Nasal inflammation in allergic rhinitis enhances bronchial Th2 driven inflammation and development of asthma. We assessed bronchial inflammation induced by natural allergen exposure during pollen season in patients with pollinosis with or without asthma to show the intensity of inflammation in asthma and rhinitis and possible persistence of inflammation in periods without allergen exposure.
MethodsSputum was induced in 52 patients with seasonal allergic rhinitis without asthma, 38 patients with seasonal allergic rhinitis and seasonal asthma and 23 healthy volunteers. Sampling was performed 6–8 weeks before the expected beginning of symptoms, during symptomatic period and 6–8 weeks after the end of symptoms. Sputum ECP was measured by means of chemiluminiscent immunometric assay and sputum cell counts were assessed by classical staining and immunocytochemistry.
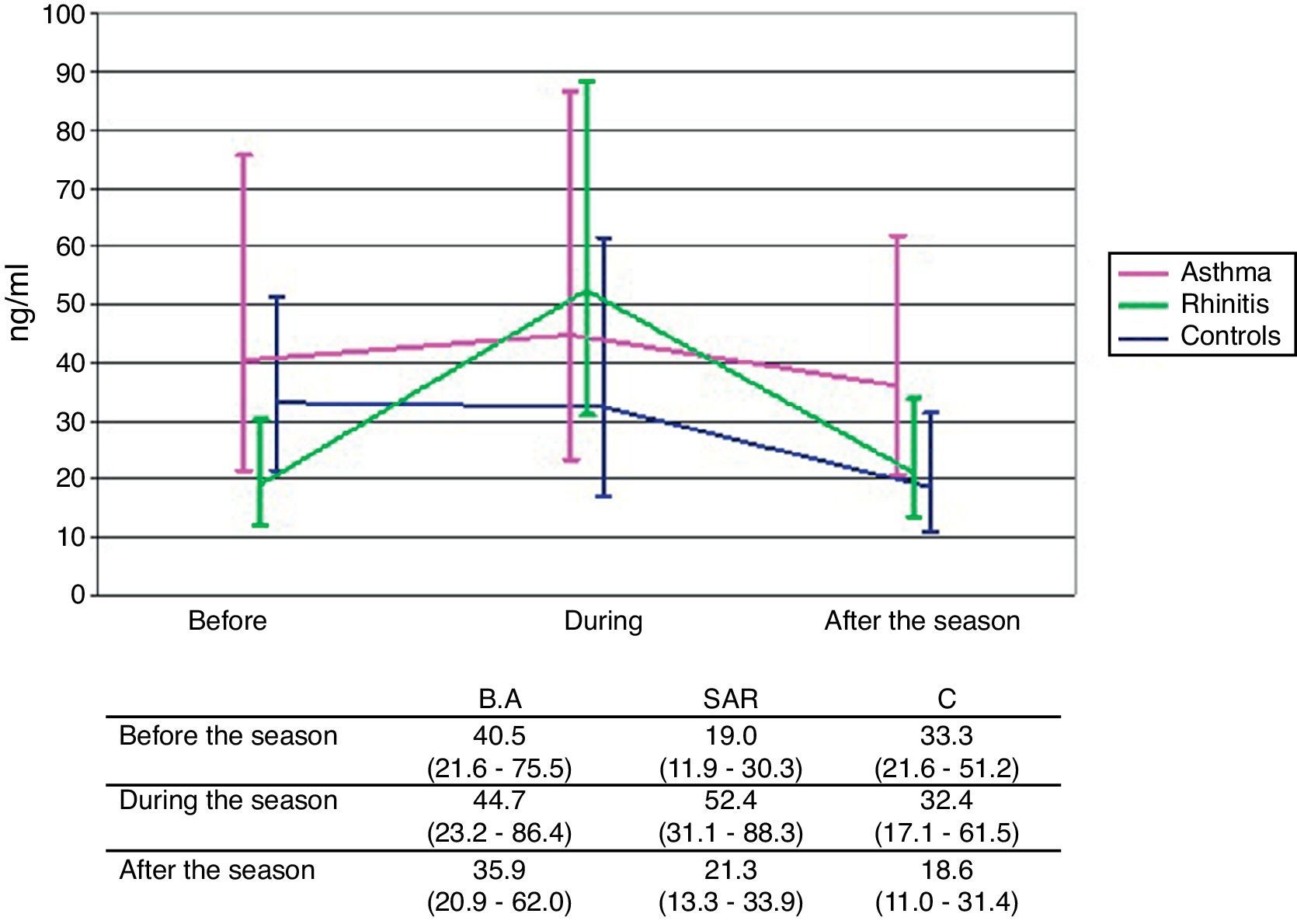
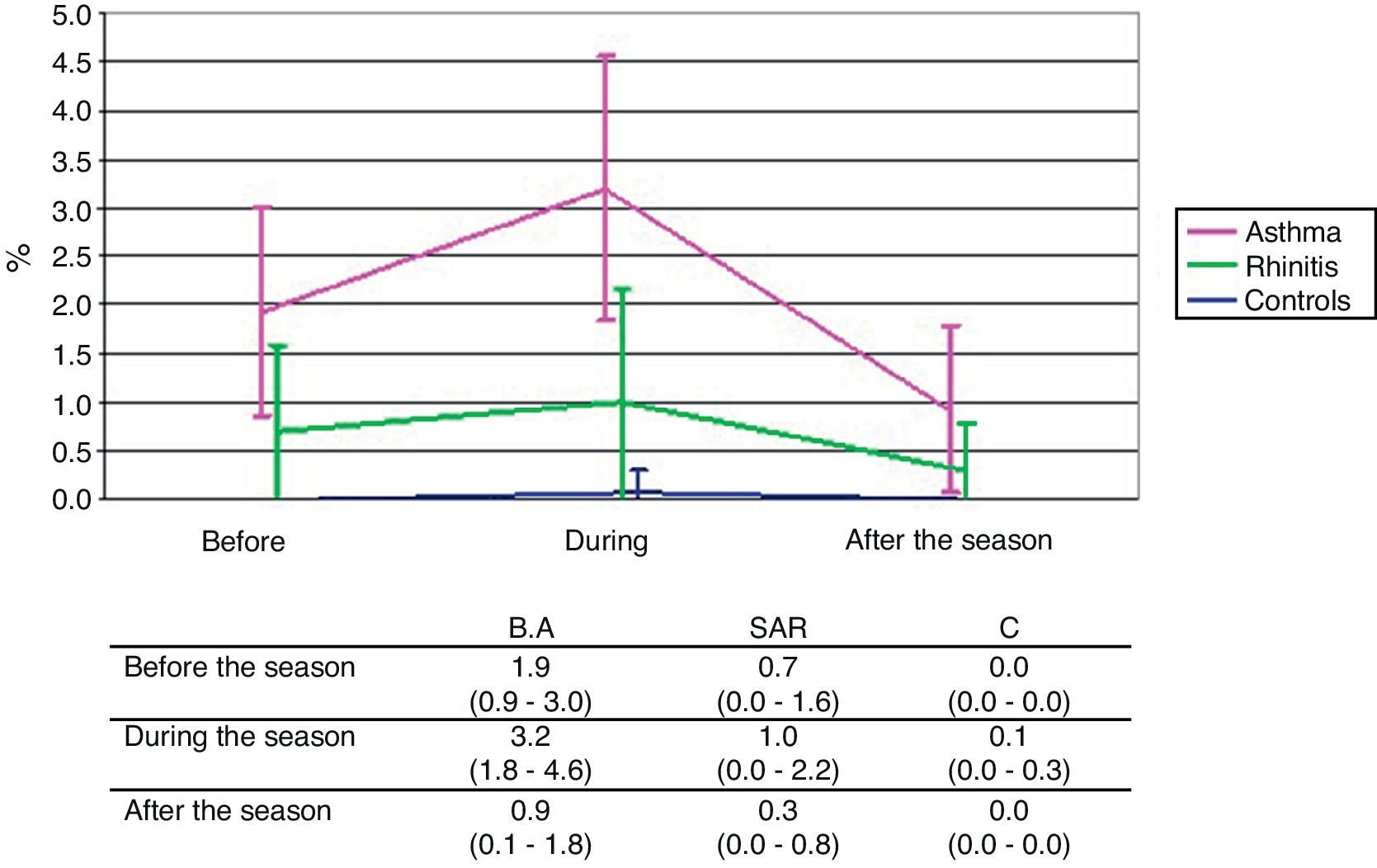
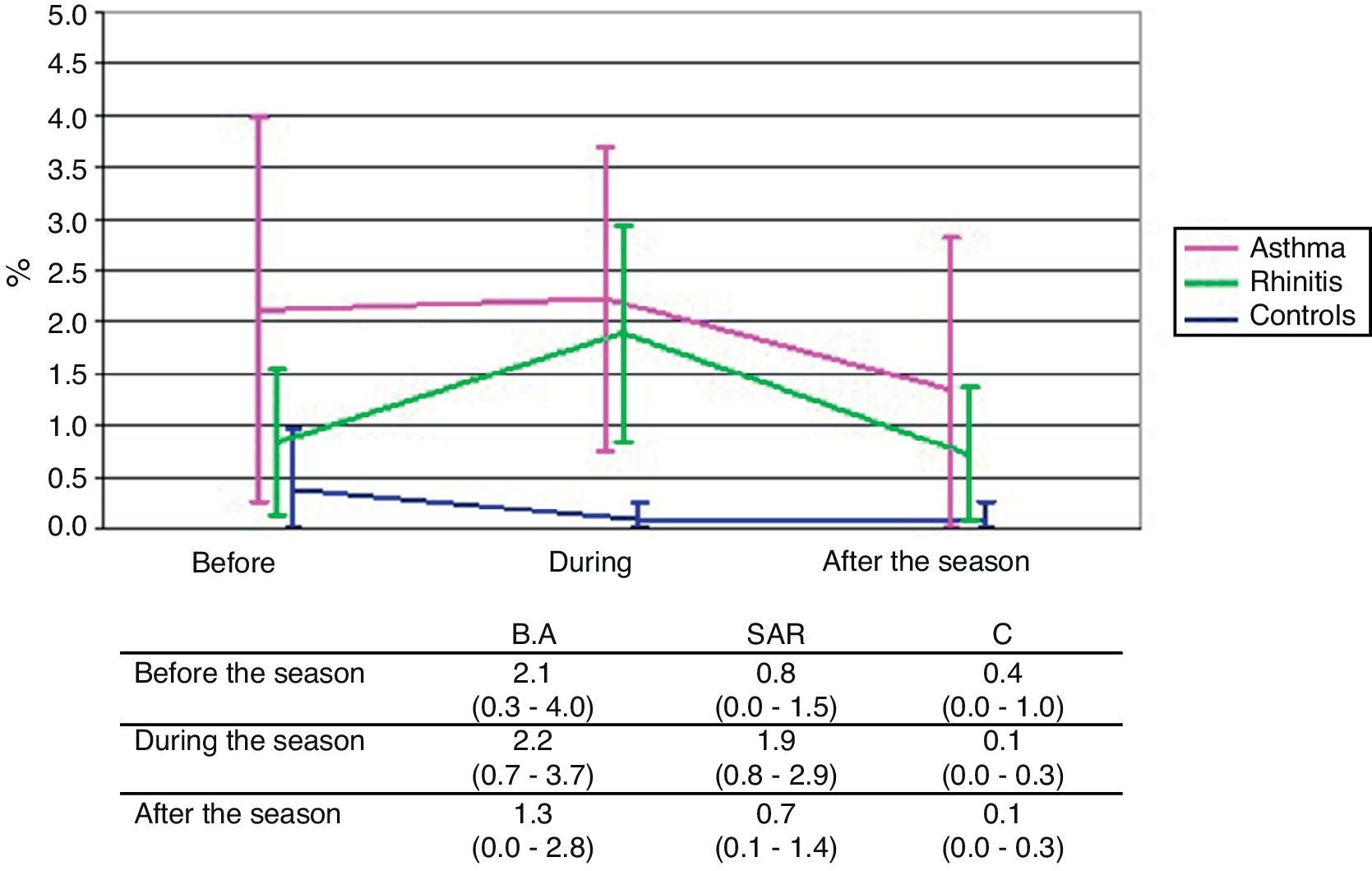
ResultsSputum eosinophils were on the whole higher in both asthma and rhinitis compared to controls (p<0.001, p=0.003). The rise of eosinophils during pollen season compared with values out of pollen season was significant in asthma (classical staining) (p=0.014) and slightly apparent in rhinitis (immunocytochemistry) (p=0.073). The seasonal rise of sputum ECP was observed only in rhinitis (p=0.006).
ConclusionsInflammation of the lower airway in patients with allergic rhinitis with and without asthma has been confirmed by means of both sputum eosinophil count and sputum ECP level. Persistent inflammation of lower airway in periods without allergen exposure was proven in seasonal asthma. This may have implications for the therapy of seasonal allergic rhinitis with and without asthma in terms of promoting long-term anti-inflammatory treatment.
Seasonal allergic rhinitis is one of the most common allergic diseases and its prevalence is steadily increasing.1 Epidemiological and pathophysiological studies suggest that allergic rhinitis and asthma are closely related diseases. They often occur together and allergic rhinitis increases the risk of asthma development.2,3 The inflammatory response in both conditions is orchestrated by Th2 lymphocytes, while other cell types may play a role, eosinophils being the most important. Asthma and allergic rhinitis are two manifestations of one common allergic respiratory syndrome. The relationship between asthma and allergic rhinitis is complex and upper and lower airways interact with each other.4
Although the relationship between allergic rhinitis and asthma has been well established, direct links between nasal and bronchial inflammation in seasonal allergic rhinitis without asthma remain to be exactly investigated. Several authors have focused on the effect of natural allergen exposure or nasal challenge on lower airway,5 but the dynamics of bronchial inflammation in patients with pollen allergic rhinitis during and out of the pollen season are not completely clear yet, as well as the exact consequences of this inflammation.
The presence of bronchial inflammation in non-asthmatic patients with seasonal allergic rhinitis has previously been reported.6,7 Natural exposure to pollen allergen induces inflammatory cell recruitment leading to bronchial eosinophilic inflammation in these patients.8 On the other hand, increased inflammatory markers in nasal mucosa from non-asthmatic patients with seasonal allergic rhinitis after segmental bronchial allergen provocation were found, suggesting a systemic cross-talk between upper and lower airways. High incidence of airway hyperresponsiveness in non-asthmatic patients with seasonal allergic rhinitis is also well known. Both natural exposure to pollen and nasal allergen challenge induce an increase in airway responsiveness to methacholin in non-asthmatic patients with seasonal allergic rhinitis.5 In studies focusing on the relationship between sputum eosinophils and airway responsiveness in general population, no correlations were found, although there was a weak correlation found when atopic subjects were considered alone.9 No significant differences were found between healthy subjects and subjects with rhinitis. Sputum eosinophil counts did not correlate with methacholine PC20 or blood eosinophils.10 Some studies describe changes in sputum ECP level after nasal allergen challenge. This parameter seems also to reflect well the inflammatory changes in bronchi.11
Seasonal allergic rhinitis could predispose to the development of chronic bronchial inflammation as observed in asthma. Direct links between nasal and bronchial inflammation and airway responsiveness in patients with seasonal allergic rhinitis without asthma are not fully understood yet.12
The aim of this study was to confirm the presence of bronchial inflammation in pollen allergic subjects with seasonal allergic rhinitis with or without asthma, to compare the intensity of the inflammation during the pollen season (natural allergen exposure) with the periods out of the pollen season (before and after the season) and to compare the intensity of the inflammation in patients with allergic rhinitis with asthma vs. patients without asthma and vs. healthy controls.
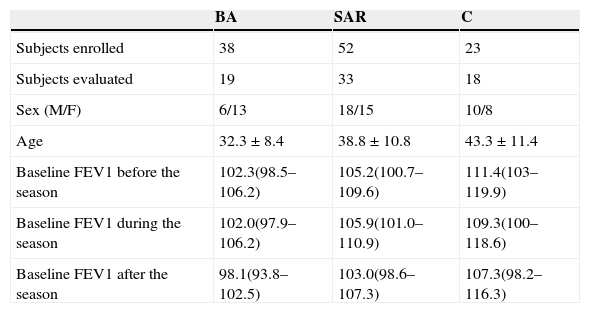
Materials and methodsPatients38 patients with seasonal allergic rhinitis and seasonal intermittent bronchial asthma (BA), 52 patients with seasonal allergic rhinitis without asthma (SAR) and 23 healthy controls (C) were recruited consecutively from patients treated in the outpatient allergology service and from healthy volunteers. The diagnosis of asthma was based on clinical history of recurrent episodes of wheezing, breathlessness and/or cough associated with reversible airway obstruction. Historical positivity of reversibility test was required (improvement in FEV1 of at least 12% 30min after inhalation of 400¿g of salbutamol). 55.8% of patients in the SAR group and 55.3% of patients in the BA group used antihistamines for symptom relief during the followed pollen season. Demographic characteristics of the subjects are described in Table 1.
Characteristics of subjects.
| BA | SAR | C | |
|---|---|---|---|
| Subjects enrolled | 38 | 52 | 23 |
| Subjects evaluated | 19 | 33 | 18 |
| Sex (M/F) | 6/13 | 18/15 | 10/8 |
| Age | 32.3±8.4 | 38.8±10.8 | 43.3±11.4 |
| Baseline FEV1 before the season | 102.3(98.5–106.2) | 105.2(100.7–109.6) | 111.4(103–119.9) |
| Baseline FEV1 during the season | 102.0(97.9–106.2) | 105.9(101.0–110.9) | 109.3(100–118.6) |
| Baseline FEV1 after the season | 98.1(93.8–102.5) | 103.0(98.6–107.3) | 107.3(98.2–116.3) |
Age – arithmetic means±s.d.
Baseline FEV1 (% predicted) – arithmetic means and 95% confidence intervals.
Seasonal allergy was defined as occurrence of seasonal symptoms during spring and/or summer and at least one positive skin prick test (mean wheal diameter ≥3mm) to a pollen allergen (trees, grass, weeds) and a positive specific serum IgE result (≥0.7IU/ml) to this pollen allergen.
All patients had a clinical history of allergic rhinitis during the previous two or more pollen seasons; all patients with asthma had a clinical history of seasonal asthma for at least the pollen season prior to the study. Asthma patients were treated only by short-acting ¿2-agonists administered as required; no treatment with systemic, inhaled or nasal corticosteroids was used before or during the study. Healthy controls did not use any form of medication influencing allergic inflammation, they had no symptoms of rhinitis, no history of asthma or other allergic diseases and they had no positivity in skin prick testing to common aeroallergens.
All subjects had no upper respiratory tract infection in the last four weeks prior to each sputum induction. Non-smoking was a prerequisite for selection. Informed consent for this study was signed by all subjects.
All included subjects were examined 6–8 weeks before the expected start of their seasonal symptoms, during the period of symptoms and 6–8 weeks after the end of symptoms. Treatment with antihistamines and cromones during the period of sampling was not restricted, as well as allergen immunotherapy in maintenance phase. The comparative analysis (results not shown) proved that none of these treatments had any influence on the followed inflammatory parameters.
Lung function measurementMeasurement of basic lung function was performed by means of a computerised system MasterScope (Jaeger, Wuerzburg, Germany) according to standard guidelines. Subjects did not use short or long acting bronchodilators 12h prior to the measurement and did not drink tea or coffee in the morning before the measurement and sputum induction.
Sputum induction and processingSputum was induced by the method described by Pizzichini.13 Briefly: patients inhaled increasing concentrations of hypertonic saline (3%, 4% and 5% NaCl) each for 7min through a mouthpiece with a nose clip, 15min after premedication with 200¿g of inhaled salbutamol. Aerosol was generated by an ultrasonic nebuliser DeVilbiss 2000 (DeVilbiss, Somerset, USA) with the output of 1ml/min. Lung function tests were performed before sputum induction and after each period of inhalation. Subjects were encouraged to cough deeply after each period and in any other time they felt the need. Samples were collected in a sterile container and kept at 4°C until processing.
Sputum was processed according to the method described by Pin and Gibson14,15 within 2h after collection. Sputum plugs were selected and treated with four times their weight of freshly prepared 0.1% dithiothreitol (Sputolysin, Calbiochem, San Diego, USA) at room temperature for 15min. The suspension was four times its volume diluted with phosphate buffered saline. Dispersed samples were filtered through a 48¿m nylon mesh to remove cell debris and mucus. The proportion of salivary squamous cells was noted and viability was determined using the trypan blue exclusion method. Only samples with cell viability ≥70% and squamous cell contamination ≤20% were considered for evaluation. Slides were prepared by centrifuging 100¿l of the cell suspension at 450rpm for 6min (Cytospin IV, Shandon, England). The remaining sample volume was centrifuged at 1500rpm for 5min and supernatant was stored at −70°C for later analysis.
Slides for classical staining were air-dried, fixed and stained with Hemacolor Rapid (Merck, Darmstadt, Germany). Slides for immunocytochemistry staining were fixed in acetone–methanol and stored at −20°C until use. Immunocytochemistry was performed using DAKO (Glostrup, Denmark) monoclonal antibodies, by the alkaline-phosphatase/anti-alkaline-phosphatase (APAAP) staining procedure. Antibodies anti-CD68 targeted macrophages, anti-neutrophil-elastase targeted neutrophils and anti-major basic protein (MBP) targeted eosinophils. Dilutions 1:50, 1:100, and 1:30 respectively, were used. Reading of slides obtained by either method was carried out by two investigators who had no knowledge about the clinical characteristic of the patients and 400cells were counted. Results were expressed as percentage of the total non-squamous cell count.
ECP in the sputum fluid phase was measured by chemiluminiscent immunometric assay (Immulite 2000, Siemens, Germany). The results were adjusted for the dilution factor. The detection threshold was 0.2ng/ml.
Statistical analysisContinuous data are presented as arithmetic mean and standard deviation values, ECP levels were log transformed and described as geometric means. Variability of the data was characterised by 95% confidence intervals. Group and time effects assessment was based on a repeated-measures analysis of variance followed by Sidak's test for multiple comparisons and orthogonal contrasts. Differences in proportions between groups were tested by Pearson's ¿2-test. Correlations between variables were analysed using Spearman's rank correlation coefficient. p-Values less than or equal to 0.05 were considered statistically significant.
ResultsThe groups of patients and controls did not differ with respect to sex and age significantly. Sputum induction was successful in 288 (85.0%) of procedures. Technical problems in sputum processing were encountered in 77 (26.7%) of these patients’ samples (cell viability ≤70%, squamous cells contamination ≥20% or high salivary content). These samples were excluded from evaluation. Paired sputum values were available in 70 (61.9%) subjects (see Table 1). Mean weight of obtained sputum samples was 0.728g, total non-squamous cell count 2.84×106/ml and cell viability 73%. These parameters were not statistically different among the groups and periods of examination.
Pulmonary functionMean FEV1 (% predicted) values in patients of all groups in the three timepoints are shown in Table 1. No significant differences and changes in baseline pulmonary function before, during and after the pollen season were observed in any group. No correlations between FEV1 and sputum eosinophils or ECP were found.
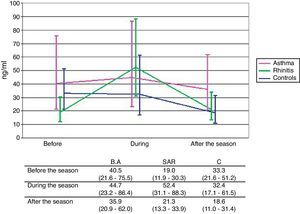
Concentration of sputum ECPNo significant differences in sputum ECP levels among the groups in any of the periods of examination were found. Significant rise of sputum ECP levels during the pollen season compared to ECP levels out of the season was observed only in patients with SAR (p=0.006). In consequence, there is an apparent although still non-significant difference in time course between the SAR group and the two other groups (p=0.105) (Fig. 1).
Differential sputum cell countsDifferential cell count was assessed by classical staining (Haemacolor Rapid) and by immunocytochemistry staining.
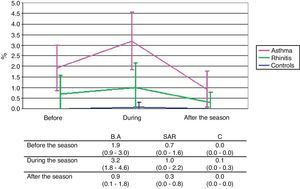
Classical stainingThe mean eosinophil counts expressed as percentage of non-squamous cells, were on the whole significantly higher in the BA group compared to the C group (p<0.001) reaching significance particularly before (p=0.005) and during (p<0.001) the pollen season. The difference between the SAR and C groups is in total on the borderline of significance (p=0.073) but is not provable at any single time point. The difference between the BA and SAR groups was significant globally (p=0.007) and in particular during the pollen season (p=0.017). The rise of eosinophil count during the pollen season compared with values out of the pollen season was significant only in the BA group (p=0.014) (Fig. 2).
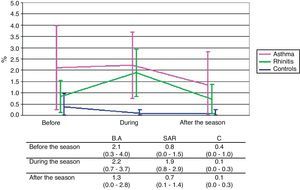
Immunocytochemistry stainingSimilar changes as in classical staining were observed also in the detection of eosinophils by immunocytochemistry. The mean eosinophil counts expressed as percentage of the non-squamous cells were on the whole significantly higher in both the BA and SAR groups compared to the C group (p<0.001 and p=0.003, respectively). The difference between the BA and C groups was significant in measurements before (p=0.046) and during (p=0.015) the pollen season and was on the borderline after the season (p=0.077). The difference between the SAR and C groups was manifested especially during the pollen season (p=0.016). No significant difference between the BA and SAR groups was observed in any timepoint of examination. The rise of eosinophil count during the pollen season was apparent only in the SAR group (p=0.073) (Fig. 3).
There were no significant differences in number of macrophages and neutrophils in sputum samples, during and out of the pollen season in any group.
DiscussionIn several studies a model of artificial allergen challenge was used to study airway inflammation in asthma and rhinitis.5 We decided to use the natural allergen exposure with its long-time duration to obtain results which reflect best the conditions in real disease. It was proven that bronchial and nasal allergen challenges give similar inflammatory response in the airway but less systemic inflammation than seasonal exposure.11 The nasal allergen challenge was shown to elevate sputum eotaxin and sputum eosinophils.16 However, other authors did not show elevation of sputum eosinophils even after subsequent multiple nasal allergen challenges which may more adequately approximate the natural exposure.17 Other studies focused on soluble inflammatory mediators only18,19 and showed similar results as our study. We confirmed the findings also by following the cellular inflammatory markers in sputum and we extended the observations to two measurements out of the period of allergen exposure – both before and after the pollen season – with the aim of following the possible persistence of eosinophilic inflammation. We also aimed to minimise the possible inconsistencies in sputum cell profiles in individual patients by repeated sampling.9,20,21
Induced sputum examination is a time consuming and technically not simple procedure, which limits its extension to the practice. Sometimes also safety issues of this procedure are raised. Inhalation of hypertonic saline aerosol carries the risk of bronchoconstriction in patients with bronchial hyperresponsiveness. We performed sputum induction in patients with baseline FEV1 higher than 90% predicted and we did not observe a considerable fall in FEV1 after inhalation of saline aerosol in any of the subjects. We confirmed that sputum induction is a reliable and safe alternative to obtain secretion from the lower airway, in order to study the presence and quality of cells and inflammatory mediators present in the airway and their changes during the course of the disease. Induced sputum can be used to monitor the presence and severity of lower airway inflammation both in asthma and allergic rhinitis.
Sputum eosinophil count seems to be the most important marker of airway inflammation.22,23 Increased number of sputum eosinophils is associated with allergen exposure and reflects the severity of airway inflammation in asthmatic patients24 and it decreases during corticosteroid therapy.25 It is estimated that more than 80% patients with asthma previously not treated with corticosteroids and more than 50% of those who used inhaled corticosteroid therapy, may have elevated sputum eosinophil numbers.24
Results of sputum cell counts obtained by means of classical staining and by immunocytochemistry staining are comparable and 89% of samples in our study were evaluated identically in the sense of elevated eosinophil count. Differences in the percentages of eosinophils obtained by these two methods were not statistically significant and may be explained by methodology aspects: immunocytochemistry is based on the detection of cell antigenic markers (MBP) and classical differential cell count is based on morphology and cellular staining.
Some studies refer the relation of airway inflammation and airway hyperresponsiveness26 or report significant inverse correlation between sputum eosinophils and lung function. Other authors did not observe this correlation, or the correlation between sputum eosinophils and bronchial responsiveness. This might be due to relatively good lung function in the included asthma patients. In our study none of the enrolled patients had FEV1 lower than 90% predicted. We suppose that this is the reason why we did not observe any correlations between FEV1 and inflammatory markers.
Sputum ECP levels are related to the inflammatory activity of the disease. ECP level in sputum supernatant reflects eosinophil degranulation and does not necessarily closely correlate with sputum eosinophil count,27 but in general, moderate correlation between sputum ECP and eosinophil count is observed.28–30 Sputum soluble inflammatory markers may sometimes reflect better asthma activity than the number of eosinophils alone.31 Elevated sputum ECP levels were also detected after single allergen challenge in patients with mild allergic asthma32 and allergic rhinitis.33 Our results correspond to these facts.
We observed the raise of sputum ECP levels during the pollen season only in SAR patients, surprisingly not in BA patients. This may be due to slightly higher levels of ECP in BA patients present also out of the pollen season, what supports the presence of persisting allergic inflammation in the bronchi of these patients also in asymptomatic periods. On the other hand this result confirms the presence of bronchial allergic inflammation during pollen exposure in patients with no bronchial symptoms (SAR).
The presence of higher numbers of eosinophils in bronchi in BA patients compared to controls confirms persisting allergic inflammation in bronchi of these patients which is present even in asymptomatic periods. This may correspond to a similar situation in asthma patients who are clinically well controlled on therapy.34 It also corresponds to the fact that asthma, even when in remission, is accompanied by airway inflammation and remodelling.35
The difference in eosinophil counts between SAR patients and controls is only borderline in classical staining and more clearly expressed in immunocytochemistry staining. The raise in eosinophil counts in SAR patients during the pollen exposure is not pronounced in classical staining, but significantly present in immunocytochemistry staining. In BA patients, this raise is significantly present only in classical staining and not pronounced in immunocytochemistry staining probably because a certain degree of inflammation is present also in periods without allergen exposure.
It seems that the immunocytochemical detection of eosinophils corresponds more closely to sputum ECP levels than the detection by means of classical staining and probably reflects better the real changes in bronchial inflammation during the followed periods.
The main findings of this study indicate that sputum eosinophil counts and ECP levels are to a certain extent and with different dynamics elevated both in BA and SAR patients as a result of natural allergen exposure during pollen season. Our results support the view that subclinical inflammatory changes in the lower airway are present in patients with allergic rhinitis without asthma and in asthma patients both in symptomatic and asymptomatic periods.
The natural courses of untreated or under-treated eosinophilic airway inflammation are unknown. Eosinophilic inflammation can heal spontaneously, especially if it is a result of exposure to an allergen, which ceases. Prolonged eosinophilic inflammation requires treatment with an anti-inflammatory agent. Optimal treatment of patients with SAR in the period of allergen exposure, which may stop the progression of SAR to asthma, is still under investigation. Some authors suggest that effective treatment may have to involve inhaled steroids for 2–3 months.36 More, it is well known that patients with SAR without asthma symptoms may experience a sudden asthma attack.37 Corticosteroid therapy was proven to be the most potent anti-inflammatory approach in allergic conditions. Anti-inflammatory effects of antihistamines are limited and their role as the first line treatment in SAR should be reconsidered from this point of view. The similar characteristics of inflammation (Th2 driven) in both BA and SAR may indicate that the therapeutic approaches should be similar as well.
At present, the asthma treatment level is mostly guided solely by clinical indices such as symptoms and lung function and the current therapeutic approach is based on the assumption that there is a relationship between clinical indices of asthma severity and bronchial inflammation. However, a major drawback of these markers is their uncertain relationship with the degree of airway inflammation.38 Several studies suggest that sputum eosinophils may be useful for tailoring the asthma treatment.39,40 Our study supports the idea that the treatment approach should consider the presence of allergic inflammation in the airways. This may concern both persistence of inflammation after the end of pollen exposure in patients with seasonal BA, and the presence of inflammation of bronchi in patients with SAR without asthma symptoms. It should be suitable to consider the treatment by anti-inflammatory drugs instead of antihistamines alone.
In summary, the presence of allergic inflammation of lower airway was detected by means of sputum ECP levels and eosinophil counts not only in patients with seasonal asthma, but also with seasonal allergic rhinitis. This inflammation persists to certain extent even in periods without allergen exposure especially in patients with seasonal asthma. Measurement of bronchial inflammation seems essential not only to achieve proper asthma control, but also to consider the optimal treatment.
Ethical disclosureProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestAll the authors declare no conflict of interest.
This research was performed with institutional support by the Faculty of Medicine in Pilsen, Charles University in Prague.