Beta-lactam antibiotics are the most frequent drugs prescribed in children worldwide. Acute rheumatic fever (ARF) is the major cause of acquired heart disease among children and adolescents. Recurrences due to inadequate penicillin prophylaxis are responsible for chronic valvular lesions requiring surgery. The fear of a severe allergic reaction is the leading cause of discontinuing prophylaxis.
ObjectiveIn this study, we aimed to reveal the frequency of adverse events and real allergic reactions to benzathine penicillin among children who are followed in our paediatric cardiology clinic with a diagnosis of ARF.
Materials methodsThe children who were followed with a diagnosis of ARF between January 2005 and December 2011 were searched for a history of penicillin allergy. Patients with a positive history were evaluated in our paediatric allergy clinic. Skin tests and provocation tests were performed with parental consent.
ResultsIn total 535 children with a diagnosis of ARF were analysed for the study. Median follow up period was 24 months (12–36) [median (%25–75)]. Eleven of our 535 (11/17.641 injection) ARF patients were suspected to have allergic reactions after 17.641 penicillin injections but only one (0.18%) was diagnosed to have penicillin allergy after detailed evaluation.
ConclusionOur data suggest that the frequency of penicillin allergy is much lower than suspected among children on penicillin prophylaxis for ARF. Consequently, penicillin prophylaxis should not be given up without proper evaluation of drug allergy.
Beta-lactam antibiotics are the most frequent drugs prescribed in children worldwide.1 Suspected allergic reactions to beta-lactams are reported by 10 to 20% of patients.2 Although a large majority of these children are labelled “penicillin-allergic” with a fear of severe reactions,3 only 5–10% are proven to have allergy when appropriate clinical investigation is performed.4–7
Acute rheumatic fever (ARF) is the major cause of acquired heart disease among children and adolescents,8,9 and a repository form of penicillin, benzathine penicillin G, remains the treatment of choice for secondary prevention of the disease.9,10
Recurrences due to inadequate penicillin prophylaxis are responsible for chronic valvular lesions requiring surgery.11 The fear of a severe allergic reaction is the leading cause of discontinuing prophylaxis.12 Most children reporting allergy-like reactions to beta-lactams are not allergic to beta-lactams and it has been shown that after further diagnostic work up the true frequency of beta-lactam allergy was 3.3% among children with suspected drug allergy.6 There are not enough data on the real frequency of proven penicillin allergy among children on penicillin prophylaxis for ARF.
In this study we aimed to reveal the frequency of adverse events and real allergic reactions to benzathine Penicillin among children who are followed in our paediatric cardiology clinic with a diagnosis of ARF.
Materials and methodsThe patient files of children followed with a diagnosis of ARF between January 2005 and December 2011 were reviewed for an immediate or non-immediate allergic reaction to prophylactic penicillin treatment. Patients with a recorded reaction to penicillin were invited for participation in the study and were evaluated retrospectively. All patients enrolled in the study fulfilled the Jones criteria for diagnosis of rheumatic fever: Major criteria include: (1) carditis; (2) polyarthritis; (3) chorea; (4) erythema marginatum; and (5) subcutaneous nodules. Minor criteria include: (1) fever; (2) arthralgia; (3) previous rheumatic fever or rheumatic heart disease; (4) leucocytosis; elevated eritrocyte sedimentation rate and C-reactive protein; and (5) prolonged P–R interval on electrocardiogram. A firm diagnosis requires that two major or one major and two minor criteria are satisfied, in addition to evidence of recent streptococcal infection.13 Benzathine Penicillin G had been given intramuscularly once every three weeks, 0.6 million unit for children under 27kg and 1.2 million unit for children over 27kg until they had a reaction to the drug. Penicillin treatment had been terminated after the reaction. The number of injections was calculated according to the follow up period until the reaction. Median follow up period was 24 months and ranged between 12 and 36 months. Detailed history of the drug reaction was recorded both from the patient files and from the patients and their parents. Skin tests (prick and intradermal) and provocation test was performed to patients who had suspected allergic reactions.14,15
Skin testsTests were performed using penicillin G (10,000U/ml), benzylpenicilloyl polylysine(PPL®) (5×10−5mmol/L), and minor determinant mixture (MDM®)(2×10−2mmol/L) (Kit DAP from Diater Laboratorios S.A., Madrid, Spain).16 Histamine (1%) was used as positive and 0.9% NaCl was used as negative control. Epidermal prick test was undertaken first and intradermal test followed when the former was negative. In the skin prick tests 15minutes after testing, a wheal larger than 3mm accompanied by erythema with a negative response to the control saline was considered positive (26). In the intradermal tests the wheal area was marked initially and 20minutes after testing, and an increase in diameter greater than 3mm was considered positive.17
Provocation testFinally, both for immediate and non-immediate reactions, a provocation test was performed if all the other investigations were inconclusive and if the provocation test was not contra-indicated. ENDA guidelines on skin and drug provocation tests were carefully respected.17,18 Oral provocation was performed with 50,000U, 100,000U and 250,000U penicillin V suspensions at 20min intervals. If there was no reaction during oral provocation, benzathine penicillin was administered intramuscularly (0.6 million unit for children under 27kg and 1.2 million unit for children over 27kg).
The study was approved by the local ethics committee (Approval number: 2012-018). Informed consent was taken from parents of patients for participation in the study.
ResultsIn total 535 children with a diagnosis of ARF were analysed for the study. Mean age of patients was 10.8±2.3 years (5–16) and 46% of them were boys. Median follow up period was 24 months (12–36) [median (%25–75)]. Three hundred and sixteen patients were diagnosed as suffering ARF while in acute period, according to Jones criteria. Two hundred and nineteen patients were attended in our clinic with late complications of ARF.
Within the 316 patients with acute symptoms, recurrence rates were 4.1% and 65.2% respectively (p<0.001) for patients who were compatible and incompatible with penicillin prophylaxis respectively. Valvular lesions were recorded in 44.6% and 68.9% of patients who were compatible and incompatible with penicillin prophylaxis respectively (p=0.003).
For all patients, 21,169.2 million units benzathine penicillin by 17,641 injections was administered. The median number of injections per patient was 32 (4–96). Eleven patients (2%) were reported to have suspected allergic reaction after penicillin injection; a suspected allergic reaction was recorded after 0.06% of all injections.
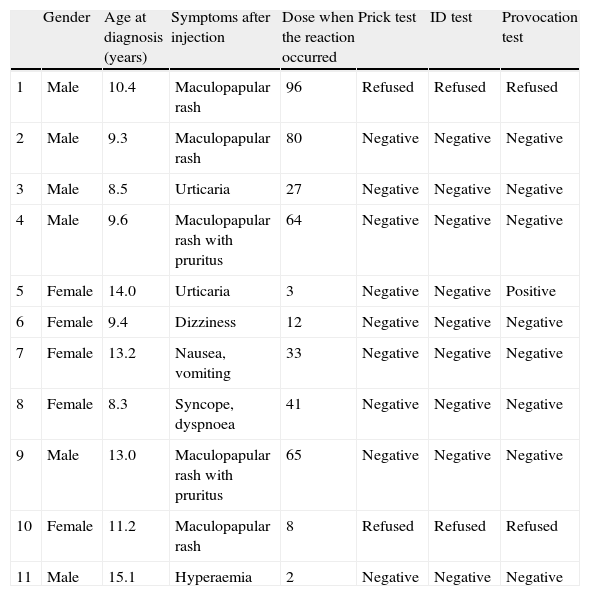
Among these 11 patients, eight had only cutaneous symptoms, one had nausea and vomiting, one had dizziness (vertigo), and one had syncope with dyspnoea. There were no fatal reactions. Nine of these patients consented to have further clinical evaluation. Two patients who had maculopapular rash after penicillin injection refused to have further evaluation for drug allergy. Among nine patients who accepted diagnostic evaluation, both skin prick tests and intradermal tests were all negative and provocation was positive in only one patient. This patient had had urticaria after her third injection and she had urticaria again after intramuscular injection during the provocation test. She was diagnosed to have immediate type hypersensitivity reaction to penicillin (Table 1). According to these results, 0.18% (1/535) of the patients had immediate type hypersensitivity reaction to penicillin and allergic reaction was positive in 0.0056% (1/17,641) of all injections.
Characteristics of patients with a history of suspected allergic reaction after penicillin injection.
| Gender | Age at diagnosis (years) | Symptoms after injection | Dose when the reaction occurred | Prick test | ID test | Provocation test | |
| 1 | Male | 10.4 | Maculopapular rash | 96 | Refused | Refused | Refused |
| 2 | Male | 9.3 | Maculopapular rash | 80 | Negative | Negative | Negative |
| 3 | Male | 8.5 | Urticaria | 27 | Negative | Negative | Negative |
| 4 | Male | 9.6 | Maculopapular rash with pruritus | 64 | Negative | Negative | Negative |
| 5 | Female | 14.0 | Urticaria | 3 | Negative | Negative | Positive |
| 6 | Female | 9.4 | Dizziness | 12 | Negative | Negative | Negative |
| 7 | Female | 13.2 | Nausea, vomiting | 33 | Negative | Negative | Negative |
| 8 | Female | 8.3 | Syncope, dyspnoea | 41 | Negative | Negative | Negative |
| 9 | Male | 13.0 | Maculopapular rash with pruritus | 65 | Negative | Negative | Negative |
| 10 | Female | 11.2 | Maculopapular rash | 8 | Refused | Refused | Refused |
| 11 | Male | 15.1 | Hyperaemia | 2 | Negative | Negative | Negative |
Eight of 11 patients continued to have prophylaxis with benzathine penicillin without any allergic reaction during subsequent penicillin injections.
DiscussionEleven of our 535 ARF patients were suspected to have allergic reactions after 17,641 penicillin injections but only one (0.18%) was diagnosed with immediate type hypersensitivity reaction to penicillin after detailed evaluation. All patients with negative results during allergy testing continued their benzathine penicillin prophylaxis without adverse events. Even the patient with a history suggesting anaphylaxis tested negative with skin test and oral provocation and did not have any allergic reaction during subsequent penicillin injections.
In an international study undertaken in 11 countries including 1790 adults and children having rheumatic fever, allergic reactions to penicillin were reported as 3.2% (57/1790) in a 3.4 year period. Anaphylaxis was present in 0.2% of patients and there was not any anaphylactic reaction among patients less than 12 years of age. The risk of fatal anaphylaxis was reported as 1/32,000 injections in that study.19 In a smaller study from Taiwan, 10 of 171 rheumatic fever patients (5.8%) were reported to have allergic reactions after benzathine penicillin injection.12 In accordance with these data, 2% of our patients had had suspected allergic reactions.Although commonly reported, most patients who have a history of a prior reaction to penicillin are not found to be allergic to penicillin upon skin testing.20 In our study only one of 11 patients with suspected allergic reaction was determined to have proven immediate type hypersensitivity reaction to penicillin. Overall, penicillin allergy was established in 0.18% of our ARF patients who were on prophylaxis with benzathine penicillin. Allergic evaluation with skin tests and oral provocation was not performed in the aforementioned studies about penicillin allergy among ARF patients. To our knowledge, this is the first study giving data on proven penicillin allergy among children with ARF. Our data suggest that the frequency of penicillin allergy is very much lower than suspected among children on penicillin prophylaxis for ARF.
One of the reasons for ceasing prophylaxis with penicillin is the suspicion that longer duration of penicillin treatment can increase the risk of allergy. The frequency of allergic reactions to beta-lactam antibiotics are reported to be 1–10%21 Weiss et al. reported a frequency of 2.24% in patients who received short term treatment with benzathine penicillin.22 Thus, according to our results and the data mentioned above, the frequency of allergic reactions among patients having long-term or short-term penicillin treatment seems to be similar. In an early study of benzathine penicillin prophylaxis for rheumatic fever, allergic reaction rates of patients receiving long term benzathine penicillin prophylaxis for ARF were similar to patients without rheumatic fever who receive short-term treatment with parenteral penicillin.19 Penicillin prophylaxis is important in the prevention of recurrences in ARF: The recurrence rate was 4% for our patients on regular penicillin prophylaxis, compared to 65.2 among patients who were incompatible with treatment. In another study, rheumatic fever recurred in eight (0.45%) of 1790 patients while on regular prophylaxis compared with 11 (11.5%) of 96 who were not.19 The remarkable increase of the recurrence rate among patients without regular penicillin prophylaxis emphasizes the importance of proper evaluation of penicillin allergy.
Avoidance of penicillin, based on self-reported allergic history alone often leads to the use of an alternate antibiotic with greater cost or side effect profile or treatment failure.23 Macrolide group antibiotics are commonly preferred for the treatment and prophylaxis for group A streptococcus with a fear of severe allergic reactions but macrolide resistance in group A Streptococcus has been well documented in several countries.24
In conclusion, allergic reactions to penicillin in patients with a history of rheumatic fever and under prophylaxis with benzathine penicillin are rare, and are no more frequent than in patients without rheumatic fever who receive short courses of parenteral penicillin for other indications. Penicillin prophylaxis is of utmost importance to decrease recurrences of ARF and only a minority of the patients with suspected reactions after injection have real penicillin allergy. Consequently, penicillin prophylaxis should not be given up without proper evaluation of drug allergy.
Ethical disclosuresProtection of human subjects and animals in researchWe declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Patients’ data protectionWe declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestAll of the authors have no conflicts of interest in the manuscript.