INTRODUCTION
Cladosporium has been known to be one of the most airborne fungi causing respiratory allergic diseases particularly asthma and rhinitis 1. Cladosporium cladosporioides is the most prevalent species 2. Cladosporium herbarum frequently dominates the outdoor mycoflora and it has been extensively studied 3-5. The direct implication of this fungus in inhalant allergy is now recognized 6-9. The lack of standardized extracts, the instability and the variability of their antigenes and allergens composition have been the most problem to progress in understanding fungal allergy. Extracts from different strains of Alternaria alternata, Aspergillus fumigatus, Candida albicans and Epicoccum nigrum vary greatly in their allergens composition 10-13. The variability between different batches of Cladosporium extracts seems very high 14,6. This variability may be due to genetical heterogeneity of fungal isolates, to extraction procedures, to the propagule used for allergen extraction, and to environment conditions (such as, climatic factors, growth media, degree of exposure). Most studies on allergenic moulds are based on culture filtrate extracts obtained from mycelium, rarely on somatic ones 15-17). Mycelium is the easiest propagule to grow in vitro. However, it is not the fungal propagule inhaled by the patients whereas fungal allergy results from exposure to spores 18. Airborne fungal spores can penetrate the lower airway and enter in contact with the mucosa. Moreover, previous studies have demonstrated that spores contain high allergenic potency when compared to the mycelium 19,20. However, a comparison of Alternaria alternata spore and mycelium extract found that mycelium extract have greater potency than that of spore extract on the basis of skin test, RAST inhibition and basophile histamine release 21. It is now acknowledged that the standardized conditions for optimal growth and sporulation and the preparation of metabolic and somatic extracts for both early and late growth phase fungal culture using the best source material containing the most relevant allergens are recommended 9,22.
In order to choose the best source material for the preparation of Cladosporium extracts, the allergenic potency of spore and mycelium extracts of different species of Cladosporium has been compared using RAST inhibition and PCA tests. RAST inhibition technique has been used in homologous and heterologous inhibitions between fungal extracts of many species of fungi imperfecti and Basidiomycetes 23-25. In the case of Cladosporium, this method has been coupled to PCA tests which have already proven to be very effecient to study the reaginicity of several Cladosporium extracts 26,7.
MATERIAL AND METHODS
Preparation of the fungal extracts
The strain LCP 404 of C. cladosporioides, LCP 406 of C. sphaerospermum (museum national d'histoire naturelle, Paris, France) and IP1679-87 of C. herbarum (Unité de Mycologie, Institut Pasteur, Paris, France) were grown on 2 % malt agar medium at room temperature. The spores (> 95 % pure for C. cladosporioides and C. sphaerospermum, > 60 % for C.herbarum) were harvested with a paint brush after 3 to 4 weeks of growth. The mycelium was obtained in a 2 liters Biolafitte fermenter containing 2 % glucose, 1 % peptone (Prolabo) and 0.1 % Rhodorsil 426 R. After 48h of growth at 25 °C, 700 rpm and 0.5 vvm, the mycelium was recovered by filtration, washed with distilled water and stored at 20 °C.
Spores or mycelia were suspended in 10 mM phosphate buffer saline pH 7.2 (PBS) or 50mM Tris pH 9.0 containing 1mM EDTA and 1 % PVP (TEP) or 50 mM NaHCO3 pH 8.0 (Bic) and disrupted in a glass bead (1 mm) MSK Braun cell homogenizer. Bic total fungal extract was stored freeze dried. PBS and TEP extracts were centrifuged (30 min, 15 000 g) and the supernatants were stored at 80 °C. Protein content was measured using the BioRad method.
Sensitization of guinea pigs and mice and PCA test
Guinea pigs were sensitized using Bic total extracts of spore and mycelium of C. cladosporioides as previously described 26. For mice sensitization spores and mycelium extracts from C. cladosporioides were used. Six groups of 5 Female Balb/c were sensitized using TEP soluble extracts as previously described 26. PCA was performed as described by Ogilvie 27 and modified by Ovary 28. PCA titer was the lowest dilution of serum giving a positive skin reaction.
RAST and RAST inhibition
Extracts of spores of C. herbarum and spores and mycelium of C. cladosporioides obtained by cell disruption in PBS were used for RAST and RAST inhibition experiments.
RAST
RAST was performed according to Ceska et al 29. RAST disks were prepared by incubating overnight at 4 °C CNBr activated cellulose disks in 100 μl aliquots containing increasing concentrations of extracts (5 to 500 μg of proteins/ml depending on the extract). After 2 successives incubations (3 and 1 h) of the disks in 0.1 M Tris pH 8.0 and 3 washes in PBS buffer at room temperture, 50 μl of a pool of sera from positive patients were added. Sera were selected on the basis of positive prick tests and RAST to Cladosporium commercial extracts. After 3h incubation, washes and successive incubation with 125 I-rabbit anti-IgE were performed according to Phadebas (Pharmacia) technical informations. IgE uptake was expressed as the percentage of total cpm measured with 50 μl of the same 125 I-anti-IgE.
RAST inhibition
The assay used was adapted from the technique developed by Yman et al 30. 100 μl of spore or mycelium extract (liquid-phase) containing increasing concentrations of proteins (0,25; 0,5; 1; 2,5; 5 μg/ml) were incubated for 3h at room temperature with 100 μl of the patient sera diluted in PBS (1:1). Cellulose disks coupled to myceliun (500 μg proteins/ml) or spore (5 μg proteins/ml) extracts were added to the serum aliquots adsorbed with the different concentrations of soluble allergens. The allergen concentrations for the coupling and the sera dilution were determined by preliminary experiments as the lowest serum and extract dilutions giving the maximal IgE uptake. IgE uptake was measured as described for RAST. The percentage of inhibition of IgE uptake was plotted against the allergen concentration in the liquid-phase.
RESULTS
Sensitized animals
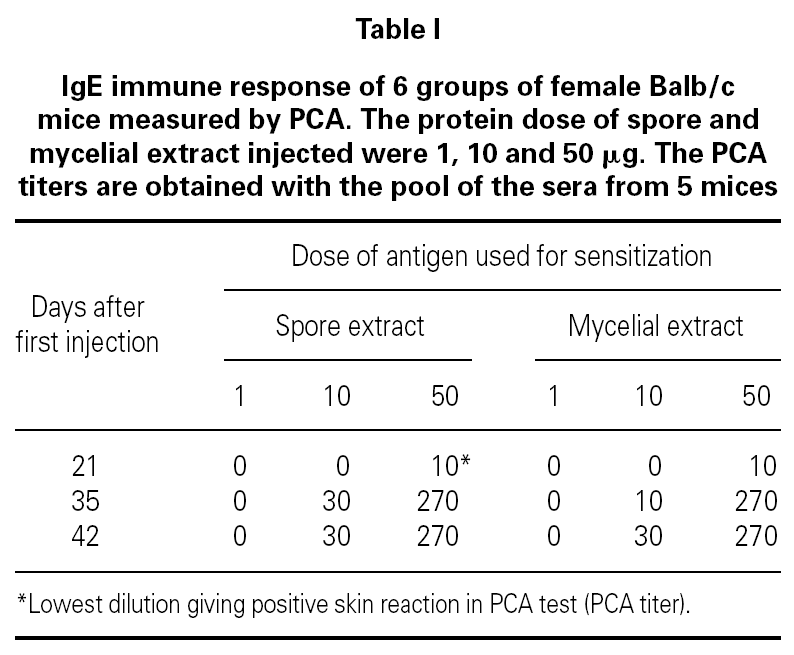
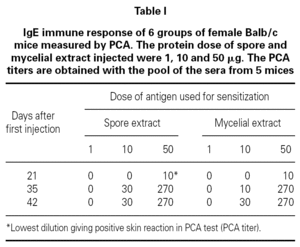
Table I showed the production of IgE antibodies in mice after sensitization with spore or mycelium extracts. No IgE response was observed after 4 injections of 1 μg proteins per mouse. A primary IgE response was only observed at day 21 when a dose of 50 μg proteins was used. The secondary IgE responses varied according to the concentration of protein used for booster. 10 μg injections induced slowly IgE increase. A titer 30 of IgE-antibodies in the serum was obtained. After 3 injections of 50 μg proteins per mouse, a maximum titer of IgE was obtained. Such sensitization protocol was used for all experiments in mouse.
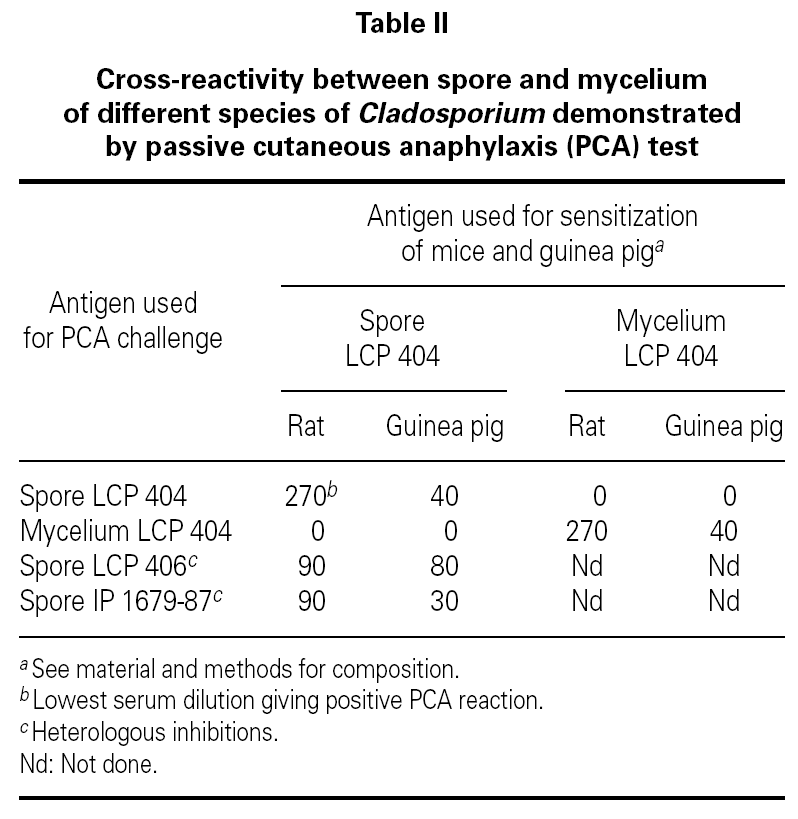
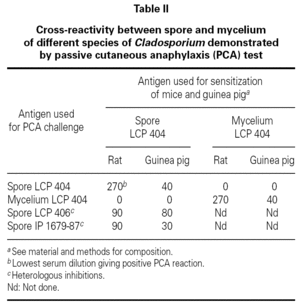
No cross-reactivity was observed when mice and guinea pigs sensitized to spores of C. cladosporioides were challenged with mycelium extracts of the same strain (table II). At the opposite, positive PCA tests were obtained when animals sensitized to spore of C. cladosporioides were challenged with spore extracts of C. sphaerospermum and C. herbarum (table II). These results indicated that spore extracts of different species of Cladosporium contained closely related allergens which looked different of the mycelial allergens even when they are extracted from the same strain.
Atopic human patients
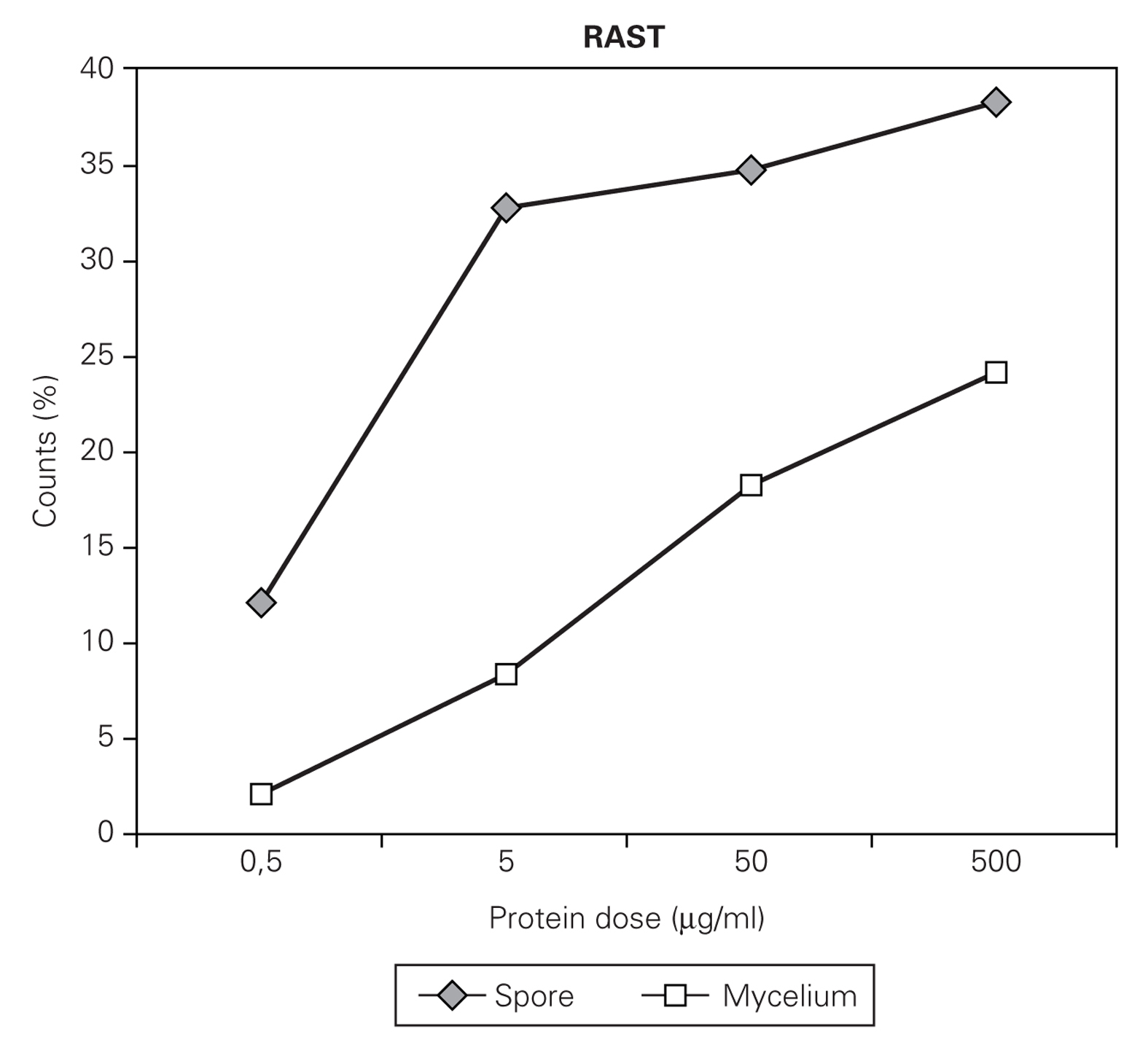
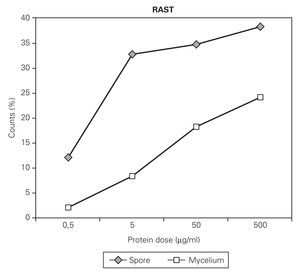
The uptake of IgE on the CNBr activated disk was dependant on the concentration used for the coupling of the allergens to the disk (fig. 1). For example, in the case of C. cladosporioides, maximal uptake was obtained when the disks were incubated respectively with spores and mycelial extracts containing, respectively 50 μg or 500 μg proteins/ml PBS (fig. 2). A proportional dose dependent immuneresponse was obtained between 0.5 to 5 μg spore extracts and 5 to 500 μg mycelial preparation. Spore and mycelium allergens of C. cladosporioides were also compared in RAST inhibition experiments
Figure 1.--RAST values obtained after incubation of CNBr-activated disks with different concentration of spore and mycelial extract of C. cladosporioides.
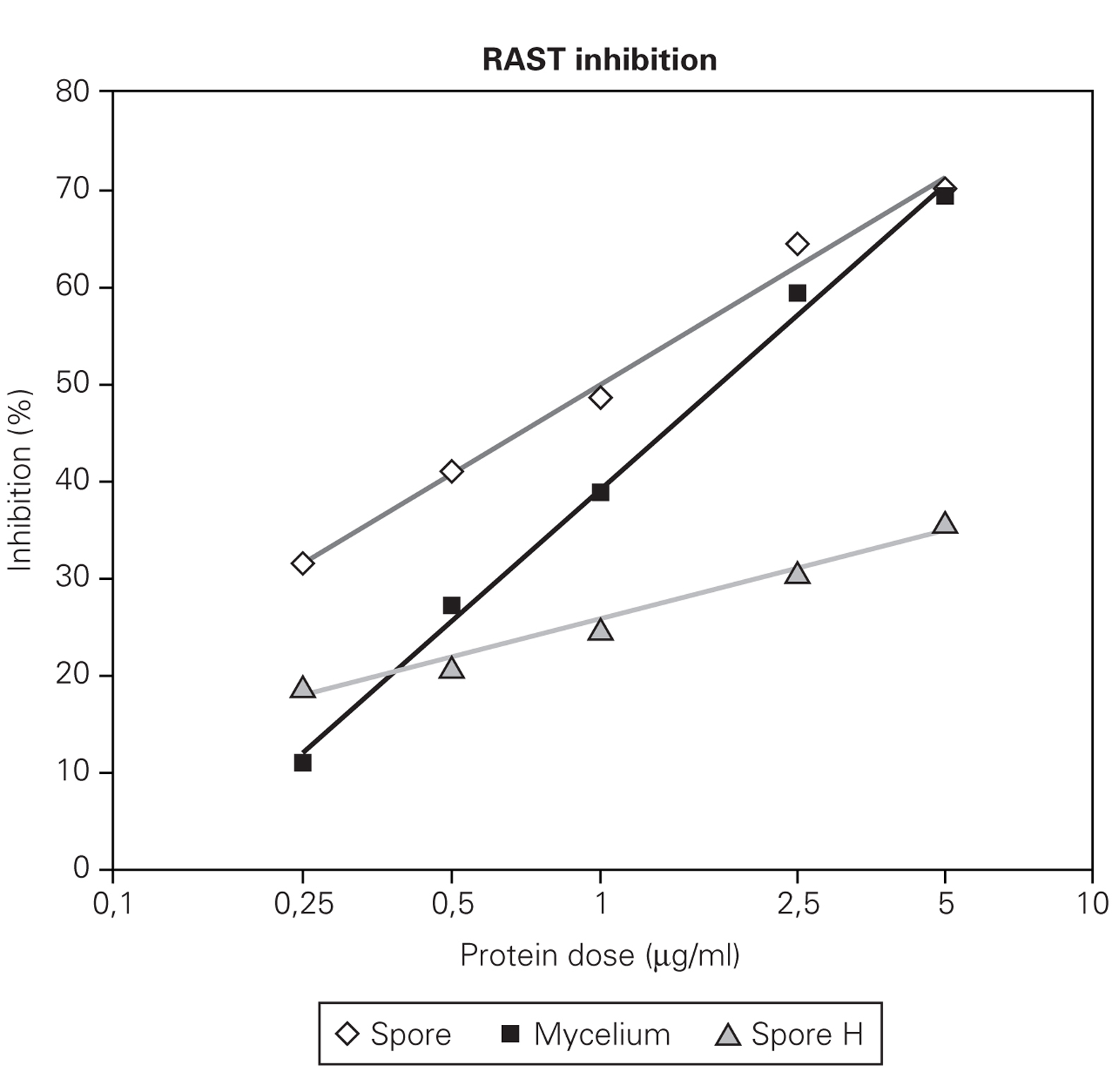
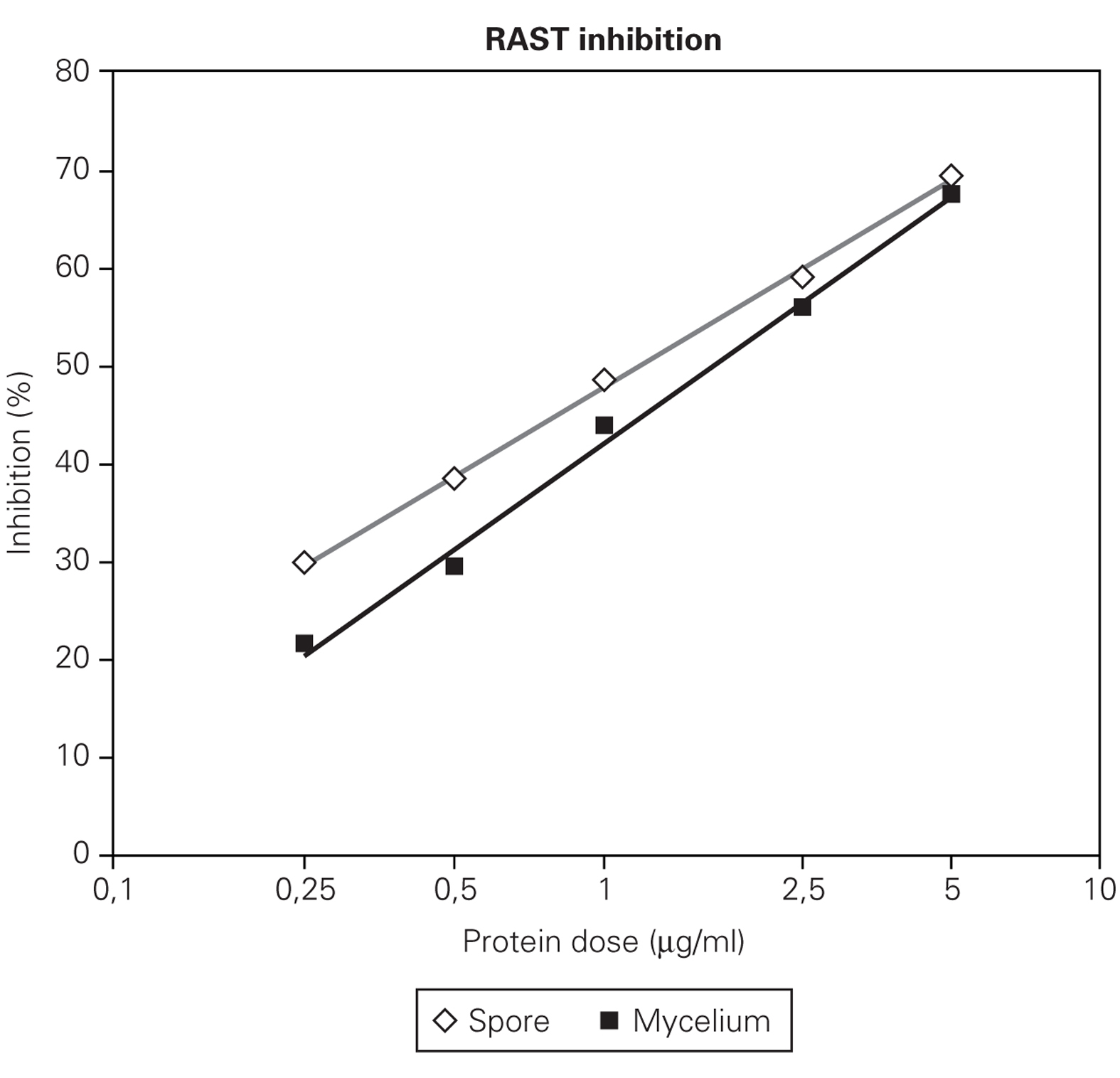
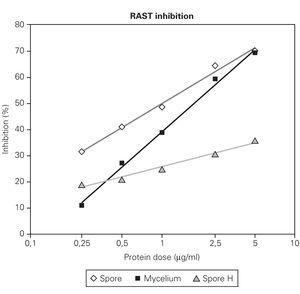
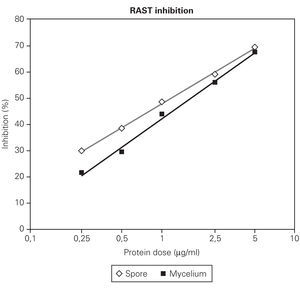
Figures 2 and 3 showed that RAST inhibition percentages were comprised between 10 % and 80 % according to the protein dose used. Logit transformation of the RAST inhibition curves produced different slopes with the three extract used. C. cladosporioides spore extract was the most potent inhibitor and C. herbarum extract was the lowest (fig. 2). At the lowest protein dose of spore or mycelial extract, the differences in RAST inhibition percentages between the two extracts were more important. At the dose of 5 μg proteins/ml, the spore and mycelial extracts of C. cladosporioides showed the same RAST inhibition response. When spores were used as solid phase (fig. 2), 50 % inhibition was obtained with 1 μg/ml of spore extract and 1.75 μg/ml of mycelium extract. When disks coupled to mycelium extracts were used as solid phase, 0.75 μg/ml of spore extract and 1.25 μg/ml of mycelium extract were necessary to obtain 50 % inhibition.
Figure 2.-- RAST inhibition curves produced by different protein dose (0,25; 0,5; 1; 5 μg/ml) of spore and mycelium extract of C.cladosporioides and by C. herbarum spore extract (H) using C. cladosporioides as the solid phase.
Figure 3.--RAST inhibition experiment with C. cladosporioides mycelium preparation as the solid phase and spore and mycelium of the same strain as the the liquid phase at increasing protein dose (0,25; 0,5; 1; 2,5; 5 μg/ml) for serum inhibition.
Spore extracts gave the highest degree of inhibition of the uptake of specific IgE antibodies on the cellulose disk when spore but also mycelium were used in the solid phase.
DISCUSSION
A classic question in mould allergy is whether spores or mycelium provide the best source of allergens 7,9. The results obtained in this study demostrated that spore and mycelium of C. cladosporioides have different allergenic potencies; the spore extracts being always much more allergenic than the mycelium. Using PCA tests no cross-reactivity was obtained between mycelium extract and spore allergens from C. cladosprioides. A comparable situation was observed in Alternaria. Using biochemical and immunochemical techniques, Hoffman et al 20 and Aukrust et al 19 demonstrated that spore extracts were clearly distinct from mycelium extracts. Although spore extracts contained many similar or identical antigens to the mycelium preparation, spore specific antigens and allergens could not be detected in the mycelium extract of A. alternata. The results of these studies suggested that mycelium extract are deficient in spore specific allergens and consequently do not seem the most best source material for diagnosis and immunotherapy of mould allergy. However, in certain cases mycelium extracts of Alternaria alternata have greater potency than that of spore 21 due probably to a higher concentration of a 31 KDa major allergen 31.
The present study was carried out using RAST, RAST inhibition and PCA tests. It confirmed previous findings 26,7 suggesting that PCA is a very helpful technique to study mould allergy in animal model. A RAST made with partially purified fungal allergens can be comparable to skin test in both sensitivity and specificity 32,33. RAST inhibition assay have been suggested as a practical way to standardize allergens because it correlates quite well with the major allergen content 22. However, this test needs reliable solid-phase allergens containing all the allergenic determinants. Such studies should be repeated with different strains and species using different extraction procedures. Moreover, for RAST inhibition one needs a suitable pool of serum containing a representative amount of IgE antibodies directed toward both major and minor allergencic determinants in the extract tested. Changes in the serum pool is unavoidable resulting differences in the measurement of allergens 22. RAST inhibition has considerable advantages according to the direct RAST because it offers the possibility to compare efficiently different allergen extracts 34,16. Considerable cross-reactivity was found between Alternaria and Cladosporium 35 indicating the presence of shared allergenic determinants in these two fungi. Significant correlation between RAST inhibition values of Aspergillus restrictus and Aspergillus fumigatus was also obtained 16. Cross-reactivity of IgE-antibodies to allergens reflects the phylogenetic relationship of these organisms. This evidence of common allergenic determinants in these fungi was not clear between select species of Basidiomycetes and Deuteromycetes since a minimal cross-reactivity was observed 36. Such method should be used more often as a guidance for fungal extract production especially to monitor the homogeneity, the stability and the presence of the major allergen in the different batch extracts.
RAST, RAST inhibition results and PCA tests have demonstrated that spore and mycelium display different allergenic potency. However, discrepancies are found when one looks at the results obtained with spore and mycelium extracts of the same species. RAST inhibition indicated an important community between the two extracts whereas PCA showed an absence of cross-reactivity between the spore and the mycelial extract. The difference with human immuneresponse could result from the way of the sensitization leading to the recognition of different allergens in spore and mycelium. These allergens may be of carbohydrate nature which do not bind to RAST disc such as the β -Galactofuranoic glycoconjugate allergen of Aspergillus niger 37.
The immunochemical comparison of conidial and mycelial allergens of Cladosporium using an electroblotting technique is currently being undertaken. Cross-inhibition transfers should be performed to look for the presence of shared allergenic components between the spores and the mycelium of C. cladosporioïdes.