0.9% | <0.001 |
| Mucolytic decongestants | 55 | 3.6% | 71 | 2.6% | 52 | 5.2% | 6 | 7.1% | 184 | 3.5% | <0.001 |
| Oral Beta 2+ | 107 | 6.9% | 347 | 12.9% | 76 | 7.6% | 1 | 1.2% | 531 | 10.0% | <0.001 |
| Inhaled Beta 2+ | 732 | 47.2% | 1386 | 51.7% | 402 | 39.9% | 19 | 22.6% | 2539 | 47.7% | <0.001 |
| Antibiotics | 213 | 13.8% | 191 | 7.1% | 74 | 7.3% | 12 | 14.3% | 490 | 9.2% | <0.001 |
| Oral corticosteroids | 253 | 16.4% | 445 | 16.5% | 87 | 8.7% | 10 | 11.9% | 795 | 14.9% | <0.001 |
| Inhaled corticosteroids | 113 | 7.3% | 62 | 2.3% | 57 | 5.8% | 7 | 8.3% | 239 | 4.5% | <0.001 |
| Ipratropium bromide | 62 | 4.0% | 20 | 0.7% | 6 | 0.6% | 0 | 0.0% | 88 | 1.7% | <0.001 |
| Montelukast | 13 | 0.8% | 8 | 0.3% | 29 | 2.9% | 0 | 0.0% | 50 | 0.9% | <0.001 |
CPAP, continuous positive airway pressure; ICU, intensive care unit; i.m., intramuscular; i.v., intravenous.
Appropriateness of the treatments in the acute and maintenance phases. Distribution according to the place of diagnosis (ICU patients excluded) and total.a
| Treatments | Hospitalisation | Emergency | Offices | Total | p | ||||
| 1576 | 2914 | 1060 | 5550 | ||||||
| n | % | n | % | n | % | n | % | ||
| Acute phase | <0.001 | ||||||||
| First choice | 130 | 8.2% | 597 | 20.5% | 158 | 14.9% | 885 | 15.9% | |
| Alternative use | 788 | 50.0% | 523 | 17.9% | 118 | 11.1% | 1429 | 25.7% | |
| Inappropriate | 658 | 41.8% | 1794 | 61.6% | 784 | 74.0% | 3236 | 58.3% | |
| Alternative use: | |||||||||
| Beta 2+ or Adrenaline in moderate-severeb | 558 | 35.4% | 446 | 86 | 8.1% | 1090 | 19.6% | ||
| Systemic corticosteroids associated with bronchodilators in moderate-severeb | 230 | 14.6% | 77 | 2.6% | 32 | 3.0% | 339 | 6.1% | |
| Inappropriate use: | |||||||||
| Beta 2+ or Adrenaline in mild | 20 | 1.3% | 1199 | 41.1% | 301 | 28.4% | 1520 | 27.4% | |
| Systemic corticosteroids in mild | 9 | 0.6% | 159 | 5.5% | 98 | 9.2% | 266 | 4.8% | |
| Other inappropriate treatmentsc | 625 | 39.7% | 219 | 7.5% | 193 | 18.2% | 1037 | 18.7% | |
| Various inappropriatec | 4 | 0.3% | 217 | 7.4% | 192 | 18.1% | 413 | 7.4% | |
| Maintenance phased | <0.001 | ||||||||
| First choice | 634 | 40.2% | 1073 | 36.8% | 496 | 46.8% | 2203 | 39.7% | |
| Alternative use | 465 | 29.5% | 291 | 10.0% | 69 | 6.5% | 825 | 14.9% | |
| Inappropriate | 477 | 30.3% | 1550 | 53.2% | 495 | 46.7% | 2522 | 45.4% | |
With some variables, there are cases of unspecified data; thus, the counts do not add up to the total.
The characteristics of our study, including the number of cases (5647 infants younger than two years of age with a first episode of bronchiolitis from 91 sanitary centres and 25 provinces from 12 autonomous communities) and location of the study (bronchiolitis cared for at different health care settings: primary care consults, emergency department, hospitalisation, and ICU), allowed us to obtain representative data of the epidemiological characteristics and diagnostic-therapeutic management of bronchiolitis in Spain. The researchers prospectively gathered consecutive cases of bronchiolitis during the epidemic period of 2007–2008. They collected the actual management of bronchiolitis in clinical practice, conducted according to their physicians’ criteria.
The most frequent types of studies regarding the treatment of bronchiolitis are surveys of physician opinions from different settings and specialities,9–18 cross-sectional studies or case series,19–24 reviews of medical records,25,26 some cohort studies,21,27,28 and interventional studies (with previous and posterior analysis to the implementation of a CPG or consensus).18,29–33
In this study, we observed a wide use of bronchodilators, corticosteroids, and other treatments of unclear efficacy (antibiotics, oral bronchodilators, inhaled steroids, ipratropium bromide, etc.). Some treatments had a greater tendency of use associated with greater disease severity; thus, hospitalised patients and ICU patients had a higher use of nebulised adrenaline, antibiotics, parenteral steroids, oxygen therapy, intravenous fluids, and other treatments of selective use (nasal continuous positive airway pressure, heliox, and mechanical ventilation). However, other treatments, including inhaled bronchodilators and inhaled and oral corticosteroids, do not reflect this tendency and were used in a similar manner within the different health care settings.
Most of the hospitalised patients received bronchodilators (69.3% inhaled beta 2 agonists and 30.1% nebulised adrenaline) and approximately one-third systemic steroids. Patients of emergency wards received in similar proportion inhaled beta 2 agonists (63.2%) but less frequently nebulised adrenaline (13.1%) and systemic steroids (13%). In ambulatory patients, we had expected a lower use of inhaled beta 2 agonists and corticosteroids because they were less severe cases. However, they mainly received inhaled beta 2 agonists (64.1%) and frequently systemic steroids (25.8%). As maintenance phase treatment, half of the patients received inhaled or oral bronchodilators, and one-fifth received corticosteroids with small, although significant, differences according to the origin of the cases.
In surveys regarding routine management of patients with bronchiolitis, there is a generalised use of beta 2 agonists (between 44% and 99% of responses), although only a portion of them in a systematic manner (between 2% and 55%).9,10,12–15,18 Adrenaline use was indicated by 20–55% of the people surveyed (1–5% for systemic use), inhaled corticosteroids by 24–54%, and antibiotics by 3–69% (0–4% systemically). The wide use of treatments reflected in the surveys contradicts the recommendations of guidelines and protocols available in their respective countries. Only two studies, carried out in Ireland and Australia, show a low use of bronchodilators and corticosteroids.16,17
Additionally, in studies regarding the standard management of patients with bronchiolitis, beta 2 agonists (42–94%), adrenaline (24–69%), corticosteroids (25–85.7% in hospitals; 5–13% in emergencies; and 18.5% in ambulatory), and antibiotics (24–65%) were predominately used.19–33 Several studies conducted in France showed a different profile with lower use of beta 2 agonists and corticosteroids and generalised use of respiratory physiotherapy.23,31,33 With respect to the use of nebulised hypertonic saline, for which recent evidence suggests a certain efficacy, neither our study nor other previously published studies allowed for the description of its implementation in clinical practice.
Due to the extensive information available, the following are well known about the treatment of bronchiolitis7,34: (1) the use of symptomatic support measures is fundamental for the management of fever, respiratory secretions, hyporexia, respiratory distress and hypoxaemia; (2) the alternative use of a therapeutic trial with inhaled beta 2 agonists or adrenaline (better with hypertonic saline solution) can be considered in selected moderate-severe cases and maintained only if there is a positive documented response (clinical severity score); (3) the use of certain drugs (heliox, surfactant, and/or ribavirin) in well selected severe cases of bronchiolitis can be considered; and (4) the use of the majority of the remaining drugs is considered inappropriate (corticosteroids, oral salbutamol, subcutaneous adrenaline, ipratropium, antibiotics, immunoglobulins, etc.). These recommendations, which were obtained from our consensus conference,7,34 are concordant with those of other guidelines previously available.1–3
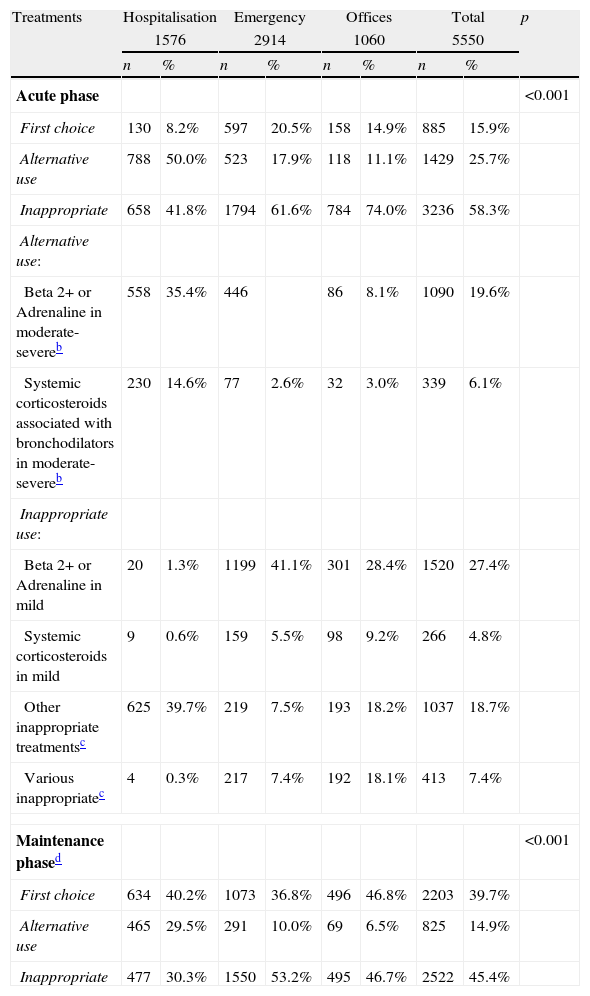
Upon classifying the appropriateness of our treatments, following the established criteria in the consensus conference7,34 and even assuming the optional or alternative use of certain interventions (trial of bronchodilators with or without corticosteroids in moderate-severe cases), we found that in our study, 58.3% of the treatments in the acute phase (somewhat higher in offices) and 45.4% in the maintenance phase (somewhat higher in emergency services) were inappropriate, which is a clear example of overuse of non-efficient treatments.
The use of bronchodilators and/or corticosteroids, the main cause of inappropriateness in our study, deserves a commentary. Adrenaline is frequently used in inpatients but it has only showed a small effect in reducing the admission risk in ambulatory patients (number needed to treat 17).35 Inhaled beta 2 agonists are also widely used, despite there being enough evidence against their efficacy. With respect to systemic corticosteroids, only some studies have shown a small improvement in clinical scores in inpatients, which has no effect in a reduction of the length of stay. The efficacy of combined nebulised adrenaline plus systemic corticosteroids was discussed in our consensus conference. According to the results of a clinical trial published by Plint et al.36 this combination could slightly reduce the risk of admission on day seven (although the main effect is seen in the first hours after a single dose of adrenaline). Nevertheless, this effect was no longer significant after adjustment for multiple comparisons (four treatment groups) and the treated group had a higher atopic risk (non-significant but of the same size as the observed effect). A recent systematic review has considered this study to support the effect of dexamethasone plus nebulised adrenaline,35 but this was not supported in previous reviews.37 Until new studies specifically designed to test this combined treatment are not available we decided to consider it only as an alternative for moderate-severe patients.
If we had accepted the widespread use of inhaled bronchodilators, associated or not with systemic corticosteroids, our estimation of appropriateness had improved. But are we sure that our patients are not harmed by this treatment? In our opinion, physicians caring for these patients try to reduce their respiratory distress or avoid their admissions. To do that, they use available treatments, which have showed limited efficacy, but they are not aware that in mild cases the benefit-risk ratio may be unfavourable. In order to justify a trial of therapy with bronchodilators, we must be aware of the limited efficacy and the associated risks, and inform the family about them. We can resort to bronchodilators if we programme them for a short period of time and if we are willing to reassess their efficacy and tolerance before extending their use.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis project was financed by a grant from the Hospital de Torrevieja Foundation between June 2007 and June 2009 (protocol code: BECA0001).
Conflict of interestNo conflicts of interest to report.
Hospital de Torrecárdenas. Almería (MD. Gámez Gómez, J. Batlles Garrido, J.E. Cabrera Servilla, I. García Escobar, F. Giménez Sánchez, L. Ruiz Tudela), C. Salud Candelaria. Sevilla (A. Fernández Valverde, M,G. Bueno Rodríguez, I. Ramón Faba, M. Praena Crespo).
C. Salud Fuentes del Ebro. Zaragoza (J.A. Castillo Laita, R. Macipe Costa), Hospital Infantil Universitario Miguel Servet. Zaragoza (C. Campos Calleja, M.C. García Jiménez, R. Pérez Delgado, Y. Romero Salas).
C. Salud Contrueces (M. López Benito), C. Salud El Llano (V. Martínez Suárez, M. García Balbuena), C. Salud Infesto (I. Mora Gandarillas), C. Salud La Magdalena (J.I. Pérez Candás), C.S. La Felguera (M. Fernández Pérez, C. Gonzavo Rodríguez), C. Salud Laviada (A. Cobo Ruisánchez, B. Yáñez Meana), C. Salud Natahoyo (A. Hernandez Encinas), C. Salud Otero (B. Domínguez Aurrecoechea), C. Salud Pravia (M. García Adaro, R. Buznego Sánchez), C. Salud Tineo (M. Fernández Francés), C. Salud Puerta La Villa (I. Franco, S. Ballesteros), C. Salud Sama (M. Benito Martín, A.J. Mira López, M. Fernández López), Hospital Cabueñes. Gijón (C. Molinos Norniella, C. Pérez Méndez, E. Fernández Fernández, J. Fernández Antuña), Hospital Central de Asturias. Oviedo (J. Rodríguez Suárez, S. Jiménez Treviño, F. Álvarez Caro).
Hospital Universitario Materno Infantil. Las Palmas de Gran Canarias (S. Todorcevic, M.R. García Luzardo).
C. Salud Buelna (A. Bercedo Sanz), Hospital Marqués de Valdecilla. Santander (M.J. Cabero Pérez, L. Álvarez Granda, E. Pérez Belmonte).
Hospital Complejo Asistencial de León. León (S. Lapeña López de Armentia, R. Morales Sánchez, L. Fernández Pérez), C. Salud Jardinillos. Palencia (S. Alberola López, I. Pérez García), C. Salud Pintor Oliva. Palencia (A.B. Camina Gutiérrez, J. G. Santos García), C. Salud Venta de Baños. Palencia (I. Casares Alonso), C. Salud Villamuriel. Palencia (A. Cano Garcinuño), Hospital Río Carrión. Palencia (C. Urueña Leal, J.M. Andrés de Llano, J.E. Fernández Alonso, J.M. Bartolomé Porro), C. Salud Ciudad Rodrigo. Salamanca (M.C. Sánchez Jiménez, M.J. Estévez Amores), C. Salud Ledesma. Salamanca (M. Mendoza Sánchez), C. Salud Miguel Armijo. Salamanca (J. López Ávila), C. Salud San Bernardo. Salamanca (A. Martín Ruano), C. Salud Santa Marta. Salamanca (J. Martín Ruano, B. de Dios Martín,), Hospital Complejo Hospitalario de Salamanca. Salamanca (S. Fernández de Miguel, J.M. Sánchez Granados, O. Serrano Ayestaran), Hospital Clínico Universitario. Valladolid (F. Conde Redondo, A. del Río López, V. Matías del Pozo), Hospital Río Hortega. Valladolid (F. Centeno Malfaz, C. Alcalde Martín, B. Bello Martínez, L. Crespo Valderrábano, C. Gutiérrez Abad), C. Salud Benavente Sur. Zamora (M.E. Vázquez Fernández), C. Salud Parada de Molino. Zamora (A. Cortés Gabaudán), C. Salud Puerta Nueva. Zamora (M.M. Miguélez Vara, P. Pérez García), C. Salud Santa Elena. Zamora (S. García Vicente), C. Salud Virgen de la Concha. Zamora (M. A. Prieto Figuero, M.J. Piorno Hernández, Mª Jesús Moro Pérez), Hospital Virgen de la Concha. Zamora (C. Ochoa Sangrador, A.F. Bajo Delgado, A. Fernández Testa), Hospital General de Segovia. Segovia (C. Ortega Casanueva).
ABS Llefiá. Badalona. Barcelona (G. Ruiz Aragón), ABS-7 La Salut. Barcelona (P. Aizpurua Galdeano), Hospital Sant Joan de Deu. Barcelona (G. Claret Teruel, S. Fernández Ureña), Hospital Universitari Germans Trias i Pujol. Badalona. Barcelona (M. Méndez Hernández, F. Brossa Guerra, J. Fàbrega Sabaté)- ABS Girona-3. Gerona (R.B. Cortés Marina, E. Fortea Gimeno), ABS Girona-4. Gerona (J.C. Buñuel Alvarez, C. Vila Pablos), Hospital Josep Trueta. Gerona (S. Uriel Prat, Ll. Mayol i Canals).
C. Salud Acequión. Alicante (C. Buhedo Gordillo, G. Rinero de Campos), C. Salud El Cabo. Alicante (M.J. Mateo Moraleda, T. Pérez Martín, A. Redondo, A. Sanguino, B. Sepulcre, B. Serra, A. Tosao), C. Salud El Campello. Alicante (J. Galiano Olivares), C. Salud Guardamar del Segura. Alicante (C.P. Rico Uriós), C. Salud Hospital Provincial. Alicante (M.C. Sirvent Mayor, M.J. Fernández Tarí), C. Salud La Mata. Alicante (M.S. Fuggini), C. Saud Mutxamel. Alicante (L. Comino Almenara, E. Gutiérrez Roble, A. Melnikova, M. Riva), C. Salud Rojales i Benijofar. Alicante (A. Bernabé Gutiérrez, I. Degtyareva), Hospital de Orihuela. Alicante (V. Cañadas Olmo, F. Goberna Burguera), Hospital de San Juan. Alicante (J.L. Mestre Ricote), Hospital de Torrevieja. Alicante (J. González de Dios, C. Rivas Juesas), C. Salud Gran Vía. Castellón (E. Fabregat Ferrer, M.J. Palomares Gimeno), Hospital de La Plana. Villarreal. Castellón (J. Colomer Pellicer), C. Salud La Eliana. Valencia (I. Úbeda Sansano, M. Romero García), C. Salud de Meliana. Valencia (A. Plaza Miranda), Consultorio Auxiliar Albalat de la Ribera. Valencia (C. Sánchez Medina), Consultorio Auxiliar Barrio de la Luz. Valencia (T. Álvarez de Laviada Mulero), C. Salud Padre Jofré. Valencia (P. Barona Zamora), C. Salud Serrería I. Valencia (M. Asensi Monzó).
C. Salud Talavera la Real. Badajoz (C.M. Gómez Málaga), C. Salud Urbano-I. Badajoz (J.J. Cuervo Valdés), C. Salud Villanueva de la Serena Sur. Badajoz (D. Barroso Espadero).
C. Salud Santa Comba. La Coruña (M.E. Amigo Ferreiro), Hospital Arquitecto Marcide. Ferrol. La Coruña (E. García Fernández, A.I. García Villar, R.M. Romaris Barca, M. Santos Tapia), Hospital Clínico de Santiago. Santiago de Compostela. La Coruña (A. Miras Veiga, F. Martinón Torres, N. Martinón Torres, L. Redondo Collazo), Hospital Virxe da Xunqueira. Cee. La Coruña (M.I. Quintela Fernández), Hospital Monforte. Monforte de Lemos. Lugo (S.A. Fernández Cebrián, M.J. Pita Pérez, F. J. Vadillo González), Hospital da Costa. Burela. Lugo (A.G. Andrés Andrés, P. Lago Manchado), Hospital Complejo Hospitalario de Ourense. Orense (C. Lorenzo Legerén, M. Berrocal Castañeda, J.M. Iglesias Meleiro), Complejo Hospitalario Universitario de Vigo. Pontevedra (E. González Colmenero, J. Antelo Cortizas, E. García Martínez, A. Ruiz Conde).
C. Salud Barrio del Pilar (P. González Rodríguez), C. Salud Canillejas (O. Cortés Rico), C. Salud Entrevías-Área 1 (M. Aparicio Rodrigo), C. Salud General Ricardos (G. Orejón de Luna, M.M. Martín Mate), C. Salud Guayaba (M. Duelo Marcos, C. Indaberea Iguaran, A. Nuñez Giralda, F. Muñoz Velasco), C. Salud Juncal (L. Perdikidis Oliveri), C. Salud Mar Báltico-Área 4 (J.L. Montón Álvarez, V. Orbe León), C. Salud Potes. Área 11 (M. Fernández Rodríguez), Hospital Gregorio Marañón (M.M. Guerrero, R. Marañón Pardillo, A. Peñalba Cítores).
C. Salud Bidebieta. Guipúzcoa (M. Callén Blecua), Hospital de Donosita. San Sebastián. Guipúzcoa (J. Korta Murua, F.J. Mintegui Aramburu, I. Olaciregui Echenique, E. Rezola Arcelus), Hospital de Basurto. Vizcaya (C. González Díaz), Hospital de Cruces. Baracaldo. Vizcaya (J. Sánchez Echaniz).